Abstract
The family of Rho GTPases are intracellular signal transducers that link cell surface signals to multiple intracellular responses. They are best known for their role in regulating actin dynamics required for cell migration, but in addition control cell-cell adhesion, polarization, vesicle trafficking, and the cell cycle. The roles of Rho GTPases in single mesenchymal cell migration are well established and rely on Cdc42- and Rac-dependent cell protrusion of a leading edge, coupled to Rho-dependent contractility required to move the cell body forward. In cells migrating collectively, cell-cell junctions are maintained, and migrating leader cells are mechanically coupled to, and coordinate, migration with follower cells. Recent evidence suggests that Rho GTPases provide multifunctional input to collective cell polarization, cell-cell interaction, and migration. Here, we discuss the role of Rho GTPases in initiating and maintaining front-rear, apical-basal cell polarization, mechanotransduction, and cell-cell junction stability between leader and follower cells, and how these roles are integrated in collective migration. Thereby, spatiotemporal fine-tuning of Rho GTPases within the same cell and among cells in the cell group are crucial in controlling potentially conflicting, divergent cell adhesion and cytoskeletal functions to achieve supracellular coordination and mechanocoupling.
Keywords: Rac, Rho, cell adhesion, cell migration, collective migration
Introduction
Cell migration is a fundamental process controlling cell position, fate decisions, and function in morphogenesis, immune function, regeneration, and cancer.1,2 To translocate the cell body, cells undergo a cyclic process to maintain bipolarity and extend a leading and trailing edge, followed by adhesive interaction with tissue substrate and actomysosin mediated contraction which leads to the forward gliding of the cell rear.1,3-5 Polarized cell extension and contraction are mediated by the actin cytoskeleton, which generates shape change and, via adhesion receptors, connects to the extracellular tissue environment.5-7 Besides single-cell migration, which propels the movement of individual cells, collective cell migration of cells that retain cell-cell adhesion and communication supports the movement of multicellular units along or within tissues.8,9 Collective migration depends upon and causes complex alterations of tissue organization and function, including the formation and regeneration of skin, tubulogenesis of epithelial tubules and glands, vascular sprouting, and complex organ remodeling, including the formation of a germ cell niche in the Drosophila ovary and development of the lateral line, the balance organ in zebrafish.10-12 In pathological contexts, collective cell migration underlies the deep tissue invasion of solid cancers.8,13 Similar to single-cell migration, collective movements depend upon actomyosin-dependent front-rear asymmetry. In multicellular groups, leading cells polarize by protruding anterior leading pseudopods, which engage with the tissue substrate by adhesive and proteolytic interactions, while the rear pole and lateral sides retain cadherin-based cell-cell adhesion and mechanocoupling to follower cells.14,15 Likewise, follower cells exhibit front-rear polarity with lateral and basal portions of cohesive cell groups form so-called cryptic lamellipodia, which extend toward the direction of migration, engage with substrate and generate traction with cell-cell junctions, which remain intact in the direct vicinity.16 This enables cells inside the group to actively migrate and generate traction toward the substrate17. Thus, collective cell migration is a specialized and complex cell migration mode that combines cell movement with “supracellular” polarity, cell-cell junction stability, and coordinated multicellular migration.10
Rho GTPases are important upstream regulators of actin polymerization and actomyosin contractility, linking outside signals received from adhesion, chemokine, and/or receptor tyrosine kinase receptors to cytoskeletal dynamics.4,14,18 Thereby Rho GTPases control mechanosensory cell functions, including cell adhesion, polarity, contractility, as well as cell-cell junction regulation in a tissue-context dependent manner. The roles of Rho GTPases in single-cell migration, particularly cell polarization and protrusion formation, and cell contractility are well established1, yet their dual role in controlling both cell kinetics and cell-cell junctions in collective cell movements adds additional complexity. We here summarize key functions of Rho GTPases in collective cell migration, with focus on their contribution to leader cell polarity, cell-cell junction stability and turnover, and multicellular coordination during morphogenesis and cancer.
Rho GTPase Regulation and Basic Functions in Cell Migration
Rho GTPases belong to the family of Ras-like GTPases, the activity of which is regulated by a cyclic switch between an inactive GDP-bound and an active GTP-bound state.18,19 Activation of Rho GTPases is controlled by guanine exchange factors (GEFs) that promote GTP-loading in response to extracellular cues. Upstream regulators of GEFs include growth factor and cytokine receptors, integrins, and cadherins.18 As antagonists to GEFs, GTPase activating proteins (GAPs) inactivate Rho GTPases through their conserved catalytic GAP domain which hydrolyses GTP to GDP. Most GAPs also execute other functions, including additional GAP function or GEF activity toward other small GTPases, or function as myosin motor. The upstream signals engaging RhoGAPs are poorly defined.20 Rho GTPases are further inhibited by Rho guanine nucleotide dissociation inhibitors (GDIs), which bind the prenyl membrane anchor of GTPases and prevent their translocation to the plasma membrane, thereby retaining Rho GTPases in inactive state and sequestered in the cytosol.21,22
Key mechanosensory cell functions controlled by Rho GTPases include protrusion formation and front-rear polarity, actomyosin contractility, and the turnover of cell-matrix and cell-cell adhesions, which jointly contribute to the type and efficacy of cell migration. In moving cells, at least three types of cell protrusions are mediated by Rho GTPases. Filopodia, thin membrane protrusions containing parallel actin bundles for mechanosensory probing of the environment, are predominantly controlled by Cdc42, through the Mammalian Diaphanous-related (mDia) formin mDia2, which nucleates and elongates actin filaments, and IRSp53, which bundles actin filaments.4 Lamellipodia, sheet-like protrusions that provide adhesion to substrate, are controlled by active Rac1, Cdc42, RhoA and RhoC4,6 (Fig. 1). Cdc42 controls cell polarization and promotes extension by stabilizing the microtubule cytoskeleton.23 Rac regulates branched actin network assembly and extension toward the leading edge through WAVE and Arp2/3.5,24 Actin branching is further promoted by cofilin, which is activated downstream of the Rac-PAK-LIMK axis25 or via RhoC-ROCK-LIMK.6 Cofilin severs actin filaments at protrusions and thereby provides free barbed ends of existing actin filaments, which enhances Arp2/3-mediated extension of lamellipodia.6 Through Pak, Rac further supports integrin-based adhesion to ECM and mechanical stabilization of forward protruding lamellipodia.26 As third principal protrusion type, membrane blebbing results from a two-step process of initial bleb-like membrane protrusion with secondary stabilization of the bleb by the cortical actin network.27,28 Membrane blebbing depends upon intracytoplasmic hydrostatic pressure, mediated by RhoA and downstream actomyosin contraction.29-31
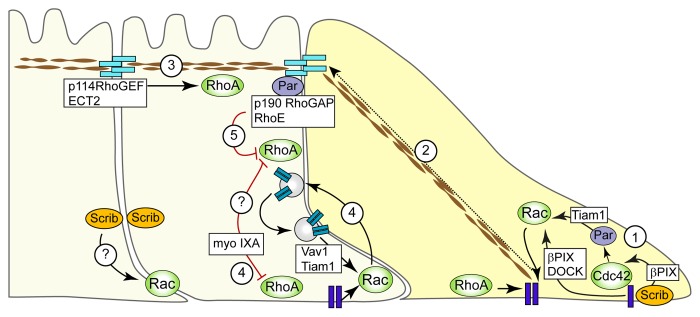
Figure 1. Spatial segregation of Rho GTPase signaling in collective migration. (1) Sustained activation of Cdc42 and Rac upstream and downstream of ECM adhesion promotes protrusion formation. (2) Mechanocoupling of traction forces to the apical junctional complex. (3) Actomyosin contractility in conjunction with Rho activation by ECT2 and p114RhoGEF. (4) Increased Rac activation at cryptic lamellipodia by a combination of ECM-mediated Rac activation and Rac activation due to increased recycling and de novo formation of adherens junctions. In parallel, Rho activity is downregulated at cryptic lamellipodia by myosin IXA, which may also inhibit Rho activity at lateral junctions. (5) Rho activation at lateral junctions is inhibited by p190 RhoGAP, or via p120 catenin or Rnd3/RhoE.
Besides cell protrusions at the leading edge, Rho GTPases control cell contractility at the trailing edge of moving cells. RhoA activates ROCK, which engages myosin light chain kinase and myosin II for actomyosin contraction, preferentially at lateral and rear cell portions.32 Thereby RhoA controls the retraction of the tail in migrating single cells and in cell groups, and likely, the lateral mechanocoupling toward cadherin-based cell-cell adhesions.32,33
In both 2D and 3D models for collective cell migration, Rho GTPases initiate and maintain leader and follower cell function, interaction with substrate, cell-cell cohesion and supracellular coordination. In individually moving cells, Rac and Cdc42 activity, controlling polarized actin polymerization, is spatiotemporally separated from Rho-dependent actomyosin engagement and cell contraction, which ensures each cell region to function simultaneously and in vicinity. Cell regions or subregions of active Rac show limited Rho activity, and vice versa, as consequence of reciprocal feed-back and mutual inhibition. Downstream of Rac and Cdc42, Par6 and atypical PKC (aPKC) engage the ubiquitin ligase Smurf1 which degrades Rho and thereby limits local Rho availability at the leading edge.34 In addition, Rac inactivates Rho by activating p190 RhoGAP, either via direct interaction,35 or indirectly, via engagement of Par6/aPKC36 or p120catenin along cell-cell junctions.37 Likewise, the Rac effectors Pak1 and Pak4 promote the phosphorylation and inactivation of a set of RhoGEFs including p115-RhoGEF,38 GEF-H1,39,40 PDZ-RhoGEF41 and Net1,42 which limits Rho activation.
Conversely, Rho limits Rac either via direct engagement of the Rac inhibitors FilGAP43 and ARHGAP22,44or via ROCK which inhibits recruitment of the Cdc42/Rac GEF βPIX to cell-matrix adhesions.45 ROCK further inhibits the assembly of the polarity complex Par3/Par6, which limits Rac and thereby prevents ectopic protrusions near the cell rear or cell-cell junctions.46 Thus, through negative cross-talk, potentially conflicting functions between Rho GTPases are distributed to and define functional subcellular compartments, including protrusions vs. cell-cell junctions, apical vs. basal zones, and actomysosin-dependent contractile vs. actomyosin-independent regions in protrusions or cell-cell junctions.
Cellular Principles of Collective Cell Migration
As initiating event of collective cell migration, cells at the edge of the cell group acquire ligand-mediated contact with tissue structures via matrix-binding adhesion receptors, particularly integrins, as well as (co)receptors, including syndecans, CD44, and chemokine and cytokine receptors engaging with ECM-bound migration-promoting chemokine and cytokines.14,47 These combined signals promote outward polarization and adhesion of leading and marginal cells to the ECM.16,47,48 Whereas in moving cell monolayer sheets all cells retain contact to the basal substrate, cells within inner portions of 3D groups lack the signaling input from ECM and adhere exclusively to neighbor cells. These cell-cell junctions mostly involve homophilic cadherin-cadherin adhesive junctions, but may also include immunoglobulin family members with homophilic or heterophilic binding, such as N-CAM, L1-CAM, ALCAM, Ephrins/Eph receptors, desmosomal proteins, and integrins.10,48 Similar to non-moving stable epithelia, moving epithelial cell sheets commonly maintain exclusive apicobasal polarity and thereby polarize toward the ECM interface by unilateral adhesion and deposition of ECM components, such as basement membrane proteins.11 Both downregulation and overexpression of cadherins inhibits collective migration,49-51 suggesting precise control of cadherin-based adherens junctions as prerequisite of collective movement. Thus, overlapping adhesion systems maintain multicellular mechanocoupling and integration between the cytoskeleton of individual cells and contribute to supracellular coordination.47,52
Rho GTPases and Front-Rear Polarity in Leader Cells
The role of Rho GTPases in leader cell functions is mostly derived from single cell migration assays, addressing general rules of front-rear polarity and cell sheets moving across 2D surfaces (Table 1). When studied in 3D models for collective cell migration or tubulogenesis,10,11 Rho GTPases and their downstream effectors likely impose additional complexity and cross-talk to accommodate the spatial constraints of 3D tissue invasion.
Table 1. Model systems for collective cell migration and identified roles of Rho GTPases.
| Model | Type of collective migration | Biological and/or application context | Roles of Rho GTPases | References |
|---|---|---|---|---|
| Cell-sheet migration (2D, in vitro) |
Migration of cohesive cells as 2D monolayer in vitro | Simplified model for epithelial and/or endothelial cell migration, wound healing | Rac, Rho in leading cells: DDR1/Rho axis reduces actomyosin contractility and stabilizes cell-cell junctions; sheet migration requires p114RhoGEF-dependent myosin II contractility | 47,49,136,137 |
| Convergent extension movement (2D in vivo) | Migration of multilayered cell sheets | Gastrulation | Wnt-driven Rac and Rho engagement controlling filopodia, protrusion, polarity and protrusion lifespan | 138,139 |
| Tubulogenesis (3D, in vitro, in vivo) | Epithelial collective sprouting and tube formation, including lumen formation. | Morphogenesis, gland development (mammary, salivary, kidney) | Rac-dependent multicellular budding tip composed of several positionally instable leader cells followed by positionally stable cells forming the duct with inner lumen and outward basement membrane deposition; Rho-dependent restriction of leader cells. Rac-dependent maintenance of cell-cell adhesion | 54,110,115,140 |
| Invasion of collective strands (3D, in vitro) | Finger-like sprouting of multicellular strand | Epithelial tubulogenesis, vascular sprouting, strand-like cancer cell invasion | Low Rho activity stabilizes cell-cell junction; active Rho favors conversion to single-cell migration | 83,103 |
| Tumor explant invasion (3D, in vitro) | 3D invasion | Cancer, personalized medicine | Rac-dependent leader cell selection; Rho dependent engagement of heterologous leader cells (fibroblasts); silencing of Rho along cell-cell junctions | 62,83,141 |
Leader cells maintain an intrinsically bipolar state, with the protruding leading edge oriented toward the ECM and the rear engaged with cell-cell connections to follower cells.14,53,54 The protruding leading edge is initially determined by Cdc42- and Rac-mediated polarization and actin polymerization and Rho-mediated actomyosin contraction for adhesion stabilization several micrometer rearward of the cell front.4,55 The rear pole of a leader cell is determined by mechanosignaling from cell-cell junctions, and higher forces at the rear support actomyosin contractility to move the cell rear and the cell-cell junction forward,15 which further minimizes potentially counterproductive ectopic protrusions.53 The process and underlying signals counteracting actin polymerization and cell protrusion formation at cell-cell junctions are termed contact-inhibition of migration.53
Leader cells, via adhesion complexes and actomyosin contraction, generate force toward the substrate and thus control tensional regulation of ECM alignment.56,57 Leader cells further reorganize ECM through degradation by surface-associated proteases, such as MT1-MMP or localized secretion of soluble proteases including MMP9, to generate space and enable the formation of ECM neo-tracks to accommodate and guide collective cell strands.58,59 In collectively migrating squamous carcinoma cells LIMK and cofilin regulate invadopodia formation and stability, and delivery of MMP9 to invadopodia, thereby promoting focal proteolysis and proteolytic path generation, possibly downstream of ROCK.59 In single tumor cells, LIMK/cofilin are regulated upstream by spatiotemporally-controlled RhoC/ROCK6 or Rac/Pak activity.25 Alternatively, local MMP-mediated proteolysis in single osteosarcoma cells depends on localized recruitment of βPIX to focal adhesions by the linker protein α-parvin followed by Rac and Pak activation.60 As special case, heterologous cells with proteolytic capacity, such as activated fibroblasts, may provide leader cell function, by generating proteolytic ECM paths in a Rho GTPase- and MMP-dependent manner.61,62
Rac and Cdc42 activity is controlled by upstream chemokine and cytokine receptor and adhesion receptor signaling. Early matrix adhesions engage integrin binding to ECM and focal adhesion formation followed by Cdc42 and Rac activation through βPIX, Tiam1 and DOCK-family GEFs, which sustains protrusive activity.4 Individually migrating or loosely connected astrocyte monolayers rely upon Cdc42 for leading edge polarization and migration63,64 and on Rac for sustained protrusion formation,23 suggesting non-redundant roles for Cdc42 and Rac in leading edge dynamics. In border cell clusters moving in the Drosophila ovary, local activation of Rac, using a photoactivatable analog of Rac, is sufficient to induce cell protrusion and maintain leader cell polarization and migration.65 This state as leader cell is transient, as decrease of Rac activity is rapidly followed by loss of guidance ability and the emergence of ectopic protrusions in other cells of the group.65,66 While the role of Cdc42 initiation of these ectopic protrusions was not addressed in these models, a recent screen suggests that both Rac and Cdc42 are sufficient to promote cell collective migration of endothelial cell sheets.47 Thus, sustained Rac and Cdc42 signaling cooperate in leader cell polarity and directional persistence of moving cell groups.
Upstream effectors of Rac and Cdc42 in maintaining front-rear polarity in both single-cell and collective cell migration include conserved Scribble and Par polarity protein complexes. The Scribble complex consists of Scribble, Discs large and Lethal giant larvae and localizes to the leading edge of migrating cells in response to integrin engagement where it controls activation of Cdc42 and recruitment of Rac via βPIX.63,67-69 The integrin/Scribble complex/Cdc42/Rac axis drives the leading edge of both 2D epithelial and endothelial cell sheets and 3D sprouting and matrix invasion models,64,69-74 indicating a key function in collective polarity. Scribble is further recruited to both adherens and tight junctions by E-cadherin75,76 and ZO1/2,77,78 where it contributes to junction stability through βPIX, Rac, and Pak signaling.75,79,80 The Par complex, composed of Par3, Par6, and aPKC, is initiated by active Cdc42 binding to Par6, which recruits Par3 and aPKC to fulfill a dual function by locally (1) activating Rac and Cdc42, and (2) inhibiting Rho.74,81-83 Par3 engages the RacGEF Tiam1, and this complex is recruited to integrin adhesions by talin, leading to a talin-dependent activation of Rac, which initiates a leading edge and promotes the formation of focal adhesions.84,85 Lastly, Par3/aPKC stabilize cell-surface integrin levels by activating Numb, which locally inhibits the endocytosis of integrins.86
Rho GTPases in Cell-Cell Junction Stability in Moving Cells
In both stable epithelia and moving cell groups, functionally stable and mechanotransducing cell-cell junctions are maintained by complementary mechanisms. These include Rac- and Cdc42-mediated formation and dynamic maintenance of junctions, Rho silencing to minimize mechanical friction along the junction, and, in subregions, Rho-mediated actin cable formation for intercellular mechanocoupling.
The apical junctional complex, located at the apical end of the lateral membrane of epithelial cells comprises adherens and tight junctions associated with contractile actin filaments. Its formation, maintenance and turnover is tightly controlled by Rho GTPases.87 E-cadherin-based adherens junctions form de novo by touching of lamillipodia of adjacent cells, followed by ligation of E-cadherins in trans. Initial E-cadherin engagement initiates transient Rac activation, via PI3 kinase and the RacGEFs Vav2 and Tiam1.88,89 Rac, in turn, activates WAVE and Arp2/3 to initiate branched actin network formation required for lateral expansion of junctions.90 Rho-mediated actomyosin contractility at the outer edges of emerging cell-cell adhesions supports junctional maturation.91,92 Likewise, tight junction formation is promoted by cell-cell adhesion through local p114RhoGEF and Rho activation and downstream myosin II and actomyosin effector activity.93 In addition, tight junction biogenesis relies on local Rac activation, which is mediated by Par3-dependent recruitment of the RacGEF Tiam1.81 How and in which sequence these pathways contribute to tight junction formation, and whether they crosstalk with each other is unclear.
As dynamic endpoint of adherens junction maturation and maintenance of junction stability, both Rac and Rho remain engaged at moderate and/or sub region-controlled level.94 To maintain junctional adhesion, established adherens junctions undergo a constant rate of cyclic remodeling by endocytosis and recycling of cadherins.95 Rac-dependent actin dynamics involved in de novo formation of adherens junctions thus remain active but are counterbalanced by RhoGTPase-mediated turnover of junctional proteins. Downstream of Cdc42 and Par6, the Arp2/3 complex and CIP4 induce initiation and scission of endocytic vesicles, thereby promoting endocytosis of E-cadherin in Drosophila embryonic epithelial cells.96,97 Likely concurrently, centralspindlin engages Rho near cell-cell junctions by two distinct mechanisms, including (1) the engagement of α-catenin to recruit the RhoGEF ECT2, which activates Rho, and (2) the inhibition of 190RhoGAP, which prevents Rho deactivation.98 Active Rho contributes to junction stabilization, perhaps by promoting formin-mediated actin nucleation.94 Consequently, the balance of Rac/Cdc42 vs. Rho activity at junctions determines junction formation, maturation and stability, or dissolution.
Collectively moving cells suppress actomyosin contractility along “inner” cell-cell junctions, likely to enable cadherin turnover and reduce friction along cell-cell junctions.51 Indeed, high activity of Rho and actomyosin contractility disrupt both adherens and tight junctions and compromise epithelial barrier functions.87,99 Inhibition of Rho activity and actomyosin contractility along cell-cell junctions depends upon Par3 and Par6, which are recruited to cell-cell junctions by E-cadherin and/or discoidin domain receptor 1 and engage RhoE/Rnd3, which in turn activates p190RhoGAP to silence the Rho-ROCK-MLC axis and actomyosin contraction.83,100 Besides through RhoE/Rnd3, Rho activity is further inhibited by active Rac and downstream p190RhoGAP, which are recruited to junctions by p120 catenin.37,83 Consistently, the Par complex is indispensable for group cohesion and coordination during collective migration of Drosophila border cells101 and of sprouting endothelia,102 although the role for silencing junctional Rho activity was not analyzed in these models. In aggregate, these data suggest that cell-cell junctions depend upon a fine balance of actin-based contractility and relaxation. As consequence of increasing either turnover or contractility, cell-cell adhesions are downregulated allowing cells to move individually.83,103
Whereas inner portions of cell-cell junctions appear to maintain low levels of tensile stress, moving cell sheets maintain substantial mechanocoupling and actomyosin contractility along cell junctions of the apical junctional complex. The resulting contractility is supracellular and allows cells within moving sheets to maintain collective mechanocoupling of directional traction forces, transmitted by cadherins.15,17,104,105 Such supracellular coordination relies on RhoA-ROCK-dependent actomyosin cables extending along apical cell-cell junctions over multiple cell bodies.106 In cell-junction free areas, such as the marginal cells of advancing sheets or wounded epithelia, relatively high Rho-ROCK-myosin II activity likely increases the formation of actomyosin cables, to coordinate cell-sheet contractility by a purse-string like mechanism and to inhibit leader cell formation.107,108 Consequently, inhibition of the Rho-ROCK axis disrupts intercellular mechanocoupling and coordination, in both 2D and 3D models of collective cell migration.107,109,110 As second, possibly related location of intercellular actomyosin cables, the circular periphery of cell clusters relies upon myosin activation downstream of Cdc42 and MRCK.83 Likely, any interface of moving cell groups toward an outward fluid or air environment depends upon mechanical rigor to shield the group and enable supracellular contractility.
Rho GTPases in Apical Basal Polarity in Moving Cells
In both stationary and moving epithelia, apical-basal polarity is important for apical lumen formation and barrier function, and basal assembly of a basement membrane.11,111 In non-moving, stable epithelia, Rho GTPases contribute to apical-basal polarity by cooperating with the Scrib, Par polarity, and Crumbs complexes. Cdc42 activates Par6, which, through downstream activation of aPKC and phosphorylation of Lgl, releases Lgl from a Par6/aPKC complex.112 This recruits Lgl to Scrib and Dlg to form the Scrib complex at the basolateral surface,112 and binding of Par6/aPKC to Par3 which forms the active Par complex at the apical and/or tight junctional area.81,112 The Par complex also interacts with the transmembrane protein Crumbs, which, together with PATJ and PALS, forms the Crumbs complex, which localizes to the apical surface by unclear mechanisms.81,113 The dichotomy of Scribble vs. Par/Crumbs distribution determines apical and basolateral membrane identity and asymmetry of membrane-associated protein complexes, organelles and cytoskeletal organization.114
Multiple upstream signals regulate polarity complex formation and distribution in collectively moving cells, including ECM-derived signals from the basement membrane which engage β1 integrins, Par3-dependent Rac or Cdc42 activation and negative cross-talk to Rho inhibiting the Rho-ROCK-myosin II axis.115-119 Mechanisms driving initial cell polarity downstream of the polarity complexes remain poorly defined, but include phosphoinosites and regulators of vesicle traffic.111 As consequence of cytoskeletal polarization, collective migration is coordinated with ECM protein deposition and remodeling required for the formation of basement membrane. Moving cell sheets or tubules deposit the basement membrane of the developing Drosophila eggchamber120 or along epithelial acini,121 highlighting how collective movement may contribute to polarization, tissue formation and patterning. Once established, both cell-cell and cell-ECM adhesions maintain and reinforce cell polarization.114 Consistently, in moving 2D epithelial sheets and Drosophila border cells, cell-cell adhesions maintain apical basal polarization of follower cells toward the underlying substrate, whereas leader cells exhibit a combination of apicobasal and front-rear polarization12,101,122 (Zegers, unpublished results).
In differentiating epithelia moving through 3D environments, apical-basal polarity is partly abandoned in early branching morphogenesis of mammary and kidney epithelial cells but reestablished in follower cells with lumen formation and stabilization of the duct.11 In tumors, apical-basal polarity is lost with dedifferentiation, resulting in non-polarized multicellular masses that extend into the connective tissue stroma.123 Likely, in collective cancer cell invasion, cell-cell junctions are loosened, as indicated by intravital studies showing increased E-cadherin dynamics and decreased adhesion strength downstream of signaling via β1 integrin, Src and Fak.51 Thus, in a context-dependent manner, collective cell migration may result in varying levels of apical-basal polarity with apical lumen formation and basolateral basement membrane assembly.
Rho GTPases in Front-Rear Polarity of Follower Cells
The forward movement of single (leader) cells is coupled to the collective motion of follower cells. The limited traction force of leader cells exerts drag force to follower cells which, on its own, is not sufficient to move a multicellular group forward.17,124,125 Individual follower cells thus maintain and integrate two types of mechanocoupling, including (1) traction from neighboring cells, mostly mediated by apical junctional complexes and (2) by actively generating traction through basal adhesion and force transmission to the substrate.17,124,126
In contrast to leader cells, which show high Rho activity and associated traction forces toward the substrate mostly at the front of the cell,107 follower cells generate both pulling- and pushing-type mechanotransduction forces to the substrate as well as varying degree of Rho activity.17,107 The forces impacting follower cell kinetics likely represent an integrated force map from leading cells pulling, follower cell mechanocoupling toward the basal substratum, and supracellular mechanocoupling along cell-cell junctions between neighboring cells in a “tug-of-war”-like manner.124,127 While moving, follower cells develop cryptic lamellipodia at their basal surface which generate protrusions along the substrate and underneath neighbor cells toward the direction of migration,12,16 the relevance of which for force generation yet remains to be resolved. In moving 2D sheets, Rac activity in both leading and trailing cells is required for basal actin polymerization and sheet migration,33,128 which indicates that Rac drives the formation of cryptic lamellipodia
The molecular integration of dynamic protrusion formation in direct vicinity to intact cell-cell junctions and apical-basal polarization signaling remains unclear. Formation of cryptic lamellipodia at the cell basis does not depend on RhoA-ROCK-myosin II pathways;16 however, mechanocoupling between leader and follower cells depends upon apical myosin II, suggesting a vertical separation between basal Rac and apical Rho dominance.129,130 Thereby cryptic lamellipodia may reflect a perpetual state of nascent cell-cell junctions with high Rac-driven cytoskeletal dynamics that simultaneously engage with and extend along ECM substrate. Consistently, the unconventional myosin IXA, an actin-binding RhoGAP involved in collective, but not single cell migration, colocalizes with myosin II in nascent adherens junctions131 and contributes to both, cryptic lamellipodia and stable cell-cell junctions.132 This function in downregulating Rho activity may enable both cryptic lamellipodia and non-contractile cell-cell junction in vicinity. The basolateral interface thus combines features of front-rear asymmetry and mechanotransduction, through cryptic lamellipodia, and apicobasal polarity with polarized protein deposition and tissue scaffold assembly.
Concluding Remarks
In contrast to single-cell migration, collective movement combines themes of mechanotransduction, epithelial biology, tissue formation and remodeling, and intercellular communication to an exciting, but also challenging interdisciplinary theme. Because interference with one signaling pathway potentially affects different modules of multicellular mechanotransduction and coordination, mechanistic insight is often blurred by complex phenotypes with yet very similar end-points, such as loss of migration efficacy or cell dispersion. Thus, although general functions of Rho GTPases in regulating supracellular shape, interaction with ECM and mechanotransduction are well established, global interference with Rho GTPases cause complex phenotypes by compromising several key functions concurrently. Spatiotemporally defined approaches such as the use of FRET-based biosensors for Rho GTPase activity or mechanotransduction, or optogenetic and laser microablation approaches enable the in-context probing for partial functions of specific Rho GTPase family members in cell mechanics and migration in a subcellular defined manner.24,106,133-135 When combined with molecular interference, RhoA biosensor imaging and the use of micropillar substrate reveal the supracellular mechanocoupling as function of RhoA in leader cells.107 Combining such precision molecular interference with mechanical and FRET-based activity readout will allow to establish the kinetic mosaic of Rac/Cdc42 and distinct Rho subfamily members in intracellular and intercellular subregions. This will delineate regions of exclusive vs. joint activity of GTPases that balance cell dynamics with cell-cell junction stability in collectively moving cells (Fig. 1).
Open questions on collective polarity further include mechanisms regulating front protrusion of budding-type invasion into 3D environments without dedicated leader cell, whereby multiple cells form the front row on a rotating, interchangeable manner54; the upstream effectors that maintain spatial separation of concurrently active Rho GTPases, including GEFs and GAPs; and the spatiotemporal specialization toward downstream effectors that execute dedicated non-redundant functions in a cell-type specific manner, including cytoskeletal regulators as well as effectors of other cellular pathways such as microtubule dynamics and cytoskeletal interaction with the nucleus.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
M.Z. is supported by the European Commission FP7-PEOPLE-2011-IIF (302067). P.F. is supported by the Netherlands Science Organization (NWO-VICI 918.11.626) and the Cancer Genomics Center, Netherlands.
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–9. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 3.Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis. 2009;26:289–98. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- 4.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–22. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 6.Bravo-Cordero JJ, Magalhaes MA, Eddy RJ, Hodgson L, Condeelis J. Functions of cofilin in cell locomotion and invasion. Nat Rev Mol Cell Biol. 2013;14:405–15. doi: 10.1038/nrm3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 2010;339:83–92. doi: 10.1007/s00441-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14:777–83. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 9.Rørth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–29. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 10.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 11.Zegers MM. 3D in vitro cell culture models of tube formation. Semin Cell Dev Biol. 2014;31:132–40. doi: 10.1016/j.semcdb.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Vasilyev A, Liu Y, Mudumana S, Mangos S, Lam PY, Majumdar A, Zhao J, Poon KL, Kondrychyn I, Korzh V, et al. Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol. 2009;7:e9. doi: 10.1371/journal.pbio.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–54. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 14.Khalil AA, Friedl P. Determinants of leader cells in collective cell migration. Integr Biol (Camb) 2010;2:568–74. doi: 10.1039/c0ib00052c. [DOI] [PubMed] [Google Scholar]
- 15.Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2012;22:104–15. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 17.Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–75. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchsbaum RJ. Rho activation at a glance. J Cell Sci. 2007;120:1149–52. doi: 10.1242/jcs.03428. [DOI] [PubMed] [Google Scholar]
- 19.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 20.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 21.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–85. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–83. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–98. doi: 10.1016/S0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 24.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–8. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, Danuser G, Bokoch GM. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell. 2007;13:646–62. doi: 10.1016/j.devcel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zegers M. Roles of P21-activated kinases and associated proteins in epithelial wound healing. Int Rev Cell Mol Biol. 2008;267:253–98. doi: 10.1016/S1937-6448(08)00606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charras GT, Hu CK, Coughlin M, Mitchison TJ. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175:477–90. doi: 10.1083/jcb.200602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paluch EK, Raz E. The role and regulation of blebs in cell migration. Curr Opin Cell Biol. 2013;25:582–90. doi: 10.1016/j.ceb.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127–37. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 30.Laser-Azogui A, Diamant-Levi T, Israeli S, Roytman Y, Tsarfaty I. Met-induced membrane blebbing leads to amoeboid cell motility and invasion. Oncogene. 2014;33:1788–98. doi: 10.1038/onc.2013.138. [DOI] [PubMed] [Google Scholar]
- 31.Poincloux R, Collin O, Lizárraga F, Romao M, Debray M, Piel M, Chavrier P. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc Natl Acad Sci U S A. 2011;108:1943–8. doi: 10.1073/pnas.1010396108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anon E, Serra-Picamal X, Hersen P, Gauthier NC, Sheetz MP, Trepat X, Ladoux B. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc Natl Acad Sci U S A. 2012;109:10891–6. doi: 10.1073/pnas.1117814109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–9. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 35.Bustos RI, Forget MA, Settleman JE, Hansen SH. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr Biol. 2008;18:1606–11. doi: 10.1016/j.cub.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Macara IG. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell. 2008;14:216–26. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–39. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeldt H, Castellone MD, Randazzo PA, Gutkind JS. Rac inhibits thrombin-induced Rho activation: evidence of a Pak-dependent GTPase crosstalk. J Mol Signal. 2006;1:8. doi: 10.1186/1750-2187-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118:1861–72. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 40.Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem. 2004;279:18392–400. doi: 10.1074/jbc.M400084200. [DOI] [PubMed] [Google Scholar]
- 41.Barac A, Basile J, Vázquez-Prado J, Gao Y, Zheng Y, Gutkind JS. Direct interaction of p21-activated kinase 4 with PDZ-RhoGEF, a G protein-linked Rho guanine exchange factor. J Biol Chem. 2004;279:6182–9. doi: 10.1074/jbc.M309579200. [DOI] [PubMed] [Google Scholar]
- 42.Alberts AS, Qin H, Carr HS, Frost JA. PAK1 negatively regulates the activity of the Rho exchange factor NET1. J Biol Chem. 2005;280:12152–61. doi: 10.1074/jbc.M405073200. [DOI] [PubMed] [Google Scholar]
- 43.Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–14. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 44.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 45.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13:383–93. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–15. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Vitorino P, Meyer T. Modular control of endothelial sheet migration. Genes Dev. 2008;22:3268–81. doi: 10.1101/gad.1725808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203–8. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- 49.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–96. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macpherson IR, Hooper S, Serrels A, McGarry L, Ozanne BW, Harrington K, Frame MC, Sahai E, Brunton VG. p120-catenin is required for the collective invasion of squamous cell carcinoma cells via a phosphorylation-independent mechanism. Oncogene. 2007;26:5214–28. doi: 10.1038/sj.onc.1210334. [DOI] [PubMed] [Google Scholar]
- 51.Canel M, Serrels A, Miller D, Timpson P, Serrels B, Frame MC, Brunton VG. Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Res. 2010;70:9413–22. doi: 10.1158/0008-5472.CAN-10-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol. 2004;48:441–9. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- 53.Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010;20:319–28. doi: 10.1016/j.tcb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–81. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubow KE, Conrad SK, Horwitz AR. Matrix microarchitecture and myosin II determine adhesion in 3D matrices. Curr Biol. 2013;23:1607–19. doi: 10.1016/j.cub.2013.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hegerfeldt Y, Tusch M, Bröcker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–30. [PubMed] [Google Scholar]
- 57.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J. 2008;95:5374–84. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 59.Scott RW, Hooper S, Crighton D, Li A, König I, Munro J, Trivier E, Wickman G, Morin P, Croft DR, et al. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J Cell Biol. 2010;191:169–85. doi: 10.1083/jcb.201002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pignatelli J, LaLonde SE, LaLonde DP, Clarke D, Turner CE. Actopaxin (α-parvin) phosphorylation is required for matrix degradation and cancer cell invasion. J Biol Chem. 2012;287:37309–20. doi: 10.1074/jbc.M112.385229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–46. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 63.Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol. 2010;191:1261–9. doi: 10.1083/jcb.201003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol. 2006;16:2395–405. doi: 10.1016/j.cub.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12:591–7. doi: 10.1038/ncb2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009;19:668–74. doi: 10.1016/j.cub.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 67.Audebert S, Navarro C, Nourry C, Chasserot-Golaz S, Lécine P, Bellaiche Y, Dupont JL, Premont RT, Sempéré C, Strub JM, et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol. 2004;14:987–95. doi: 10.1016/j.cub.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 68.Nola S, Sebbagh M, Marchetto S, Osmani N, Nourry C, Audebert S, Navarro C, Rachel R, Montcouquiol M, Sans N, et al. Scrib regulates PAK activity during the cell migration process. Hum Mol Genet. 2008;17:3552–65. doi: 10.1093/hmg/ddn248. [DOI] [PubMed] [Google Scholar]
- 69.Bahri S, Wang S, Conder R, Choy J, Vlachos S, Dong K, Merino C, Sigrist S, Molnar C, Yang X, et al. The leading edge during dorsal closure as a model for epithelial plasticity: Pak is required for recruitment of the Scribble complex and septate junction formation. Development. 2010;137:2023–32. doi: 10.1242/dev.045088. [DOI] [PubMed] [Google Scholar]
- 70.Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, Russell SM, Humbert PO. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–82. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- 71.Eastburn DJ, Zegers MM, Mostov KE. Scrib regulates HGF-mediated epithelial morphogenesis and is stabilized by Sgt1-HSP90. J Cell Sci. 2012;125:4147–57. doi: 10.1242/jcs.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hunter MP, Zegers MM. Pak1 regulates branching morphogenesis in 3D MDCK cell culture by a PIX and beta1-integrin-dependent mechanism. Am J Physiol Cell Physiol. 2010;299:C21–32. doi: 10.1152/ajpcell.00543.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michaelis UR, Chavakis E, Kruse C, Jungblut B, Kaluza D, Wandzioch K, Manavski Y, Heide H, Santoni MJ, Potente M, et al. The polarity protein Scrib is essential for directed endothelial cell migration. Circ Res. 2013;112:924–34. doi: 10.1161/CIRCRESAHA.112.300592. [DOI] [PubMed] [Google Scholar]
- 74.Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–87. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- 75.Yates LL, Schnatwinkel C, Hazelwood L, Chessum L, Paudyal A, Hilton H, Romero MR, Wilde J, Bogani D, Sanderson J, et al. Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Dev Biol. 2013;373:267–80. doi: 10.1016/j.ydbio.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–71. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Métais JY, Navarro C, Santoni MJ, Audebert S, Borg JP. hScrib interacts with ZO-2 at the cell-cell junctions of epithelial cells. FEBS Lett. 2005;579:3725–30. doi: 10.1016/j.febslet.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 78.Ivanov AI, Young C, Den Beste K, Capaldo CT, Humbert PO, Brennwald P, Parkos CA, Nusrat A. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol. 2010;176:134–45. doi: 10.2353/ajpath.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nola S, Daigaku R, Smolarczyk K, Carstens M, Martin-Martin B, Longmore G, Bailly M, Braga VM. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J Cell Biol. 2011;195:855–71. doi: 10.1083/jcb.201107162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tay HG, Ng YW, Manser E. A vertebrate-specific Chp-PAK-PIX pathway maintains E-cadherin at adherens junctions during zebrafish epiboly. PLoS One. 2010;5:e10125. doi: 10.1371/journal.pone.0010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–59. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 82.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–6. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 83.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–7. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- 85.Wang S, Watanabe T, Matsuzawa K, Katsumi A, Kakeno M, Matsui T, Ye F, Sato K, Murase K, Sugiyama I, et al. Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. J Cell Biol. 2012;199:331–45. doi: 10.1083/jcb.201202041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–24. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukuyama T, Ogita H, Kawakatsu T, Inagaki M, Takai Y. Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway. Oncogene. 2006;25:8–19. doi: 10.1038/sj.onc.1209010. [DOI] [PubMed] [Google Scholar]
- 89.Malliri A, van Es S, Huveneers S, Collard JG. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem. 2004;279:30092–8. doi: 10.1074/jbc.M401192200. [DOI] [PubMed] [Google Scholar]
- 90.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–17. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–27. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–66. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ratheesh A, Yap AS. A bigger picture: classical cadherins and the dynamic actin cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:673–9. doi: 10.1038/nrm3431. [DOI] [PubMed] [Google Scholar]
- 95.de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci U S A. 2009;106:7010–5. doi: 10.1073/pnas.0811253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–8. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 97.Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–48. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 98.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14:818–28. doi: 10.1038/ncb2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Popoff MR, Geny B. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim Biophys Acta. 2009;1788:797–812. doi: 10.1016/j.bbamem.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Wennerberg K, Forget M-A, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, Der CJ, Hansen SH. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr Biol. 2003;13:1106–15. doi: 10.1016/S0960-9822(03)00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinheiro EM, Montell DJ. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development. 2004;131:5243–51. doi: 10.1242/dev.01412. [DOI] [PubMed] [Google Scholar]
- 102.Oubaha M, Lin MI, Margaron Y, Filion D, Price EN, Zon LI, Côté J-F, Gratton J-P. Formation of a PKCζ/β-catenin complex in endothelial cells promotes angiopoietin-1-induced collective directional migration and angiogenic sprouting. Blood. 2012;120:3371–81. doi: 10.1182/blood-2012-03-419721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hosono Y, Yamaguchi T, Mizutani E, Yanagisawa K, Arima C, Tomida S, Shimada Y, Hiraoka M, Kato S, Yokoi K, et al. MYBPH, a transcriptional target of TTF-1, inhibits ROCK1, and reduces cell motility and metastasis. EMBO J. 2012;31:481–93. doi: 10.1038/emboj.2011.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012;109:12568–73. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maître JL, Heisenberg CP. Three functions of cadherins in cell adhesion. Curr Biol. 2013;23:R626–33. doi: 10.1016/j.cub.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heisenberg CP, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–62. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 107.Reffay M, Parrini MC, Cochet-Escartin O, Ladoux B, Buguin A, Coscoy S, Amblard F, Camonis J, Silberzan P. Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat Cell Biol. 2014;16:217–23. doi: 10.1038/ncb2917. [DOI] [PubMed] [Google Scholar]
- 108.Tamada M, Perez TD, Nelson WJ, Sheetz MP. Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J Cell Biol. 2007;176:27–33. doi: 10.1083/jcb.200609116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci U S A. 2003;100:10788–93. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu W, O’Brien LE, Wang F, Bourne H, Mostov KE, Zegers MM. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell. 2003;14:748–63. doi: 10.1091/mbc.E02-06-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roignot J, Peng X, Mostov K. Polarity in mammalian epithelial morphogenesis. Cold Spring Harb Perspect Biol. 2013;5:5. doi: 10.1101/cshperspect.a013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Humbert PO, Dow LE, Russell SM. The Scribble and Par complexes in polarity and migration: friends or foes? Trends Cell Biol. 2006;16:622–30. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 113.Schlüter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20:4652–63. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–57. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 115.Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–45. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu W, Shewan AM, Brakeman P, Eastburn DJ, Datta A, Bryant DM, Fan QW, Weiss WA, Zegers MM, Mostov KE. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep. 2008;9:923–9. doi: 10.1038/embor.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li R, Pendergast AM. Arg kinase regulates epithelial cell polarity by targeting β1-integrin and small GTPase pathways. Curr Biol. 2011;21:1534–42. doi: 10.1016/j.cub.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Daley WP, Gervais EM, Centanni SW, Gulfo KM, Nelson DA, Larsen M. ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development. 2012;139:411–22. doi: 10.1242/dev.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–4. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang H, Lacoche S, Huang L, Xue B, Muthuswamy SK. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proc Natl Acad Sci U S A. 2013;110:163–8. doi: 10.1073/pnas.1201141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee M, Vasioukhin V. Cell polarity and cancer--cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121:1141–50. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- 123.Pearson HB, Perez-Mancera PA, Dow LE, Ryan A, Tennstedt P, Bogani D, Elsum I, Greenfield A, Tuveson DA, Simon R, et al. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J Clin Invest. 2011;121:4257–67. doi: 10.1172/JCI58509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ. Physical forces during collective cell migration. Nat Phys. 2009;5:426–30. doi: 10.1038/nphys1269. [DOI] [Google Scholar]
- 125.Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell. 2006;10:673–80. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 126.Vitorino P, Hammer M, Kim J, Meyer T. A steering model of endothelial sheet migration recapitulates monolayer integrity and directed collective migration. Mol Cell Biol. 2011;31:342–50. doi: 10.1128/MCB.00800-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ladoux B. Biophysics: Cells guided on their journey. Nat Phys. 2009;5:377–8. doi: 10.1038/nphys1281. [DOI] [Google Scholar]
- 128.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol. 2000;10:831–8. doi: 10.1016/S0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- 129.Georgiou M, Baum B. Polarity proteins and Rho GTPases cooperate to spatially organise epithelial actin-based protrusions. J Cell Sci. 2010;123:1089–98. doi: 10.1242/jcs.060772. [DOI] [PubMed] [Google Scholar]
- 130.Ng MR, Besser A, Danuser G, Brugge JS. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J Cell Biol. 2012;199:545–63. doi: 10.1083/jcb.201207148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Omelchenko T. Regulation of collective cell migration by RhoGAP myosin IXA. Small GTPases. 2012;3:213–8. doi: 10.4161/sgtp.20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Omelchenko T, Hall A. Myosin-IXA regulates collective epithelial cell migration by targeting RhoGAP activity to cell-cell junctions. Curr Biol. 2012;22:278–88. doi: 10.1016/j.cub.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Friedl P, Wolf K, Zegers MM. Rho-directed forces in collective migration. Nat Cell Biol. 2014;16:208–10. doi: 10.1038/ncb2923. [DOI] [PubMed] [Google Scholar]
- 134.Bacchus W, Fussenegger M. The use of light for engineered control and reprogramming of cellular functions. Curr Opin Biotechnol. 2012;23:695–702. doi: 10.1016/j.copbio.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Campinho P, Behrndt M, Ranft J, Risler T, Minc N, Heisenberg CP. Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat Cell Biol. 2013;15:1405–14. doi: 10.1038/ncb2869. [DOI] [PubMed] [Google Scholar]
- 136.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–44. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Terry SJ, Elbediwy A, Zihni C, Harris AR, Bailly M, Charras GT, Balda MS, Matter K. Stimulation of cortical myosin phosphorylation by p114RhoGEF drives cell migration and tumor cell invasion. PLoS One. 2012;7:e50188. doi: 10.1371/journal.pone.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–35. doi: 10.1016/S0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 139.Tada M, Heisenberg CP. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development. 2012;139:3897–904. doi: 10.1242/dev.073007. [DOI] [PubMed] [Google Scholar]
- 140.Pirraglia C, Jattani R, Myat MM. Rac function in epithelial tube morphogenesis. Dev Biol. 2006;290:435–46. doi: 10.1016/j.ydbio.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Rizqiawan A, Tobiume K, Okui G, Yamamoto K, Shigeishi H, Ono S, Shimasue H, Takechi M, Higashikawa K, Kamata N. Autocrine galectin-1 promotes collective cell migration of squamous cell carcinoma cells through up-regulation of distinct integrins. Biochem Biophys Res Commun. 2013;441:904–10. doi: 10.1016/j.bbrc.2013.10.152. [DOI] [PubMed] [Google Scholar]