Abstract
Rho GTPases oscillate between an inactive GDP-bound state and an active GTP-bound state. They are activated by Rho Guanine nucleotide Exchange Factors (GEF), which accelerate the GDP to GTP exchange. RhoGEFs fall into two different classes: the Dbl family and the DOCK family of proteins. In this review, we focus on the function and regulation of the Dbl family RhoGEF Trio. Trio and its paralog Kalirin are unique within this family in that they display two GEF domains of distinct specificity. Trio is a major regulator of neuronal development, and its function is conserved through evolution. Moreover, Trio plays an important role in cell adhesion and in signaling pathways elicited by Gαq protein-coupled receptors. Combined, these observations suggest that Trio has a major role in cellular physiology. Of note, Trio is an essential gene for mouse development, with a prominent role in the development of the nervous system. Finally, Trio expression is significantly increased in different types of tumors and it has been proposed that it could participate in oncogenesis.
Keywords: Rho GTPases, GEF, Trio, UNC-73, actin cytoskeleton remodeling, neuronal differentiation, cell adhesion, GEF inhibitors, migration and invasion, cancer
Introduction
The small GTPases of the Rho (Ras homologous) family represent a major branch of the Ras superfamily of GTPases and comprise around 20 members in mammals. Rho GTPases control many different cellular processes including actin cytoskeleton remodeling, microtubule dynamics, gene transcription, and phospholipid metabolism. By controlling this wide range of basic cellular processes, Rho GTPases are involved in major functions such as cell polarity, adhesion, cell motility, growth, and differentiation.1 It is thus not surprising that deregulation of their activities has been associated with different kinds of diseases, including cancer, neurological, cardiovascular, and infectious diseases.
Rho GTPases are basic switches that oscillate between an inactive GDP-bound state and an active GTP-bound state (Fig. 1). This cycle is tightly regulated by three types of proteins2: Rho Guanine nucleotide Exchange Factors (GEF) accelerate the exchange of GDP for GTP; GTPase Activating Proteins (GAP) catalyze the hydrolysis of GTP, rendering the GTPase inactive; the family of Guanine Dissociation Inhibitors (GDI) sequesters the GTPases in the cytosol before they are targeted to the plasma membrane where they are activated by GEFs. Once bound to GTP, GTPases specifically bind to numerous effectors that trigger various cellular responses.
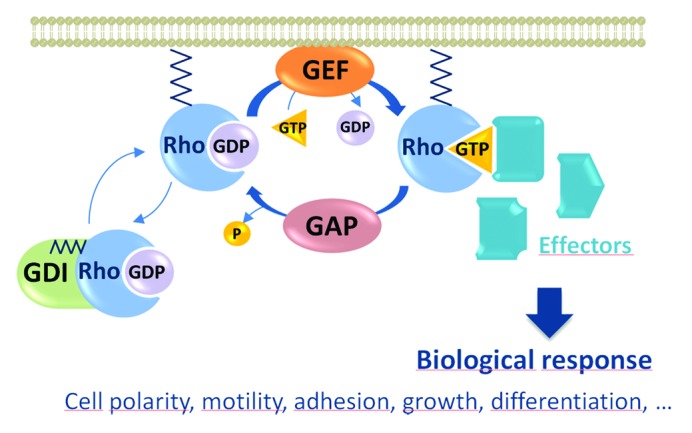
Figure 1. Regulation of RhoGTPase activity by three classes of proteins: GEFs, GAPs, and GDIs. Rho GTPases are basic switches that oscillate between an inactive GDP-bound state and an active GTP-bound state. The family of GDIs sequesters the GTPases in the cytosol before they are targeted to the plasma membrane where they are activated by GEFs. GEFs, which receive activation by upstream signals, promote GDP/GTP exchange on the GTPases. Once bound to GTP, GTPases specifically bind to numerous effectors that trigger various cellular responses. GAPs catalyze the hydrolysis of GTP, rendering the GTPase inactive.
Rho GTPases respond to a wide range of external stimuli, such as growth factors, hormones, and cytokines.3 Among the GTPase regulators, RhoGEFs have received a lot of attention because they relay the external stimuli leading to activation of Rho GTPases. RhoGEFs fall into two different classes: the family of Dbl proteins4 and the more recently identified family of DOCK proteins (DHR-2/CZH).5
The family of Dbl proteins comprises 70 members that all share a catalytic domain, called Dbl Homology (DH) domain, in reference to Dbl, the first RhoGEF identified as an oncogene in mammalian cells, and a Pleckstrin-Homology (PH) domain that plays a role in GEF activation and localization.6 In addition to this invariant module, Dbl family GEFs display a number of different catalytic or protein-protein interaction motifs, leading to the concept that RhoGEFs connect Rho GTPase signaling to upstream receptors or intracellular pathways.7
The 11 members of the DOCK family of RhoGEFs display a catalytic domain completely different from the Dbl domain, the DHR-2 or CZH domain, which is also conserved through evolution.8 The specificity of DOCK proteins has so far been found to be restricted to the GTPases Rac1 and Cdc42, in contrast to the Dbl family members that can also act as GEFs for other GTPases including RhoA.
The mode of regulation of RhoGEFs is quite diverse within the two families of RhoGEFs. One explanation for this diversity is that RhoGEFs display a complex structural organization with various accessory domains, which can participate in RhoGEF regulation in different ways (reviewed in Cherfils et al.2). Indeed, RhoGEFs can be regulated by protein-protein or intra-molecular interactions, or by control of their subcellular localization such as membrane targeting or nuclear sequestration.2 For example, a small sub-family of RhoA-specific RhoGEFs contains RGS (regulator of G-protein signaling) domains. Binding of the α-subunits of heterotrimeric G proteins to the RGS domain promotes RhoGEF activity, allowing the control of RhoA activation downstream of heterotrimeric-coupled receptors.9 Another example is DOCK180, whose activity toward Rac1 is regulated by binding to its partner ELMO, which might aid targeting DOCK180 to the plasma membrane.10 In addition, RhoGEF activity can be also controlled by post-translational modifications such as phosphorylation. This is the case for the Vav proteins where tyrosine phosphorylation mediated by Src-family tyrosine kinases results in RhoGEF activation.11,12
The striking observation that the number of RhoGEFs (more than 80, including both Dbl and DOCK families of proteins) far exceeds the number of GTPases (22) has led to the hypothesis that there is redundancy within the Rho regulators. However, the divergence in the accessory protein domains found beside the catalytic domains and the tissue- and/or developmental-specific distribution of the different members possibly rather reflect their involvement in specific pathways to control Rho GTPase activity.
In this review, we will focus on the RhoGEF Trio, giving an overview of its function in mediating various biological processes. Trio and its paralog Kalirin are the only members of the mammalian Dbl family that display two GEF domains of distinct specificity. Trio is a complex molecule that harbors various protein-protein interaction domains, indicating its integration in numerous signaling pathways. Trio is a major regulator of cell motility, axon outgrowth, and guidance, mainly through the activation of Rac1 and RhoG, and this function is conserved through evolution. Moreover, numerous lines of evidence support an important role of Trio in cell adhesion during different physiological processes and in signaling pathways elicited by Gαq protein-coupled receptors. In addition, Trio is an essential gene for mouse development, with a prominent role in the development of the nervous system. Finally, Trio expression is significantly increased in different types of tumors and it has been proposed that it could participate in oncogenesis.
Function and Regulation of the RhoGEF Trio
The family of Trio proteins
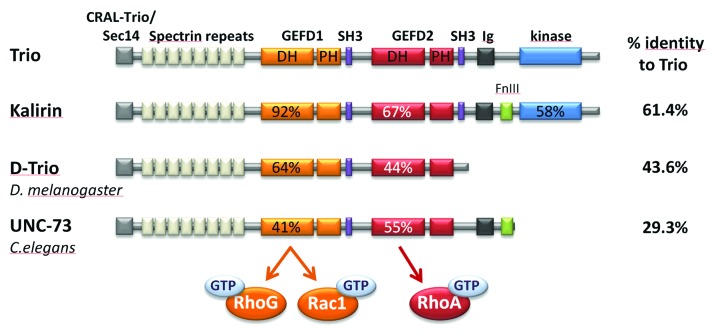
Trio has originally been discovered in a two-hybrid screen as an interactor of the transmembrane tyrosine phosphatase LAR.13 The name of Trio refers to the fact that Trio displays three enzymatic domains, two GEF, and one serine kinase domains (Fig. 2). In addition, Trio harbors two SH3 motifs, a CRAL-Trio/Sec14 motif, an Ig motif, and several spectrin-like repeats. The GEFD1 domain activates both GTPases Rac1 and RhoG,13,14 while the GEFD2 domain acts specifically on RhoA.13,15 Kalirin, the other member of this sub-family in mammals, was identified as an interactor of the peptidylglycine α-amidating monooxygenase, an enzyme involved in the secretory pathway of neuroendocrine peptides.16 Trio expression is ubiquitous, while the one of Kalirin is mostly restricted to the nervous system.13,17 Two orthologs of Trio/Kalirin exist in invertebrates, the unc-73 gene in C. elegans and D-Trio in Drosophila. The overall organization of Trio family proteins in different organisms is remarkably conserved (Fig. 2). The main divergence between the proteins occurs after the second GEF domain, where only vertebrate Trio and Kalirin proteins harbor a serine kinase domain. In addition, Trio and Kalirin are similarly distant to D-Trio (43.6% and 43.2% of amino-acid identity, respectively) and to UNC-73 (29.3% and 29.6% of amino-acid identity, respectively).
Figure 2. The family of Trio proteins. The name of Trio refers to the fact that Trio displays three enzymatic domains, two GEF and one serine kinase domains. In addition, Trio harbors numerous accessory domains. Listed from N-terminus to C-terminus, these include: a CRAL-Trio/Sec14 motif, several spectrin-like repeats, two SH3 motifs and an Immunoglobulin (Ig) domain. The Trio family consists of two paralogs in mammals, Trio and Kalirin. Two orthologs of Trio/Kalirin exist in invertebrates, UNC-73 in C. elegans and D-Trio in D. melanogaster. The overall organization of Trio family proteins in different organisms is remarkably conserved. The main divergence between the proteins occurs after the second GEF domain, where only vertebrate Trio and Kalirin proteins harbor a serine kinase domain. The percentage of identity at the protein level between Trio and the other members is shown.
Trio isoforms
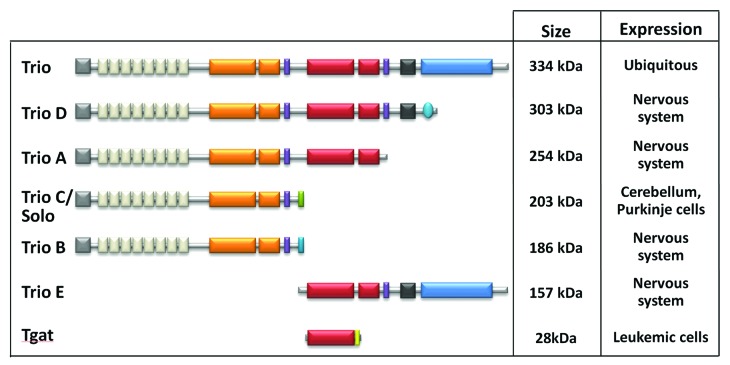
In addition to its complex structure, the trio gene can be alternatively spliced and, as a result, can encode several isoforms named TrioA-E and Tgat, ranging from 28 to 334 kDa in apparent molecular weight (Fig. 3). Five isoforms (TrioA-E) that differ in their C-terminus have been described to be specific to the nervous system.18,19 These isoforms display either one or both GEF domains, suggesting that they could differentially regulate Rac1 and RhoA. Interestingly, TrioC/Solo, containing only the Rac1-specific GEF domain, is specifically expressed after birth in the Purkinje cells of the developing cerebellum.19 TrioC has been shown to modulate endosome dynamics and neurite outgrowth in Purkinje cells, suggesting that it could play an essential role in cerebellar development through its role in Purkinje cell differentiation.20 In addition, a sixth, oncogenic isoform of Trio, named Tgat, has been isolated from ATL (adult-T cell leukemia) patients. Tgat displays only the RhoA specific DH domain followed by a short C-terminal peptide and is able to trigger tumor formation following xenografts in nude mice.21
Figure 3. Trio isoforms. In addition to its complex structure, the trio gene can encode several isoforms as a result of alternative splicing. Five isoforms (TrioA to E) that differ in their C-terminus have been described to be specific of the nervous system, with TrioC/Solo expressed only after birth in the Purkinje cells of cerebellum. In addition, a sixth, oncogenic isoform of Trio, named Tgat, has been isolated from ATL (adult-T cell leukemia) patients.
Regulation of Trio RhoGEF activity
Regulation by protein-protein interaction
The activity of the two RhoGEF domains of Trio can be regulated by the different protein-protein interaction domains present in its sequence, as illustrated in the examples described hereafter.
The DH1 activity of GEFD1 requires the presence of its associated PH1 domain for a full activation of RhoG/Rac1. In addition to playing a role in the catalytic reaction, the PH1 domain also targets the protein to the cytoskeleton, via its binding to the actin cross-linker Filamin A.22,23 Trio may also be targeted to the cytoskeleton by binding to Trio-associated repeat on actin (Tara) protein through the GEFD1 domain.24 In contrast to the positive role played by the PH1 domain on the GEF activity of DH1, the PH2 domain plays an inhibitory role on the in vitro DH2 activity on RhoA.23 Interestingly, the molecular mechanisms of this PH-mediated inhibition have been solved by structural studies performed on the RhoA-specific p63RhoGEF, which is closely related to Trio. p63RhoGEF mediates Gαq signaling by activating RhoA25 and its GEF activity is also inhibited by its PH domain.26 The resolution of the crystal structure of p63RhoGEF with Gαq shows that Gαq directly activates p63RhoGEF by binding to an extension of the PH domain.27 Interestingly, this domain is conserved in the PH2 domain of Trio, and likewise, Gαq binds to and activates the Trio GEFD2 domain.28
The spectrin-like domains also play a major role in inhibiting Trio activity toward Rac1.29 The exact mechanism of this inhibition is not fully understood, but different studies support a model whereby proteins binding the spectrin-like domains would relieve the proposed auto-inhibitory constraint. The first example is the schizophrenia susceptibility gene Disrupted-in-Schizophrenia 1 (DISC1), which has been shown to be involved in axon guidance in C. elegans through a Trio/Rac/PAK pathway.30 DISC1 specifically binds to the spectrin-like repeats, releasing inhibition of the GEF domain and thereby facilitating the recruitment of Rac1 to Trio. The second example is the integral membrane protein Kidins220/ARMS, a downstream target of neurotrophins, which also binds to Trio spectrin-like repeats. It has been proposed that this binding may localize Trio to specific membrane sites and regulate its activity, leading to Rac1 activation and neurite outgrowth.31
Regulation by tyrosine phosphorylation
Like other Dbl RhoGEFs, Trio has been shown to be regulated by phosphorylation. For example, phosphorylation of Trio by the Src-family tyrosine kinase Fyn is essential for Rac1 activation and axon outgrowth induced by the guidance cue netrin-1.32 The Abl tyrosine kinase has also been proposed as a candidate regulator of Trio function in Drosophila. Trio and Abl cooperate in regulating axon outgrowth in the fly embryonic central nervous system (CNS) and physically interact.33,34 Trio tyrosine phosphorylation increases dramatically with co-expression of Abl, but it remains to be determined whether Trio is a direct target of the Abl kinase, and whether phosphorylation by Abl modulates Trio function during axon guidance.33,34 Together, these data suggest that Abl and Trio are integrated into a complex signaling network that regulates axon guidance at the CNS midline.
Trio function and upstream regulatory pathways
Trio in neuronal development
Evolutionary conserved function of Trio in nervous system development
The first indication of Trio function came from C. elegans studies, when it was discovered that unc-73, a major regulator of axon guidance and cell motility, was the ortholog of mammalian Trio.35 Two years later, the Drosophila trio gene was shown in four distinct studies to be essential for axon guidance in the central and peripheral nervous system of the fly via activation of Rac through its first GEF domain.34,36-38 Trio k.o. mice were shown to be embryonic lethal and presented an aberrant organization in different brain regions and defects in secondary myogenesis.39 The specific deletion of Trio in the nervous system induces severe defects, as 90% of the trio-deleted pups died within 1 day after birth. The few pups surviving up to one month display an aberrant granule cell migration in the developing cerebellum as well as abnormal neurite outgrowth, generating a severe ataxia in these animals.40 It remains to be determined whether Trio also plays an essential role in the development of the Purkinje cells where the isoform Trio C is highly expressed after birth.20 The dramatic phenotypes observed in the trio k.o. mice demonstrate that Kalirin, the other member of the Trio family, is not capable of replacing Trio in neuronal development. This can easily be explained by the fact that Kalirin-7 is preferentially expressed in the adult brain, where it has been shown to play a key role in the morphogenesis of dendritic spines.41 Consistently, kalirin k.o. mice are viable and display different behavioral phenotypes related to Kalirin’s established role in spine morphogenesis.42,43 This suggests that Trio and Kalirin functions might have diverged in mammals during evolution. Finally, Trio is one of the few RhoGEFs described so far to be essential for the development of the nervous system in mammals.39,40,44
Trio and axon outgrowth and guidance
Several studies demonstrate a role for mammalian Trio in axon outgrowth downstream of different extracellular signals, such as Nerve Growth Factor (NGF) and netrin-1.29,31,32,45 Trio was first shown to induce neurite outgrowth in response to NGF in PC12 cells. This is mediated by RhoG activation by Trio’s first RhoGEF domain.29 Trio interacts with the integral membrane Kidins220/ARMS, a downstream target of neurotrophins, and both cooperate for Rac1 activation and neurite outgrowth.31
Netrin-1 is a major guidance cue that regulates axon attraction and guidance through its receptor DCC (Deleted in Colorectal Cancer).46 Genetic and physical interactions between D-Trio and the fly ortholog of DCC, Frazzled (Fra), have been described during axon pathfinding at the Drosophila embryonic CNS midline,33 suggesting that Trio could take part in netrin-1/DCC signaling. Consistently, trio k.o. mice display axon guidance defects in netrin-1/DCC-dependent axonal projections.45 In addition, netrin-1-induced Rac1 activation and axon outgrowth are impaired in the absence of Trio.45 All together, these studies show that the RhoGEF Trio mediates Rac1 activation and axon outgrowth and guidance in response to netrin-1.45 A more precise analysis of the netrin-1/DCC/Trio signaling pathway shows that phosphorylation of Trio by the Src-family tyrosine kinase Fyn is essential for Rac1 activation by netrin-1 and for the proper targeting of DCC to the cell surface of growth cones, in order to mediate netrin-1-induced cortical axon outgrowth (Fig. 4A).32 The role of Trio in targeting DCC to the membrane is consistent with studies in C. elegans that support the model whereby UNC-73 acts upstream of UNC-40 (ortholog of DCC) and of SAX-3 (ROBO) to positively regulate their levels at the cell membrane.47,48 In these pathways, UNC-73 cooperates positively with the kinesin-like protein VAB-847,48 and, in the SAX-3 pathway, negatively with CRML-1, the C. elegans ortholog of CARMIL (capping protein, Arp2/3, and Myosin-I linker), a protein controlling actin dynamics in lamellipodia in mammals (Fig. 4B).49
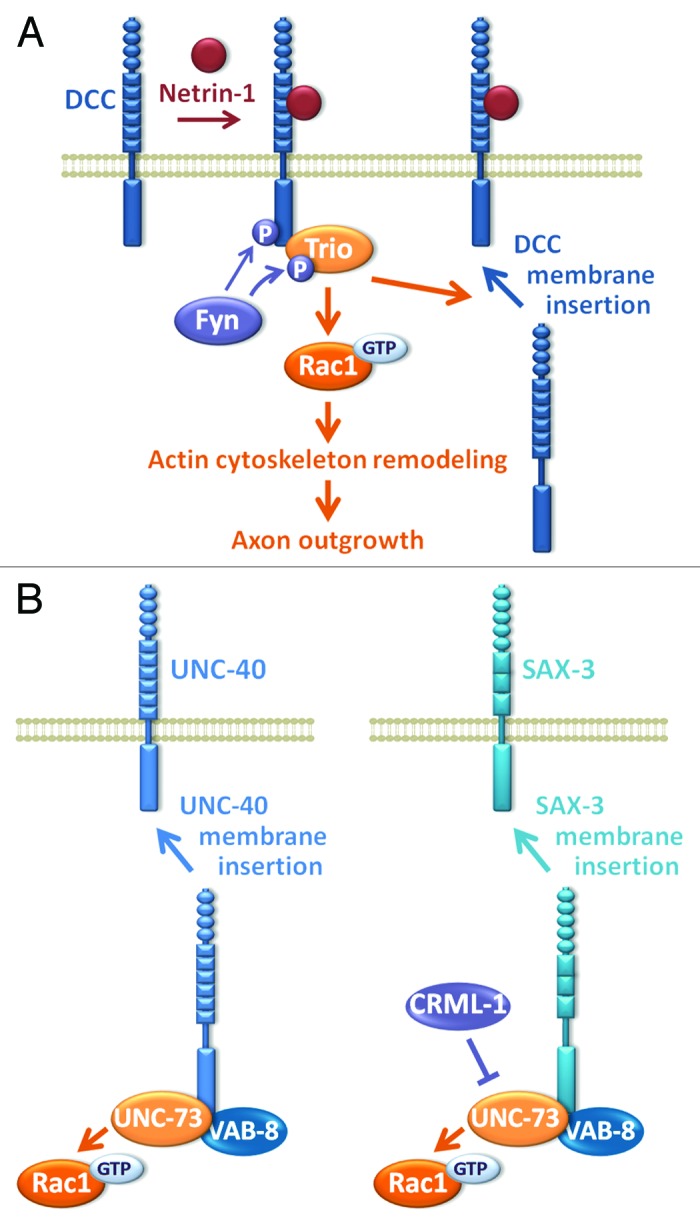
Figure 4. Trio and upstream regulators in axon outgrowth and guidance. (A) Trio mediates Rac1 activation and axon outgrowth and guidance in response to netrin-1 in mammals. Phosphorylation of Trio by the Src-family tyrosine kinase Fyn is essential for Rac1 activation by netrin-1 and for the proper targeting of DCC to the cell surface of growth cones in order to mediate netrin-1-induced cortical axon outgrowth. (B) In C. elegans, UNC-73 acts upstream of UNC-40 (ortholog of DCC) and of SAX-3 (ortholog of Robo) to positively regulate their levels at the cell membrane. UNC-73 cooperates positively with the kinesin-like protein VAB-8 and, in the SAX-3 pathway, negatively with CRML-1, the C. elegans ortholog of CARMIL (capping protein, Arp2/3, and Myosin-I linker), a protein controlling actin dynamics in lamellipodia in mammals.
Trio and synapse physiology
In addition to its main effects on axon outgrowth and guidance, studies have shown that Trio also plays important roles in other aspects of neuronal development. Trio mutants display a reduction in dendritic branching in sensory neurons of the Drosophila embryo, and this effect of D-Trio is mediated by Rac1.50,51 The role of mammalian Trio in this process has not yet been investigated, while, as described above, Kalirin has been shown to be a major player of dendritic spine morphogenesis and synapse formation.43,52-54
D-Trio has also been shown to play an important role in Drosophila larval neuromuscular junction (NMJ), which is under the control of BMP signaling. BMP regulates D-Trio at the transcriptional level, and D-Trio, together with Rac, participates in pre-synaptic growth and neurotransmitter release at the NMJ.55
Trio in cell adhesion
In addition to the established role of Trio in neuronal development, other functions have been ascribed to Trio more recently, which is not surprising considering its ubiquitous expression. Numerous lines of evidence suggest that Trio is activated by adhesive receptors, especially cadherins, and thereby participates in cell adhesion-mediated physiological processes56-59 (Fig. 5A). For instance, Trio promotes Rac1 activation triggered by M-cadherin during fusion of myotubes in vitro, which is in agreement with the defects in secondary myogenesis observed in trio k.o. embryos.56
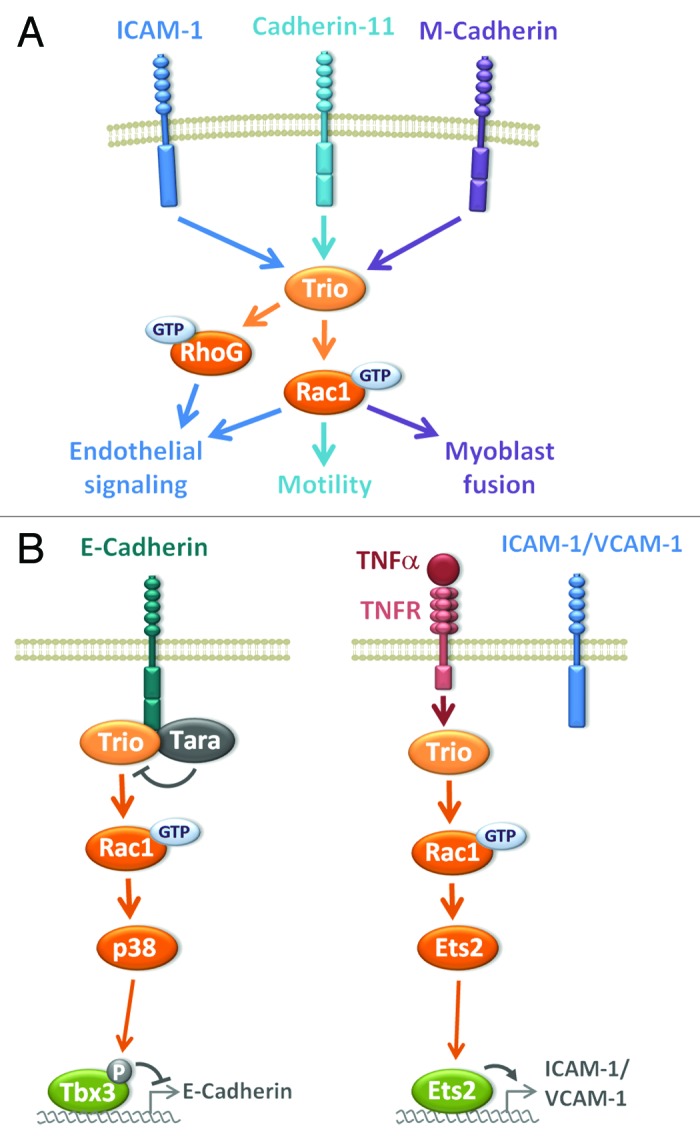
Figure 5. Trio in cell adhesion. (A) Trio is activated by binding to adhesive receptors, such as cadherins and integrins of the ICAM family and thereby participates to several cell adhesion-mediated physiological processes, mainly through Rac1 activation and actin cytoskeleton remodeling. (B) Trio impinges on adhesive receptor expression at the transcriptional level through Rac1 activation. Left panel: Trio-mediated Rac1 signaling induces the phosphorylation of the transcriptional repressor Tbx3, leading to a decrease in E-cadherin expression. Tara upregulates E-cadherin transcription, by inhibiting Trio. Right panel: Trio regulates a novel pro-inflammatory pathway during transendothelial migration by controlling the expression of VCAM-1/ICAM-1 through Rac1-mediated activation of the transcription factor Ets2 in response to the cytokine TNFα.
Different studies have revealed that cadherin-11 represents an important partner for Trio. Trio binds to cadherin-11 in the mouse hindbrain where it controls the mature organization of neuronal clusters.57 The cranial neural crest (CNC) is a highly migratory mesenchymal cell population that migrates during development from the neural plate to contribute to the formation of cartilages and bones of the vertebrate face.60-62 Kashef and colleagues report that Xenopus x-cadherin-11 is essential for the motility of the CNC. They show that Trio is a downstream target of x-cadherin-11 in these CNC cells, mediating protrusive activity through Rho GTPase activation and cell migration.58 Surprisingly, rescue experiments show that both GEF activities of Trio toward Rac1 and RhoA are required in this process. Trio has also recently been involved in the regulation of cell-cell contact during contact inhibition of locomotion (CIL) of the CNC, a process by which, after cell-cell contact, a cell reorients and migrates in another direction.63 This process is essential for morphogenesis during development, and its failure is thought to participate in cancer invasion.64 Moore and colleagues described the essential role of the cell polarity protein Par3 during CIL, where Par3 binds to Trio and inhibits its GEF activity toward Rac1 at cell-cell contacts.59 Interestingly, the inhibition of Rac1 induced by Par3 at cell-cell contacts leads to microtubule catastrophe and re-orientation of the CNC migration.59 This study highlights a novel pathway where Trio is involved in modulating microtubule dynamics during the collective and directional migration of neural crest cells.59 It remains to be determined whether cadherin-11 is also part of this Par3/Trio signaling pathway.
In addition to being downstream of cadherin signaling, Trio has been proposed to impige on E-cadherin expression in epithelial cells (Fig. 5B). In a screen aimed at identifying proteins enriched in adherens junctions (AJs) in epithelial cells, Yano and colleagues identified the Trio-associated repeat on actin (Tara) protein, a binding partner of Trio described as a F-actin binding protein that increases cell spreading.24,65 Tara is enriched at AJs through its association with Trio and E-cadherin. Tara upregulates E-cadherin transcription, by inhibiting Trio-mediated Rac1 signaling (Fig. 5B). The molecular mechanism by which Tara inhibits Trio activation remains unclear. Given the fact that Tara binds GEFD1, we can speculate that Tara binding impairs Rac1 recruitment to Trio. This study describes a novel signaling pathway wherein Tara, Trio and Rac1 modulate E-cadherin expression at the transcriptional level, highlighting a novel signaling pathway controlling E-cadherin expression initiated at AJs.65
In addition to cadherin-mediated adhesion processes, Trio has also been involved in integrin-mediated cell adhesion. A good example is leukocyte transendothelial migration (TEM), a process in which the endothelial cells participate actively, by forming a ring-like actin-rich structure around the adherent leukocyte. This process is dependent on the binding of leukocytic β2-integrin to the endothelial adhesion molecule ICAM-1, which triggers ICAM-1 clustering and thereby endothelial signaling, including actin cytoskeleton remodeling.66 A recent study shows that ICAM-1 binds to Trio, and that Trio promotes leukocyte transendothelial migration in a filamin-dependent manner through Rac1 and RhoG, by controlling the formation of the endothelial docking structure (Fig. 5A).67 During inflammation, cytokines are produced and play a key role in leukocyte recruitment by stimulating the expression of adhesion molecules on the endothelium. Interestingly, Trio has been shown to be involved in the TNF-α inflammatory pathway by controlling the expression of VCAM-1/ICAM-1 through the transcription factor Ets2 (Fig. 5B).68
Trio in other developmental functions
Phagocytosis of cells undergoing apoptosis is essential during development, cellular turnover and wound healing. The adaptor protein ELMO and its ortholog CED-12 in C. elegans play a critical role in engulfment in mammals and worms.69 ELMO functions together with the RhoGEF DOCK180 to activate Rac1 during this process. An interesting study has shown that Trio is activating RhoG, which in turn binds to ELMO, leading to DOCK180-mediated Rac1 activation during engulfment in worms and mammals.70 The link between RhoG and Rac1 through ELMO/DOCK-180 was confirmed during RhoG-induced neurite outgrowth and during integrin-mediated activation of Rac1.71
Despite the fact that Trio harbors two GEF domains activating RhoG/Rac and RhoA respectively, the different functions of Trio described so far in this review seem to be mediated mainly by Rac1/RhoG activation via the GEFD1 domain. The physiological function and the regulation of the RhoA-specific GEFD2 domain of Trio have remained elusive for a long time. The first data uncovering a role of the activation of RhoA by Trio in vivo came from studies in C. elegans.72 In the worm, 8 UNC-73 isoforms exist, including isoforms containing only the GEFD2 domain, which do not participate in the axon guidance process. Genetic studies have shown that these UNC-73 isoforms control pharynx and vulva musculature and modulate synaptic neurotransmission.72 As described before, in vitro binding of Gαq to Trio releases the PH2-mediated inhibition of DH2, and thereby activates the GEFD2 activity toward RhoA. The in vivo understanding of this Gαq/Trio signaling pathway was elucidated in C. elegans. Indeed, UNC-73 isoforms are activated by Gαq and are major effectors of the Gαq pathway leading to egg laying, locomotion, and growth of the animal.73 The activation of RhoA by UNC-73 in the Gαq pathway therefore plays a major role in the development and the physiology of the worm (Fig. 6A).
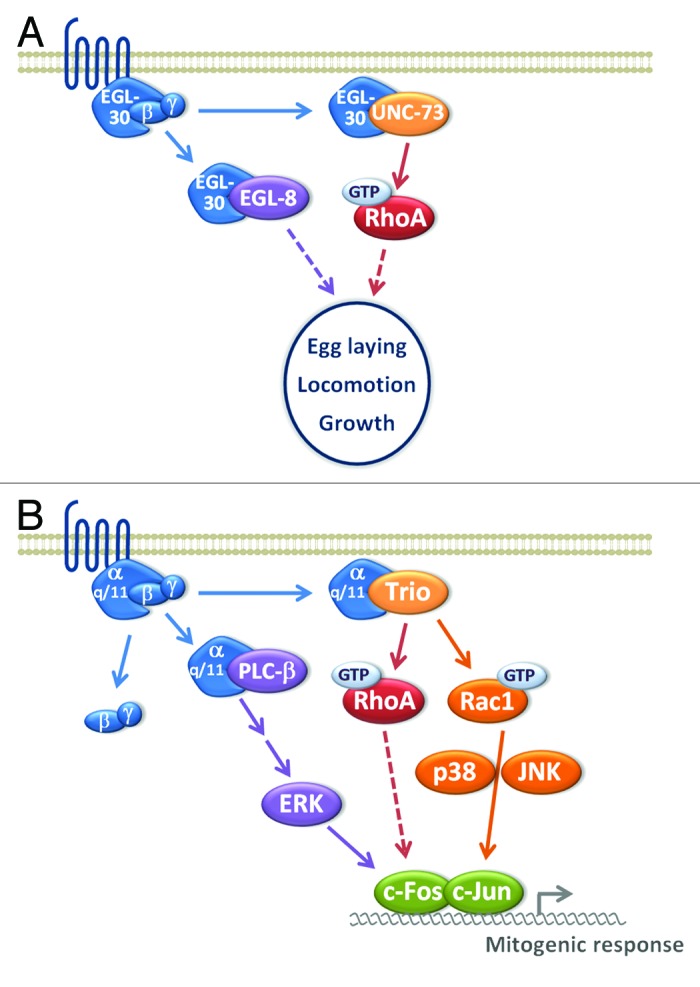
Figure 6. Trio in physiological processes initiated by Gαq-protein coupled receptors. In vitro binding of Gαq to Trio releases the PH2-mediated inhibition of DH2, and thereby activates the GEFD2 activity toward RhoA. (A) In C. elegans, EGL-30/Gαq binding to UNC-73 isoforms activates RhoA, leading to egg laying, locomotion and growth of the animal together with the EGL-8/PLCβ pathway. UNC-73 isoforms are therefore major effectors of the Gαq pathway and play a major role in the development and the physiology of the worm. (B) In mammals, Trio mediates the Gαq-elicited mitogenic response through Rac1 and RhoA activation, by acting on p38 and Jun kinase (JNK), which control nuclear AP-1 activity.
Recently, this pathway has also been described in mammals. Vaqué and colleagues designed a screen to identify components of the signaling pathways transducing mitogenic cues, initiated by G-protein coupled receptors. They show that Trio mediates the Gαq-elicited mitogenic response by acting through Rho GTPase-mediated activation of p38 and Jun kinase (JNK), which control nuclear AP-1 activity.74 Surprisingly, both Trio GEF activities targeting Rac1 and RhoA are necessary for mediating this Gαq protein-coupled receptor-induced mitogenic signaling, suggesting that they both impinge on the AP-nuclear signaling pathway74 (Fig. 6B).
Finally, a recent study shows an essential role for Trio-mediated RhoA activation during development of the early eye, when the lens placode forms the lens pit.75 This process is accompanied by cell shape changes, called apical constriction, and is known to be dependent on the cytoskeletal protein Shroom3.75 Plageman and co-authors now show that apical constriction during epithelial invagination requires a Trio/RhoA/Shroom3 pathway.75
Trio and oncogenesis
Since Trio is controlling a wide range of cellular processes through the activation of Rho GTPases, it is not surprising that deregulation of its activity is associated with the development of diseases. Indeed, in various tumors, perturbations in Trio activity have been observed, arising by different mechanisms, such as deregulation of its expression, somatic mutations, and aberrant alternative splicing.
A growing number of studies have described the upregulation of Trio expression in different types of tumors, including urinary bladder, breast, lung and oral cancers, glioblastoma, and soft tissue sarcoma (Schmidt and Debant76). Upregulation of Trio expression due to gene amplification or overexpression of Trio mRNA has often been associated with poor patients’ survival. However, the signaling pathways by which Trio overexpression contributes to tumor progression and/or metastasis have remained unclear, until recent studies have started to shed some light on this process.
Glioblastoma are highly malignant and infiltrative primary brain tumors in adults. In these tumors, Trio overexpression is observed in the most aggressive ones and is associated with poor patients’ survival.77 Trio plays an essential role in the capacity of glioblastoma to invade in an ex vivo organotypic rat brain slice model and is required for tumor cell proliferation.77 Several growth factors or cytokines such as the TNF-like weak inducer of apoptosis (TWEAK) cytokine promote glioblastoma invasion. A recent study shows that Trio mediates TWEAK-induced migration and invasion of glioblastoma cells through Rac1 activation.78 Interestingly, RhoG, one of Trio’s targets, has also been involved in HGF-induced invasion of glioblastoma cells.79
Upregulation of Trio gene expression has also been observed in cervical and in head and neck carcinoma.76 As described above, Trio has recently been proposed as a key player in the proliferative signaling induced by Gαq that directly binds to and activates Trio.74 These results prompted the authors to explore the possibility that Trio may also participate in aberrant proliferation. Indeed, they show that Trio overexpression is critical for aberrant growth of cervical and oral squamous carcinoma cells in vitro and in vivo. Notably, this requirement was even more evident in uveal melanoma, in which activating mutations in Gαq drive the growth of this aggressive human malignancy. Vaque and colleagues therefore propose that, in response to activated Gαq, Trio triggers via its targets Rac1 and RhoA the activation of JNK and p38 kinases leading to a sustained AP-1-dependent nuclear signaling and aberrant proliferation.74
In addition to the alteration of gene expression, mutations in the trio gene have also been described in a numbers of different tumors. A systematic sequencing of cancer genomes led to the identification of more than thousand somatic mutations in the coding exons of kinase genes.80 In this study, nine mutations were found in the trio gene in tumors, including melanoma and glioma. In addition, a whole-genome sequencing of neuroblastoma identified mutations in members of the family of Rho GTPase regulators, including a mutation generating a truncated Trio protein.81 It will be interesting to decipher the functional consequences of these events on Trio-induced Rho GTPase activation and to determine whether these events are driver mutations in cancer progression. On the same line, Rac1 has recently been discovered as a novel melanoma gene by analysis of large-scale melanoma exome data. Indeed the authors show that melanoma present a recurrent Rac1 P29S mutation, which turns out to be an activating mutation by increasing the binding of the GTPase to downstream effectors.82,83 This re-affirms the importance of the Rac1 pathway in the development of cancer malignancy.
The identification of an oncogenic isoform of Trio, as a result of an aberrant alternative splicing, reinforced the involvement of Trio in cancer malignancy. Yoshizuka and colleagues screened for novel oncogenes that could participate in the malignant progression of adult T-cell leukemia (ATL).21 They isolated one gene with transforming properties, which turned out to be an alternative splice variant of Trio, harboring only the catalytic DH2 domain activating RhoA and a unique 15 amino acid C-terminal sequence. Overexpression of Tgat induced cell transformation and tumor formation in nude mice. Tgat has been proposed to enhance tumor invasion by stimulating Matrix MetalloProteinases (MMPs) via the RECK protein84 and by activating the transcription factor NF-kappaB, which plays a crucial role in tumorigenesis, including ATL.85 However, the function of Tgat in the progression of this malignancy remains to be determined.
Trio: A therapeutic target for cancer research?
A growing number of studies have described the upregulation of Trio expression in tumors, suggesting that Trio may be involved in cancer development.76 Trio thus represents a challenging target for the development of inhibitors. Very few GEF inhibitors exist so far, because it is difficult to target protein-protein interactions that are not yet well characterized. The first RhoGEF inhibitor targeting the GEFD2 domain of Trio was identified using a peptide aptamer screening strategy.86 Optimization of this first peptidic GEF inhibitor led to the identification of a series of new peptides, including TRIPE32G 87. TRIPE32G specifically inhibits Tgat-induced RhoA activation and reduces the formation of Tgat-induced tumors.88
Two different approaches were used to develop inhibitors targeting the Rac1/RhoG-specific GEFD1 domain of Trio. First, a computer-assisted virtual screening identified the NSC23766 compound, based on structure-function information of the Rac1/Tiam1 complex. This powerful molecule inhibits specifically Rac1-induced events in vitro and in vivo, but the targeted associated RhoGEFs include at least Tiam1 and Trio GEFD1.89 Second, a modified yeast exchange assay allowed the identification of compounds specifically targeting the GEFD1, NPPD and its analog ITX3,90,91 which specifically inhibit several Trio-dependent cellular processes.65,91
Concluding Remarks
In this review, we have overviewed recent findings about the function and the regulation of the RhoGEF Trio. Trio is involved in a panel of physiological and pathological processes, and represents a good example of the participation of GEFs in a wide range of signaling pathways. Trio has originally been described as a key player in axon outgrowth and guidance in different organisms. More recently, emerging data implicate Trio in cell adhesion-mediated processes and in signaling pathways elicited by Gαq-protein coupled receptors. In addition to its involvement in Rho GTPase activation downstream of different types of signals mediated by receptors, Trio has emerged recently as an upstream regulator of these same receptors. Indeed, Trio seems to control their subcellular localization or their expression through Rho GTPase-mediated transcriptional activity. Additional experiments will be required to determine whether Trio controls other receptors than adhesive receptors through Rac1-mediated transcriptional control. This new concept in Trio regulation may help to gain insight not only in the physiological function of Trio, but also in the contribution of Trio to oncogenesis. These findings warrant further studies investigating the contribution of Trio-induced transcriptional activation in oncogenesis.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
Work in the authors’ laboratory was supported by grants from Fondation ARC (Association pour la Recherche Contre le Cancer), Ligue Nationale Contre le Cancer, Fondation de France (comité tumeurs). We apologize to colleagues whose work we did not cite, due to space limitations.
References
- 1.Hall A. Rho family GTPases. Biochem Soc Trans. 2012;40:1378–82. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 2.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 3.Kjoller L, Hall A. Signaling to Rho GTPases. Exp Cell Res. 1999;253:166–79. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- 4.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 5.Côté JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci. 2002;115:4901–13. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 7.Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2013 doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Côté JF, Vuori K. In vitro guanine nucleotide exchange activity of DHR-2/DOCKER/CZH2 domains. Methods Enzymol. 2006;406:41–57. doi: 10.1016/S0076-6879(06)06004-6. [DOI] [PubMed] [Google Scholar]
- 9.Loirand G, Scalbert E, Bril A, Pacaud P. Rho exchange factors in the cardiovascular system. Curr Opin Pharmacol. 2008;8:174–80. doi: 10.1016/j.coph.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–93. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aghazadeh B, Lowry WE, Huang XY, Rosen MK. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–33. doi: 10.1016/S0092-8674(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 12.Yu B, Martins IR, Li P, Amarasinghe GK, Umetani J, Fernandez-Zapico ME, Billadeau DD, Machius M, Tomchick DR, Rosen MK. Structural and energetic mechanisms of cooperative autoinhibition and activation of Vav1. Cell. 2010;140:246–56. doi: 10.1016/j.cell.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debant A, Serra-Pagès C, Seipel K, O’Brien S, Tang M, Park SH, Streuli M. The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains. Proc Natl Acad Sci U S A. 1996;93:5466–71. doi: 10.1073/pnas.93.11.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouvière C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113:729–39. doi: 10.1242/jcs.113.4.729. [DOI] [PubMed] [Google Scholar]
- 15.Bellanger JM, Lazaro JB, Diriong S, Fernandez A, Lamb N, Debant A. The two guanine nucleotide exchange factor domains of Trio link the Rac1 and the RhoA pathways in vivo. Oncogene. 1998;16:147–52. doi: 10.1038/sj.onc.1201532. [DOI] [PubMed] [Google Scholar]
- 16.Alam MR, Johnson RC, Darlington DN, Hand TA, Mains RE, Eipper BA. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine alpha-amidating monooxygenase, an integral membrane peptide-processing enzyme. J Biol Chem. 1997;272:12667–75. doi: 10.1074/jbc.272.19.12667. [DOI] [PubMed] [Google Scholar]
- 17.Ma XM, Johnson RC, Mains RE, Eipper BA. Expression of kalirin, a neuronal GDP/GTP exchange factor of the trio family, in the central nervous system of the adult rat. J Comp Neurol. 2001;429:388–402. doi: 10.1002/1096-9861(20010115)429:3<388::AID-CNE3>3.0.CO;2-I. [In Process Citation] [DOI] [PubMed] [Google Scholar]
- 18.McPherson CE, Eipper BA, Mains RE. Multiple novel isoforms of Trio are expressed in the developing rat brain. Gene. 2005;347:125–35. doi: 10.1016/j.gene.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Portales-Casamar E, Briançon-Marjollet A, Fromont S, Triboulet R, Debant A. Identification of novel neuronal isoforms of the Rho-GEF Trio. Biol Cell. 2006;98:183–93. doi: 10.1042/BC20050009. [DOI] [PubMed] [Google Scholar]
- 20.Sun YJ, Nishikawa K, Yuda H, Wang YL, Osaka H, Fukazawa N, Naito A, Kudo Y, Wada K, Aoki S. Solo/Trio8, a membrane-associated short isoform of Trio, modulates endosome dynamics and neurite elongation. Mol Cell Biol. 2006;26:6923–35. doi: 10.1128/MCB.02474-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshizuka N, Moriuchi R, Mori T, Yamada K, Hasegawa S, Maeda T, Shimada T, Yamada Y, Kamihira S, Tomonaga M, et al. An alternative transcript derived from the trio locus encodes a guanosine nucleotide exchange factor with mouse cell-transforming potential. J Biol Chem. 2004;279:43998–4004. doi: 10.1074/jbc.M406082200. [DOI] [PubMed] [Google Scholar]
- 22.Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat Cell Biol. 2000;2:888–92. doi: 10.1038/35046533. [DOI] [PubMed] [Google Scholar]
- 23.Bellanger JM, Estrach S, Schmidt S, Briançon-Marjollet A, Zugasti O, Fromont S, Debant A. Different regulation of the Trio Dbl-Homology domains by their associated PH domains. Biol Cell. 2003;95:625–34. doi: 10.1016/j.biolcel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Seipel K, O’Brien SP, Iannotti E, Medley QG, Streuli M. Tara, a novel F-actin binding protein, associates with the Trio guanine nucleotide exchange factor and regulates actin cytoskeletal organization. J Cell Sci. 2001;114:389–99. doi: 10.1242/jcs.114.2.389. [DOI] [PubMed] [Google Scholar]
- 25.Lutz S, Freichel-Blomquist A, Yang Y, Rümenapp U, Jakobs KH, Schmidt M, Wieland T. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem. 2005;280:11134–9. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 26.Souchet M, Portales-Casamar E, Mazurais D, Schmidt S, Léger I, Javré JL, Robert P, Berrebi-Bertrand I, Bril A, Gout B, et al. Human p63RhoGEF, a novel RhoA-specific guanine nucleotide exchange factor, is localized in cardiac sarcomere. J Cell Sci. 2002;115:629–40. doi: 10.1242/jcs.115.3.629. [DOI] [PubMed] [Google Scholar]
- 27.Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, et al. Structure of Galphaq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science. 2007;318:1923–7. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- 28.Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J. Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem. 2007;282:29201–10. doi: 10.1074/jbc.M703458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estrach S, Schmidt S, Diriong S, Penna A, Blangy A, Fort P, Debant A. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr Biol. 2002;12:307–12. doi: 10.1016/S0960-9822(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen SY, Huang PH, Cheng HJ. Disrupted-in-Schizophrenia 1-mediated axon guidance involves TRIO-RAC-PAK small GTPase pathway signaling. Proc Natl Acad Sci U S A. 2011;108:5861–6. doi: 10.1073/pnas.1018128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neubrand VE, Thomas C, Schmidt S, Debant A, Schiavo G. Kidins220/ARMS regulates Rac1-dependent neurite outgrowth by direct interaction with the RhoGEF Trio. J Cell Sci. 2010;123:2111–23. doi: 10.1242/jcs.064055. [DOI] [PubMed] [Google Scholar]
- 32.DeGeer J, Boudeau J, Schmidt S, Bedford F, Lamarche-Vane N, Debant A. Tyrosine phosphorylation of the Rho guanine nucleotide exchange factor Trio regulates netrin-1/DCC-mediated cortical axon outgrowth. Mol Cell Biol. 2013;33:739–51. doi: 10.1128/MCB.01264-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development. 2005;132:1983–94. doi: 10.1242/dev.01736. [DOI] [PubMed] [Google Scholar]
- 34.Liebl EC, Forsthoefel DJ, Franco LS, Sample SH, Hess JE, Cowger JA, Chandler MP, Shupert AM, Seeger MA. Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio’s role in axon pathfinding. Neuron. 2000;26:107–18. doi: 10.1016/S0896-6273(00)81142-3. [see comments] [DOI] [PubMed] [Google Scholar]
- 35.Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz Morales A, Hogue CW, Pawson T, Culotti J. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell. 1998;92:785–95. doi: 10.1016/S0092-8674(00)81406-3. [DOI] [PubMed] [Google Scholar]
- 36.Awasaki T, Saito M, Sone M, Suzuki E, Sakai R, Ito K, Hama C. The Drosophila trio plays an essential role in patterning of axons by regulating their directional extension. Neuron. 2000;26:119–31. doi: 10.1016/S0896-6273(00)81143-5. [see comments] [DOI] [PubMed] [Google Scholar]
- 37.Bateman J, Shu H, Van Vactor D. The guanine nucleotide exchange factor trio mediates axonal development in the Drosophila embryo. Neuron. 2000;26:93–106. doi: 10.1016/S0896-6273(00)81141-1. [see comments] [DOI] [PubMed] [Google Scholar]
- 38.Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, Dickson BJ. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–94. doi: 10.1016/S0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc Natl Acad Sci U S A. 2000;97:12074–8. doi: 10.1073/pnas.97.22.12074. [In Process Citation] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng YJ, He WQ, Tang J, Tao T, Chen C, Gao YQ, Zhang WC, He XY, Dai YY, Zhu NC, et al. Trio is a key guanine nucleotide exchange factor coordinating regulation of the migration and morphogenesis of granule cells in the developing cerebellum. J Biol Chem. 2010;285:24834–44. doi: 10.1074/jbc.M109.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–56. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, Weiss C, Radulovic J, Sweatt JD, Disterhoft JF, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106:13058–63. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, Eipper BA, Mains RE. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008;28:12368–82. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo S, Kim Y, Lee H, Park S, Park S. A gene trap knockout of the Tiam-1 protein results in malformation of the early embryonic brain. Mol Cells. 2012;34:103–8. doi: 10.1007/s10059-012-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Briançon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, Fromont S, Piché C, Enslen H, Chebli K, Cloutier JF, et al. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol. 2008;28:2314–23. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–85. doi: 10.1016/S0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 47.Levy-Strumpf N, Culotti JG. VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat Neurosci. 2007;10:161–8. doi: 10.1038/nn1835. [DOI] [PubMed] [Google Scholar]
- 48.Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G. C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci. 2007;10:169–76. doi: 10.1038/nn1834. [DOI] [PubMed] [Google Scholar]
- 49.Vanderzalm PJ, Pandey A, Hurwitz ME, Bloom L, Horvitz HR, Garriga G. C. elegans CARMIL negatively regulates UNC-73/Trio function during neuronal development. Development. 2009;136:1201–10. doi: 10.1242/dev.026666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iyer SC, Wang D, Iyer EP, Trunnell SA, Meduri R, Shinwari R, Sulkowski MJ, Cox DN. The RhoGEF trio functions in sculpting class specific dendrite morphogenesis in Drosophila sensory neurons. PLoS One. 2012;7:e33634. doi: 10.1371/journal.pone.0033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shivalkar M, Giniger E. Control of dendritic morphogenesis by Trio in Drosophila melanogaster. PLoS One. 2012;7:e33737. doi: 10.1371/journal.pone.0033737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE, Huganir RL. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–74. doi: 10.1016/S0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 53.Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–42. doi: 10.1016/S0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 54.Ma XM, Wang Y, Ferraro F, Mains RE, Eipper BA. Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J Neurosci. 2008;28:711–24. doi: 10.1523/JNEUROSCI.5283-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 2010;66:536–49. doi: 10.1016/j.neuron.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 56.Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouvière C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell. 2007;18:1734–43. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Backer S, Hidalgo-Sánchez M, Offner N, Portales-Casamar E, Debant A, Fort P, Gauthier-Rouvière C, Bloch-Gallego E. Trio controls the mature organization of neuronal clusters in the hindbrain. J Neurosci. 2007;27:10323–32. doi: 10.1523/JNEUROSCI.1102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kashef J, Köhler A, Kuriyama S, Alfandari D, Mayor R, Wedlich D. Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev. 2009;23:1393–8. doi: 10.1101/gad.519409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore R, Theveneau E, Pozzi S, Alexandre P, Richardson J, Merks A, Parsons M, Kashef J, Linker C, Mayor R. Par3 controls neural crest migration by promoting microtubule catastrophe during contact inhibition of locomotion. Development. 2013;140:4763–75. doi: 10.1242/dev.098509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Lièvre CS. Participation of neural crest-derived cells in the genesis of the skull in birds. J Embryol Exp Morphol. 1978;47:17–37. [PubMed] [Google Scholar]
- 61.Le Lièvre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–54. [PubMed] [Google Scholar]
- 62.Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806–18. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- 63.Stramer BM, Dunn GA, Davis JR, Mayor R. Rediscovering contact inhibition in the embryo. J Microsc. 2013;251:206–11. doi: 10.1111/jmi.12045. [DOI] [PubMed] [Google Scholar]
- 64.Powell DR, Blasky AJ, Britt SG, Artinger KB. Riding the crest of the wave: parallels between the neural crest and cancer in epithelial-to-mesenchymal transition and migration. Wiley Interdiscip Rev Syst Biol Med. 2013;5:511–22. doi: 10.1002/wsbm.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yano T, Yamazaki Y, Adachi M, Okawa K, Fort P, Uji M, Tsukita S, Tsukita S. Tara up-regulates E-cadherin transcription by binding to the Trio RhoGEF and inhibiting Rac signaling. J Cell Biol. 2011;193:319–32. doi: 10.1083/jcb.201009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heemskerk N, van Rijssel J, van Buul JD. Rho-GTPase signaling in leukocyte extravasation: An endothelial point of view. Cell Adh Migr. 2014;8:8. doi: 10.4161/cam.28244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Rijssel J, Kroon J, Hoogenboezem M, van Alphen FP, de Jong RJ, Kostadinova E, Geerts D, Hordijk PL, van Buul JD. The Rho-guanine nucleotide exchange factor Trio controls leukocyte transendothelial migration by promoting docking structure formation. Mol Biol Cell. 2012;23:2831–44. doi: 10.1091/mbc.E11-11-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Rijssel J, Timmerman I, Van Alphen FP, Hoogenboezem M, Korchynskyi O, Geerts D, Geissler J, Reedquist KA, Niessen HW, Van Buul JD. The Rho-GEF Trio regulates a novel pro-inflammatory pathway through the transcription factor Ets2. Biol Open. 2013;2:569–79. doi: 10.1242/bio.20134382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Wu YC, Fadok VA, Lee MC, Gengyo-Ando K, Cheng LC, Ledwich D, Hsu PK, Chen JY, Chou BK, et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science. 2003;302:1563–6. doi: 10.1126/science.1087641. [DOI] [PubMed] [Google Scholar]
- 70.deBakker CD, Haney LB, Kinchen JM, Grimsley C, Lu M, Klingele D, Hsu PK, Chou BK, Cheng LC, Blangy A, et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol. 2004;14:2208–16. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 71.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–4. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 72.Steven R, Zhang L, Culotti J, Pawson T. The UNC-73/Trio RhoGEF-2 domain is required in separate isoforms for the regulation of pharynx pumping and normal neurotransmission in C. elegans. Genes Dev. 2005;19:2016–29. doi: 10.1101/gad.1319905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams SL, Lutz S, Charlie NK, Vettel C, Ailion M, Coco C, Tesmer JJ, Jorgensen EM, Wieland T, Miller KG. Trio’s Rho-specific GEF domain is the missing Galpha q effector in C. elegans. Genes Dev. 2007;21:2731–46. doi: 10.1101/gad.1592007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaqué JP, Dorsam RT, Feng X, Iglesias-Bartolome R, Forsthoefel DJ, Chen Q, Debant A, Seeger MA, Ksander BR, Teramoto H, et al. A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol Cell. 2013;49:94–108. doi: 10.1016/j.molcel.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plageman TF, Jr., Chauhan BK, Yang C, Jaudon F, Shang X, Zheng Y, Lou M, Debant A, Hildebrand JD, Lang RAA. A Trio-RhoA-Shroom3 pathway is required for apical constriction and epithelial invagination. Development. 2011;138:5177–88. doi: 10.1242/dev.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt S, Debant A. TRIO (triple functional domain (PTPRF interacting)) Atlas Genet Cytogenet Oncol Haematol. 2011;15:490–8. [Google Scholar]
- 77.Salhia B, Tran NL, Chan A, Wolf A, Nakada M, Rutka F, Ennis M, McDonough WS, Berens ME, Symons M, et al. The guanine nucleotide exchange factors trio, Ect2, and Vav3 mediate the invasive behavior of glioblastoma. Am J Pathol. 2008;173:1828–38. doi: 10.2353/ajpath.2008.080043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fortin SP, Ennis MJ, Schumacher CA, Zylstra-Diegel CR, Williams BO, Ross JT, Winkles JA, Loftus JC, Symons MH, Tran NL. Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells. Mol Cancer Res. 2012;10:958–68. doi: 10.1158/1541-7786.MCR-11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwiatkowska A, Didier S, Fortin S, Chuang Y, White T, Berens ME, Rushing E, Eschbacher J, Tran NL, Chan A, et al. The small GTPase RhoG mediates glioblastoma cell invasion. Mol Cancer. 2012;11:65. doi: 10.1186/1476-4598-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–8. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, van der Ploeg I, Hamdi M, van Nes J, Westerman BA, van Arkel J, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–93. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 82.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–14. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mori T, Moriuchi R, Okazaki E, Yamada K, Katamine S. Tgat oncoprotein functions as a inhibitor of RECK by association of the unique C-terminal region. Biochem Biophys Res Commun. 2007;355:937–43. doi: 10.1016/j.bbrc.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 85.Yamada K, Moriuchi R, Mori T, Okazaki E, Kohno T, Nagayasu T, Matsuyama T, Katamine S. Tgat, a Rho-specific guanine nucleotide exchange factor, activates NF-kappaB via physical association with IkappaB kinase complexes. Biochem Biophys Res Commun. 2007;355:269–74. doi: 10.1016/j.bbrc.2007.01.147. [DOI] [PubMed] [Google Scholar]
- 86.Schmidt S, Diriong S, Méry J, Fabbrizio E, Debant A. Identification of the first Rho-GEF inhibitor, TRIPalpha, which targets the RhoA-specific GEF domain of Trio. FEBS Lett. 2002;523:35–42. doi: 10.1016/S0014-5793(02)02928-9. [DOI] [PubMed] [Google Scholar]
- 87.Bouquier N, Fromont S, Debant A, Schmidt S. Random mutagenesis of peptide aptamers as an optimization strategy for inhibitor screening. Methods Mol Biol. 2012;928:97–118. doi: 10.1007/978-1-62703-008-3_8. [DOI] [PubMed] [Google Scholar]
- 88.Bouquier N, Fromont S, Zeeh JC, Auziol C, Larrousse P, Robert B, Zeghouf M, Cherfils J, Debant A, Schmidt S. Aptamer-derived peptides as potent inhibitors of the oncogenic RhoGEF Tgat. Chem Biol. 2009;16:391–400. doi: 10.1016/j.chembiol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–23. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blangy A, Bouquier N, Gauthier-Rouvière C, Schmidt S, Debant A, Leonetti JP, Fort P. Identification of TRIO-GEFD1 chemical inhibitors using the yeast exchange assay. Biol Cell. 2006;98:511–22. doi: 10.1042/BC20060023. [DOI] [PubMed] [Google Scholar]
- 91.Bouquier N, Vignal E, Charrasse S, Weill M, Schmidt S, Léonetti JP, Blangy A, Fort P. A cell active chemical GEF inhibitor selectively targets the Trio/RhoG/Rac1 signaling pathway. Chem Biol. 2009;16:657–66. doi: 10.1016/j.chembiol.2009.04.012. [DOI] [PubMed] [Google Scholar]