Abstract
Podosomes and invadopodia (collectively known as invadosomes) are specialized plasma-membrane actin-based microdomains that combine adhesive properties with matrix degrading and/or mechanosensor activities. These organelles have been extensively studied in vitro and current concerted efforts aim at establishing their physiological relevance and subsequent association with human diseases. Proper functioning of the bone, immune, and vascular systems is likely to depend on these structures while their occurrence in cancer cells appears to be linked to tumor metastasis. The elucidation of the mechanisms driving invadosome assembly is a prerequisite to understanding their role in vivo and ultimately to controlling their functions. Adhesive and soluble ligands act via transmembrane receptors that propagate signals to the cytoskeleton via small G proteins of the Rho family, assisted by tyrosine kinases and scaffold proteins to induce invadosome formation and rearrangements. Oncogene expression and cell-cell interactions may also trigger their assembly. Manipulation of the signals that regulate invadosome formation and dynamics could therefore be a strategy to interfere with their functions in a multitude of pathological settings, such as excessive bone breakdown, infections, vascular remodeling, transendothelial diapedesis, and metastasis.
Keywords: RhoGTPases, extracellular matrix, cytoskeleton, podosomes, invadopodia, invadosomes
Introduction
Remodeling of the actin cytoskeleton heavily relies on the regulation of RhoGTPases which have important and conserved roles in almost all its functions. Their activity is tightly regulated by three classes of molecules: guanine nucleotide exchange factors, GEFs, which exchange GDP for GTP hence activating the GTPase; GTPase activating proteins, GAPs, which assist in the hydrolysis of bound GTP to GDP thus contributing to inactivation; and guanine dissociation inhibitors, GDIs, which sequester membrane-anchored GTPases into the cytosol and thus affect their distribution, localization and protein levels.1 Typically, stress fibers are induced in response to RhoA activation, lamellipodium formation is promoted by Rac activity, and filopodia are formed upon Cdc42 stimulation.2 Less common cytoskeletal structures are podosomes and their tumor cell counterpart invadopodia (collectively called invadosomes).3,4 Why and how these structures are generated is now the focus of intense research. Podosomes form spontaneously upon cell adhesion in several subsets of the myelomonocytic lineage such as osteoclasts (OCs), macrophages and immature dendritic cells (iDCs). They can also be formed by other cells like endothelial (ECs) and vascular smooth muscle cells (VSMCs) in a context dependent manner.4 Invadopodia are related structures found in invasive cancer cells displaying aberrant signaling.3 Cells transformed by oncogenic Src in vitro also display invadosomes with mixed features of podosomes and invadopodia.5 Invadosomes differ significantly from other cytoskeletal structures in terms of molecular composition, dynamics, architecture, subcellular distribution, and presumed functions, and these characteristics largely vary in accordance with the cell type and microenvironment considered (Fig. 1).
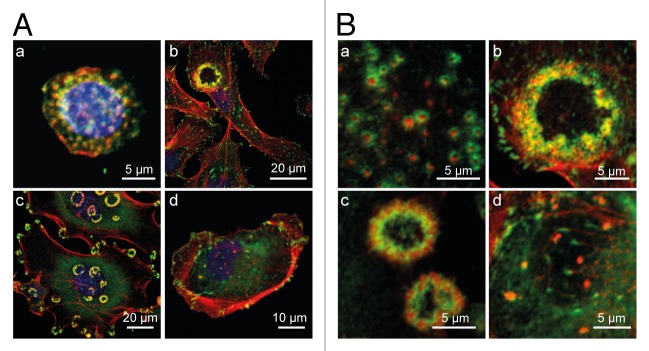
Figure 1. Invadosome architecture and patterning. An unusual feature of invadosomes is the extreme diversity of their architectural presentation and subcellular distribution in cells of different cells types. (A) Members of the invadosome family arise with various architectures and in various types of subcellular arrangement: (a) a stationary macrophages with numerous individual podosomes covering the whole substrate-attached cell side; (b) an endothelial cell with a typical rosette superstructure consisting of interlinked podosomes; (c) RSV–transformed fibroblasts form numerous rosettes of invadosomes in the cell periphery or in the perinuclear area. (d) A cancer cell with several irregularly sized invadopodia located in the vicinity of the nucleus. Such diversity suggests that podosome functions depend on podosome subcellular arrangement within the cells. (B) Invadosome architectural organization showing the actin core and the adhesive apparatus shown at higher magnification, corresponding to the cells presented in (A). Cells seeded on glass coverslips, fixed and stained for F-actin (red), vinculin (green), and the nucleus (blue).
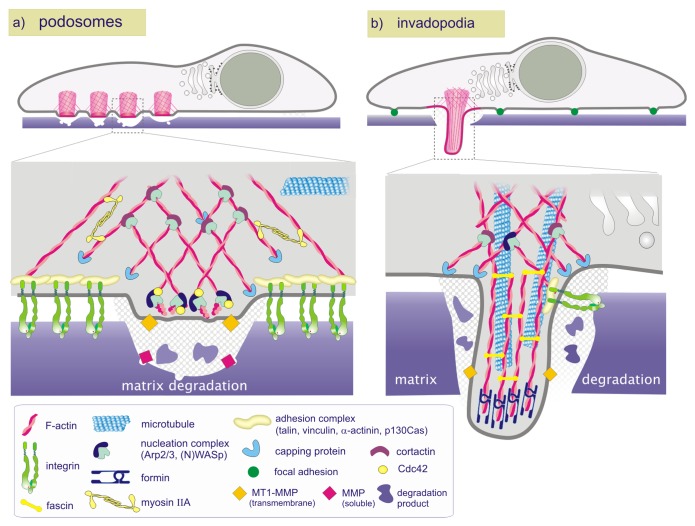
Structurally, all types of invadosomes are visualized as punctate dynamic microdomains formed at the ventral membrane, highly enriched with filamentous actin (F-actin) and orientated perpendicularly to the substratum (Fig. 2). In close association with F-actin, Arp2/3, WASp/N-WASp, cortactin, dynamin, gelsolin, and cofilin are consistently found in invadosomes and account for their dynamic behavior. The scaffolding protein and Src substrate Tks5 also colocalizes with F-actin and constitutes a reliable invadosome marker.6 Beside these common traits, podosomes and invadopodia differ from each other in several aspects. Podosomes clearly appear as bipartite entities where the actin core is surrounded by a ring structure containing integrins and other focal adhesion proteins such as actin binders, signaling molecules, and adaptors including vinculin, talin, paxillin, and p130Cas among others.7 This ring complex links cell surface integrins to an interconnected network of actin filaments radiating from the F-actin cores, allowing collective dynamical behaviors.8-10 The ring structure is less prominent for invadopodia and the focal adhesion proteins often colocalize with the core (Fig. 1B).11,12 Podosomes extend upwards from the ventral cell surface into the cytoplasm whereas invadopodia are long filopodial-like membrane extensions that penetrate into the extracellular matrix (ECM). Thus, the architecture, properties and subcellular arrangement of invadosomes may reflect, at least in part, the cellular processes in which they are involved.
Figure 2. Schematic representation of podosomes and invadopodia. Podosomes consist of a core column of actin filaments that extends upwards from the ventral cell surface into the cytoplasm whereas invadopodia are long filopodial-like membrane extensions that penetrate into the ECM. (A) Schematic representation of a podosome in a macrophage: Cdc42/WASp/Arp2/3 drives actin polymerization at the face of the actin core, generating forces that push against the core and consequently promotes podosome growth. Radial actin filaments emanate from the actin core and link it to cell surface integrins and associated proteins that form the basis of the adhesive ring complex. (B) Schematic representation of an invadopodium in a cancerous cell: The N-WASp/Arp2/3 complex is present at the base and along the length of shafts of invadopodia but absent from the tips. The Rho effector mDia is involved in both initiation of invadopodia formation through actin nucleation and subsequent growth of invadopodia through the elongation of actin filaments. The protease activity is essential for the assembly of protrusive actin structures. Invadopodia lack the strict organization of core and ring structure found in podosomes.
One of the most distinctive features of invadosomes is the presence of metalloproteases (MMPs), notably MT1-MMP, endowing them with ECM degrading activities.4 Recent studies have challenged this traditional view because focal adhesions in tumor cells may also be enriched with matrix degrading proteinases that, although less potently, alter ECM integrity.13 Invadosomes are thus commonly seen as cellular organelles specifically involved in cell migration and invasion processes. However, above all, podosomes are the primary adhesion structures of monocyte-derived cells that do not form focal adhesions.14 They develop evenly at the cell ventral membrane in the first steps of integrin-mediated cell spreading and cover the whole substrate-attached cell side in stationary cells3 (Fig. 1A part a). These organelles seem to be endowed with a matrix sensor function involving oscillations of the actin cores.16-18 Upon exposure to chemotactic factors, they relocalize at the leading edge14,15 enabling cells to migrate through environments that otherwise act as barriers to most cell types. Highly dynamic podosomes may thus continuously probe the substrate and serve as guidance organelles during cell migration. Eosinophils,19 natural,20 or lymphokine-activated21 killer cells also interact with endothelial cell surfaces via structures presenting podosome characteristics. The observations made in the model of three dimensional (3D) transcellular diapedesis suggest a situation of migratory pathfinding in which dynamic “invadosome-like protrusions” formed by leukocytes have a central role in both identifying and exploiting endothelial locations that are permissive for intravasation and extravasation.22
Podosomes may also cluster to build up cellular devices involved in other types of functions. The most meaningful illustration of this situation is encountered with OCs. Here, the nature of the substratum represents a key element in their organization. When OCs are plated on glass slides, podosomes are seen in their generic configuration consisting of the actin core flanked by the adhesive ring.14 However, when these cells are seeded on bone slices, podosomes are found organized into dense arrays of podosome cores highly interconnected through the radial network of actin filaments.18 The resulting structure, the sealing zone (SZ), encloses a bone-resorption compartment, which is the site of intense degrading activity. As the SZ mediates firm adhesion of OCs to the bone surface and seals off the resorption pit, the overall function of podosome cores at the SZ differs from that of individual podosomes.23 Once osteoclast-mediated bone resorption is complete, osteoblasts come into play to regenerate bone tissue. Similarly, vessel homeostasis may involve rings of podosomes (rosettes) (Fig. 1A part b) that assemble in arterial ECs in response to TGFβ,24 a cytokine known to be involved in vessel maintenance. By concentrating metalloprotease activities, podosome rosettes may strip the basement membrane, allowing the renewal of ECM components by matrix-producing cells.
In primary tumor cells or in cell lines derived from them, invadopodia often appear as irregular perinuclear isolated dots in the vicinity of the Golgi apparatus.3 (Fig. 1A part d). In fibroblasts transformed by oncogenic Src in vitro, the structures cluster in circular arrays (rosettes) (Fig. 1A part c).25 Here, invadosomes may be part of drilling machinery that creates paths in which the cell body subsequently penetrates, allowing cells to make their way through the stroma. By analogy, a similar function may be performed by endothelial invadosomes in microvessels (TD, unpublished data). In this situation, endothelial podosomes may be endowed with functions similar to those performed by aggregated invadopodia seen in cells isolated from primary tumors or cell lines, using clustered invadosomes as a cellular device for invasive migration as required for the accomplishment of the angiogenic program.
Considerable progress has been made in recent years in understanding the ultrastructural and functional features of invadosomes. The elucidation of the molecular mechanisms driving the assembly of these organelles is a prerequisite to understanding their role in vivo and to controlling their functions in physiological and pathological processes. The objective of this review is to highlight how GTPases of the Rho family contribute to invadosome biology. Integrin signaling, cytokine stimulation, oncogene expression, cellular stress, and cell-cell interactions represent a series of inducers able to drive invadosome assembly through a dramatic reorganization of the cell actin cytoskeleton which relies on GTPases of the Rho family. When their induction requires de novo protein synthesis, RhoGTPases are also involved, as components of the transcriptional machinery. The matrix remodeling function depends on other GTPases, the Rab and Arf families, regulating the traffic of specific proteinases through several subcellular compartments and their release at invadosomes. The description of the invadosome mechanosensing activity is still in its infancy but it is a safe bet that cycling of RhoGTPases will be involved in the dynamic aspects of this function. We first present an overview of the history, architectural characteristics and functions of invadosomes. Given the close interplay between invadosome formation and cell-associated functions, we review the role of GTPases, GEFs, and GAPs, as well as some of their canonical effectors in the main cell types where these organelles have been described. We also discuss these regulations in the context of invadosome-assigned functions such as adhesion, actin-based motility or invasion, matrix remodeling and mechanosensing, focusing on the model specificities that have led to important findings in the field. In the last part of this review, we seek to identify the functions of individual GTPases that are shared by the different types of invadosomes and those that confer specificity.
History of the Invadosome Family
Podosomes (from podos, feet and soma, body: foot bodies) were initially discovered in the early 1980s in Rous sarcoma virus transformed fibroblasts.26 Src was the first signaling element to be discovered for podosome formation and remains the master regulator of all types of structures within the family. Podosomes were subsequently shown to be sites of cell adhesion5 and matrix degradation.27 Interest in these structures gained ground when it was discovered that normal cells also have the intrinsic ability to assemble podosomes, first OCs,28 then macrophages,29 and lastly iDCs,30,31 all bone marrow-derived cells. Research on podosomes expanded in the late 1990s when defective podosome formation in macrophages was linked to the mutant gene that causes Wiskott-Aldrich Syndrome (WAS),32 thus laying the foundations for studying the connection between podosomes and diseases. Intensified research in the field led to the observation that non-myelomonocytic cells are also endowed with a podosome-forming capacity induced under appropriate stimulation, which may be a soluble factor,19,33,34 a matrix receptor,35 or cell stress.36,37 It was then found that melanoma, glioma, breast, carcinoma, and some leukemic cells assemble actin structures that are absent from their normal counterparts and that this process can be stimulated further by certain cytokines such as EGF or TGFβ.38,39 With the discovery of a connection between podosomes and the metastatic potential of tumor cells,40 podosomes found in cancer cells were progressively renamed invadopodia and further characterization of podosomes and invadopodia added a list of specificities (see above) for each type of structure for distinguishing the two founding members of the invadosome family. However, some recent studies have reported novel situations that call such classifications into question. Organelles with invadopodial features have now been reported in normal cells.41 In addition, there is no evidence for an invariable association between mechanosensing activities and matrix degrading-functions because MT1-MMP is not an obligate component of podosomes.42 The substratum palping-function of invasive protrusions described in leucocytes involves cell-cell interactions, not cell cell-matrix ones.22 Direct contacts between macrophages and tumor cells trigger tumor cell intravasation mediated by invadopodial structures.43
Early studies to explore RhoGTPase signaling in the formation, characteristics and functions of invadosomes relied on the use of dominant negative and active mutants, whose limitations in terms of specificity are known.44 The development of small interfering RNA approaches in vitro and gene knockout strategies in mice, targeting individual GTPases has refined the pioneering studies. More recently, sophisticated imaging techniques using fluorescence resonance energy transfer (FRET)-based biosensors for RhoGTPases has provided information on the spatio-temporal regulation of this class of regulators.
Cells of the Myelomonocytic Lineage: Spontaneous Podosomes
Cells of myeloid lineage are apparently unique in their capacity to form podosomes spontaneously following cell adhesion. Interactions with the ECM through matrix receptors trigger the activation of RhoGTPases and Src via a variety of mechanisms. In addition, adhesion allows actomyosin dependent contractility, which is regulated by matrix stiffness. Thus, actin-dependent adhesion and tension may suffice to regulate the formation and organization of these podosomes.42 Spontaneous podosomes arise with a functional matrix degrading apparatus. Localized matrix degradation at podosomes relies on the targeted delivery of MT1-MMP via microtubules.45 In these cells, podosomes are small shallow structures characterized by a high turnover and superficial and widespread degradation of the underlying matrix.
Osteoclasts
OCs are large, multinucleated bone cells that conduct the process of bone resorption. In this remodeling process, podosomes play an integral role. As mentioned above, the sealing zone (SZ), formed by tightly packed podosomes provides firm attachment to bone and seals off the resorption lacunae where proteolytic enzymes and protons are secreted to dissolve the bone matrix.46 The resulting degradation products are then endocytosed, transported through the OC body by transcytosis, and finally released at the apical side. Excessive bone resorption resulting from unbalanced OC activity is the main cause of osteoporosis in the elderly, causing bone fragility and fractures. Podosomes have thus been extensively studied in OCs with the ultimate goal of identifying targets for therapeutic interventions aiming at controlling the formation of the bone-degrading apparatus of these cells. In the in vitro osteoclastogenesis model, immature OCs exhibit clustered podosomes, which first rearrange into dynamic short-lived rings, then into a single stable belt around the cell periphery47 (also known as the SZ-like structure, SZL). The SZL is reminiscent of the SZ found in OCs seeded on bone.18
Cdc42 stands as a central player in the regulation of podosome dynamics as it orchestrates podosome actin polymerization via its canonical effector, WASp. When activated by a GEF, Cdc42 binds to WASp. This binding, together with phosphorylation of WASp on tyrosine, induces a dramatic conformational change. The hydrophobic core is disrupted, releasing the VCA (Verprolin Homology domain-cofilin homology domain–Acidic region) domain and enabling its interaction with the Arp2/3 complex, thereby promoting actin nucleation.48 Although microinjection of active Cdc42 into OCs does not promote podosome formation,49 OCs derived from mice with increased Cdc42 activation due to knockout of its negative regulator Cdc42-GAP show increased SZ formation and bone resorption compared with wild type cells.50
The essential role of WASp in podosome formation has been demonstrated in WASp deficiencies. OCs from WASp knockout mice exhibit a reduction in podosome formation. WASp interacts with WIP (WASp-interacting protein), which induces its localization to podosomes and protects against calpain-dependent degradation.51 Consequently, WIP deficiency induces a reduction in WASp protein level in OCs. Interestingly, deletion of WIP only affects podosome core formation but not the actin radial fibers to which scaffolding and adhesion proteins are recruited. Both of these changes can be restored by the clustering of CD44 receptors.52 This collagen and osteopontin receptor may restore some level of Cdc42 activity either by neutralizing the inhibitory effect of Cdc42 interacting partner IQGAP through direct binding or indirectly, or by releasing the GTPase from RhoGDI.53
In OCs, podosome reorganization, a prerequisite for bone resorption, is critically dependent on Rho proteins. The microinjection of C3 enzyme (a clostridium botulinum toxin which inhibits RhoA/B/C) in murine OC-like cells was found to induce the rapid disassembly of the SZ.54 When the analysis was refined, it appeared that Rho interferes with the OC maturation process by controlling the level of microtubule acetylation and actin organization through the Rho effector formin mDia2, which in turn regulates the histone deacetylase HDAC6. Lowering Rho activity prevents mDia2 from activating HDAC6, thus inducing microtubule stabilization and promoting the switch from podosome rings to podosome belts, but inhibiting further maturation.55 Although microinjection of active RhoA may increase podosome formation to some extent,56 active RhoA does not promote SZ formation in the absence of signals emanating from the mineralized matrix.55 It is likely that cycling of RhoA plays here an essential role.44 Thus, in OCs, RhoA is involved in many aspects of podosome dynamics influencing the formation, stability and patterning of podosomes during cytoskeletal maturation. This may be achieved by engaging with distinct RhoA effectors, or by tuning the amplitude of the RhoA signal which can have distinct outcomes. Of note, the role of RhoB and RhoC in these events remains to be explored.
Another member of the Rho GTPase family involved in the regulation of OC podosomes is RhoU (also known as Wrch1). RhoU shares significant sequence and functional similarity with Cdc42, but unlike Cdc42, it has an extremely rapid, intrinsic guanine nucleotide exchange activity. In contrast to Cdc42 (expected but not formally demonstrated at the podosome core in these cells), RhoU colocalizes with vinculin around the podosome actin core.57 In agreement with this, RhoU does not bind to the isolated CRIB (Cdc42/Rac-interacting binding) domain of WASp in vitro.58 However, RhoU regulates proteins of the PAK family and this interaction may control its localization at podosomes.57 RhoU overexpression destabilizes the podosome belt and increases the formation of actin clusters but has no effect on the formation of the SZ or on bone resorption.57 The regulatory effect of RhoU on the RhoA pathway may account for its effect on podosome organization.59
The last and most recent member of the Rho GTPase family being studied in this model is RhoE (Rnd3).60 This GTPase-deficient and thus constitutively active protein is a RhoA antagonist through its interaction with ROCKI. Although RhoE does not localize at podosomes, RhoE-deficient OCs show defective podosome organization and small abnormal SZ when seeded on bone. This is due to the fact that RhoE stimulates actin filament severing at podosomes by preventing cofilin phosphorylation through ROCKI. This regulatory mechanism ensures the fast actin turnover at podosomes required for their organization, enabling OC migration, SZ formation and ultimately, bone resorption. Altogether, these novel findings describing the role of RhoE on actin turnover support an emerging model that dissociates podosome dynamics from its adhesive function.9
Both Rac1 and Rac2 are expressed in OCs. The two proteins have overlapping roles in podosome assembly and SZL formation by localizing Arp2/3 at podosome sites during osteoclastogenesis.61 OCs generated from the Rac double knockout mouse are devoid of podosomes and SZ, and show impaired bone resorption capacities. However, these defects are observed only if Rac deletion occurs at the early OC precursor stage since Rac1 deletion in cathepsin-K+ differentiating Rac2 deficient OCs has no effect on actin ring formation.61 Nevertheless, the importance of Rac1/2 on OC podosome organization and bone resorption capacities has been confirmed by introducing intracellular blocking antibodies against either Rac1 or Rac2.62
Rac activity in OCs is regulated by at least three GEFs, Dock5, Vav3 and FARP2.63,64 Vav3, which is relatively OC-specific, is stably expressed during osteoclastogenesis and promotes cell spreading and SZ formation. In contrast, expression of Dock5 is upregulated during RANKL-induced osteoclastogenesis and localizes at podosomes in SZLs. Both Vav3 and Dock 5 null mice present an osteopetrotic phenotype accounted for by decreased Rac activity, absence of podosome formation and SZL patterning, leading to reduced adhesion and bone resorption.63,64 However, in contrast to Dock5, a late step in OC differentiation is blocked in cells lacking Vav3. Altogether, it seems that Dock5 and Vav3 regulate Rac1 activation at distinct locations in OCs and at different phases of the bone-resorption cycle.64 Like Dock 5, FARP2 (FRG), a Dbl family GEF specific for Rac1, is important for cytoskeletal organization, is upregulated during osteoclastogenesis and localizes to the actin core of podosomes. Overexpression of a dominant negative form of FARP2 lacking the RacGEF domain results in the disorganization of the actin belt and in impaired bone resorption.65
Macrophages
Macrophages are terminally differentiated cells of the mononuclear phagocytic lineage and develop under the stimulus of their primary growth and differentiation factor CSF-1. Although they differentiate into heterogeneous populations depending upon their tissue of residence, motility is always an important aspect of their function. Like OCs, podosomes constitute the most prominent part of the actin cytoskeleton in macrophages and enable them to rapidly respond to migratory stimuli. As well as triggering growth and differentiation, CSF-1 is also a chemokine that regulates macrophage migration. Unraveling these pathways may help to identify drug targets for diseases in which macrophages contribute to adverse outcomes.
Human primary macrophages are the cells where the essential role of Cdc42 in podosome formation was uncovered. In a seminal paper, Linder and colleagues reported for the first time a connection between podosomes and diseases. WASp mutations are responsible for the immunodeficiency disorder Wiskott-Aldrich syndrome (WAS). Macrophages from patients expressing truncated forms of WASp completely lack podosomes. WASp normally colocalizes with Cdc42 and F-actin in the core of podosomes. However, microinjection of constitutively active Cdc42 or a constitutively active WASp fragment (the VCA domain) leads to the disassembly of podosomes.32 This reflects the fact that WASp activity, stability and subcellular localization are tightly controlled in the process.66 Activated Cdc42 and WASp associate with scaffolding F-bar proteins, which regulate both membrane and actin dynamics at podosomes. One of these, FBP17 (F-BAR-domain-containing formin-binding protein 17) recruits WASp, WIP and dynamin-2 to the plasma membrane during podosome formation in macrophages.67 By stimulating membrane curvature, FBP17 promotes the molecular interactions that facilitate actin polymerization.68 Another member of the family, CIP4 (Cdc42 interacting protein 4) links WASp to microtubules.69 Macrophages microinjected with CIP4 deficient in either the microtubule- or the WASp-binding domain, fail to assemble podosomes.69 In contrast to Arp2/3, formins drive the formation of linear (unbranched) actin filaments. The formin FMNL1 may be another downstream effector of Cdc42 at macrophage podosomes. FMNL1 is specifically upregulated during monocyte differentiation to macrophages where it localizes at actin cores. In a 3D reconstruction of podosomes, FMNL1 was visualized at the tip of podosome cores suggesting that actin filaments are organized into cables at this location. Targeted disruption of FMNL1 by siRNA results in disruption of podosome dynamics, suggesting that FMNL1 alters actin turnover at podosomes in these cells.70 The contribution of formins to podosome formation requires further confirmation.
Macrophages are the only podosome-forming cells in which the role of RhoB has been investigated. As in OCs, C3 transferase reduces the number of cells with podosomes and affects podosome distribution, promoting the clustering of scattered podosomes into circular arrays, as observed upon CSF-1 treatment.15 It is possible that the cell spreading associated with these treatments promotes the assembly of podosome rosettes. Macrophages do not express RhoC. Since podosome shape, number and organization are not affected in RhoB null cells,71 it can be concluded that inhibition of RhoA function is responsible for the effect of C3 toxin in macrophages. RhoA contributes to but is not essential for podosome assembly in these cells.
In contrast to OCs, Rac1 and Rac2 play distinct roles in macrophage podosome formation. Rac1-deficient macrophages show impaired podosome formation with fewer cells displaying podosomes that lack the adhesion ring, but migrate at a speed similar to that of wild-type macrophages. Rac2 deficiency results in complete loss of podosomes in macrophages. Surprisingly however, these cells are still able to invade the ECM. Rac1/2 deficient macrophages remain motile but with an altered mode of migration. Downstream of Rac, IRSp53 and WAVE2 are not required for podosome formation72 whereas PAK4 and its regulator αPIX play a central role. As a kinase, PAK4 regulates podosome size and numbers in these cells. αPIX, which plays a dual role in relation to RhoGTPases, as a GEF for Rac and as a downstream effector of Cdc4273,74 serves two functions here: localization to podosomes (independent of its GEF activity) and regulation of podosome size and number (dependent on its GEF activity). It is likely that PAK4 and αPIX form a complex at the interface between the ring and the core and regulate F-actin levels by influencing the actin filament severing activity of cofilin or by phosphorylating cortactin.75
Macrophages patrol tissues to engulf and destroy bacteria, dead cells, and other waste materials by phagocytosis. During their migration, they may use dynamic podosomes to sample the microenvironment. Initial formation of podosomes is influenced by the composition and structure of the underlying substratum including the abundance and distribution of specific ligands.10 Once formed, the matrix substratum dictates the lifespan of the podosomes with increased stiffness leading to longer endurance and closer spacing between podosomes.103 Exploring the structure/function of podosomes in living macrophages, Labernardie and collaborators demonstrated that podosomes harbor two types of overlapping periodic stiffness oscillations throughout their lifespan, which depend on F-actin and myosin II activity.16 The regulation of myosin II activity by the GTPase network was subsequently analyzed in more detail in iDCs.
Immature dendritic cells
Dendritic cells are the most potent antigen-presenting cells for activating naïve T cells, a process facilitated by the ability of iDCs to mature and home to lymph nodes after activation by an antigen or inflammatory cytokine. Formation of podosomes is restricted to cells with an immature phenotype, indicating a specific role for these structures during the early migratory phase31 such as the breakdown of connective tissue barriers. Maturation stimuli induce podosome dissolution, allowing cells to undergo a transition to a higher migratory phenotype. The transition is thus associated with extensive changes in cell adhesion and cytoskeletal organization.
The analysis of the role of each GTPase individually on podosome formation in iDCs has not revealed any significant differences with macrophage or OC models when using mutants of Cdc42 or Rac, depletion of WASp or WIP, or C3 transferase treatment.31 In this model, the novelties in the podosome field came from the study of the interplay between Cdc42-driven actin polymerization and RhoA-controlled myosin IIA contractility. Although the podosome core can be observed in the absence of podosome rings, and vice versa,52,76 the two structures are functionally connected. Super-resolution imaging of podosomes in iDCs has revealed how the dynamics of protrusive cores and adhesive rings are coordinated by the actomyosin apparatus.17,77 Vinculin preferentially localizes proximal to the core and along the radiating actin filaments whereas integrin and talin islets are homogeneously distributed more distally. Myosin IIA localizes in and around the adhesive ring. Mechanistically, core growth resulting from actin polymerization induces podosome growth and provides tension within the network of actin filaments. The tension transmitted to the ring recruits vinculin and zyxin mechanosensitive proteins, preserving the overall podosome integrity. Conversely, myosin IIA contracts the network of actin filaments and applies tension to the vinculin molecules, counterbalancing core growth and eventually reducing podosome size and protrusion. The interplay between actin and myosin IIA at podosomes accounts for their dynamic behavior and places RhoA as a likely orchestrator of podosome dynamics in iDCs.
Upon encountering an antigen, iDCs become activated, turn into mature DCs and migrate to a regional lymph node, where they present the antigen to T lymphocytes, thereby initiating an immune response. Maturation is associated with podosome loss in response to prostaglandin E2 (PGE2) as a result of a rapid increase in activated RhoA levels. RhoA signals to ROCK, which by regulating myosin IIA, promotes actomyosin-based contractility, leading to podosome dissolution and the formation of focal adhesions. Concomitant to RhoA activation, levels of active Rac1 and Cdc42 are decreased. This may occur either as a result of crosstalk between the three GTPases or through independent spatio-temporal regulation of each GTPase, but changes in the podosome-ring structure precede dissolution of the actin-rich core.78 Interestingly, RhoA activity cannot be raised in 3D contexts and podosomes are maintained. These studies demonstrate the importance of substrate dimensionality in controlling RhoA regulation and hence podosome dissolution in the performance of iDC functions.79
Podosomes in Non-Myelomonocytic Cells: Inducible Podosomes
Apart from the myelomonocytic lineage, a couple of cell types such as ECs and VSMCs are also able to assemble podosomes. Whereas podosome formation is minimal in response to cell adhesion, a vigorous response occurs upon induction.33 The stimuli, which appear to be cell-type specific, initiate a program involving podosomes. N-WASp replaces WASp at the podosome cores, questioning whether or not WASp expression confers the spontaneous occurrence of podosomes in myelomonocytic lineage cells. When podosomes are induced, focal adhesions are maintained and both structures are intimately involved in cell motility, with podosomes specifically involved in cell invasion. Invasiveness is achieved through transmembrane MT1-MMP at the podosome cores and secreted MMPs which degrade the basement membrane, a physical barrier that divide tissues into compartments. This thin (80–400 nm thick) one-layered ECM sheet is composed of a network of collagen IV connected to self-assembled laminin.80 The basal localization of the basement membrane as well as the extracellular proteins and growth factors contained in this tissue are thought to provide instructive cues for invadosome assembly in these invading cells. Since basement membranes are very thin and basically 2D, studies performed on planar surfaces are relevant to in vivo situations
Endothelial cells
Whereas expression of a constitutive form of Cdc42 triggers the formation of filopodia in most cells, it induces the assembly of podosomes in ECs.81,82 However, podosomes seem restricted in their functions because they are unable to degrade the underlying matrix (EG, unpublished data). They appear as isolated dots, a pattern that may be a consequence of the inhibition of Rho activity when that of Cdc42 remains at a high level. Alternatively, since biological functions require cycling of GTPases between their active and inactive states, the constitutively active GTPase may inhibit functions for which the cognate endogenous GTPase is required.44 The same phenotype is induced upon expression of the active form of the two Cdc42 related GTPases RhoJ (TC10) and RhoQ (TC10-like, TCL).83 When Cdc42 activity is stimulated by cytokines such as the canonical angiogenic factor VEGF or by TNFα33 in umbilical vein derived ECs (HUVECs), or by TGFβ in aortic ECs (BAEc),34 podosomes arise in various arrangements such as arrays or rosettes and efficiently degrade the ECM.
Fgd1 has been identified as the GEF mediating Cdc42 activation and subsequent podosome formation in TGFβ-stimulated BAE cells.84 Fgd1 activation involves tyrosine phosphorylation by Src and translocation to the subcortical cytoskeleton via a cortactin-dependent mechanism. Small interfering RNA-mediated Fgd1 knockdown inhibits TGFβ-induced Cdc42 activation and reduces podosome formation and associated ECM degradation. Although overexpression of Fgd1 does not promote podosome formation per se, it enhances TGFβ-induced matrix degradation. Fgd1 is the first GEF shown to be involved in the process of cytokine-induced podosome formation.84 By revealing the involvement of Fgd1 in endothelial podosome formation, these results open up new avenues to study its role in vascular pathophysiology.
The involvement of RhoGTPases in the formation of these inducible podosomes is dependent both on the cellular context and on the triggering stimulus. In arterial ECs, podosome induction in response to GTPase regulators such as GTPase GAPs depends on Cdc42 and Rac and their formation is promoted when Rho is inhibited.85 In venous ECs, PMA-induced podosome formation is dependent on Cdc42 and Rho but does not involve Rac.86 However, these types of stimulus may bypass the need for some signaling proteins otherwise required when podosomes arise in response to cytokines. In TGFβ-treated BAEc, Rac is involved in the induction of the structures (EG, unpublished data) but this may be due, at least in part, to its role in transcription as podosome formation here requires protein synthesis in this model.34
As in other models, C3 toxin reduces the number of cells with podosomes and silencing RhoA expression produces the same effect in all types of ECs.34,85 Upon TGFβ stimulation, the expression of RhoA increases and active RhoA relocalizes to podosome rosettes in BAE cells.34 In HUVECs, depletion of either of the Rho regulators p190RhoGAP-A and p190RhoGAP-B, has no effect on global Rho activity.87 However, whereas the knockdown of p190RhoGAP-A stimulates podosome formation, that of RhoGAP-B does not. In addition, depletion of p190RhoGAP-B decreases MT1-MMP mRNA levels and subsequent expression at the protein at the cell surface. Thus, p190RhoGAP-B may be involved in targeting MT1-MMP to the plasma membrane.87
Vascular smooth muscle cells
VSMCs in the medial layer of healthy arteries are differentiated, quiescent, highly specialized cells whose principal functions are contraction and regulation of the vessel diameter that controls blood flow distribution. In response to vessel wall damage, VSMCs undergo significant phenotypic modulation. This phenotypic transition is characterized by the loss of contractility and the acquisition of a proliferative, migratory, and synthetic phenotype, as well as the ability to degrade the ECM. The ability to breach tissue barriers allows synthetic VSMCs to migrate to the sub-endothelial space, where they proliferate and secrete ECM and pro-inflammatory molecules. This transition plays a critical role in pathological vascular remodeling such as atherosclerosis, post-angioplasty restenosis and in age-related vascular decay. The potential for VSMCs to migrate and invade has a direct impact on atheromatous plaque formation and stability. In cultured VSMCs, podosomes can be induced by phorbol-esters such as PMA.88-90 The formation of podosomes in response to these agents might be a reversible dedifferentiation process that could occur in vivo under specific conditions. Indeed, when embedded in a 3D collagen matrix, VSMCs spontaneously assemble numerous podosome-like structures.91 In addition, VSMC transition from a differentiated, contractile state to a de-differentiated, migratory state is associated with an increased propensity to form podosomes in vitro.92 Importantly, immunoelectron microscopy analysis of these de-differentiated VSMCs in the mouse aorta provided the first demonstration of the occurrence of podosomes in vivo.92
As with ECs, expression of constitutively active Cdc42 induces podosome formation in VSMCs.91 In these cells, active Rac1 also promotes their formation.91 N-WASp, together with cortactin and Arp2/3 are the proteins involved in the early steps of podosome assembly. Ectopic expression of cytoskeletally active constructs of PAK1 mimics this effect. The kinase activity of PAK1 plays a role in the regulation of the turnover rates of these structures but is not essential for their formation. The ability of PAK to interact with PIX but not with Rac1 or Cdc42, however, is required for podosome initiation. Overexpression of βPIX induces podosome formation and the protein localizes to the actin core.93 This situation is reminiscent of that of PAK4 and αPIX in macrophage podosomes.75
In VSMCs, initiation of podosome formation in response to phorbol esters requires rapid and spatially restricted remodeling of the actin cytoskeleton.88 Podosomes arise in a region at the intersection of focal adhesion and stress fibers.90 The process is linked to the restricted inhibition of actomyosin-based contractile activity,94 involving local de-activation of RhoA via the specific accumulation and activation of p190RhoGAP95 and the disassembly of the actin tropomyosin and myosin contractile machinery.88 Accordingly, ectopic expression of active RhoA suppresses podosome formation in response to phorbol ester by global inhibition of actin turnover.95
Most studies on VSMC podosomes have been performed using phorbol esters to induce the structures. However, PDGF was recently shown to be a physiological inducer of podosome formation in these cells. Vascular stress induces cells to respond to PDGF through the regulation of Src, PKC, miR-143, miR-145, and p53, thereby stimulating the formation of podosomes and migratory capacities.92 Although the contribution of RhoGTPases to these pathways has not yet been demonstrated, the involvement of Src and PKC strongly suggests that Cdc42 participates in the signaling cascade leading to podosome assembly in VSMCs, like it does in HUVECs exposed to phorbol esters.85
Cancer Cells and Src-Transformed Cells
Invasion is one of the most characteristic features of cancer malignancy. Cancer cells break away from their site or organ of origin, degrading the adjacent ECM and migrating into surrounding tissues. Accumulating evidence suggests that invadopodia play a major role in this process.96,97 Invadopodia spontaneously form in tumor cells directly cultured from patient samples and as Src is rarely mutated in tumors, it is thought that high levels of endogenous Src promote their assembly. In agreement with this, Src overexperession induces invadopodia similar to those found in tumor cells.98 Nonetheless, a model commonly used to study invadopodia formation is that of Src-transformed cells, which uses either v-src (truncated Src from RSV) or a Src mutant lacking the negative regulatory site (SrcY530).99 Oncogenic forms of Src do not induce single invadopodia, but invadosome rosettes, indicating that differences in Src structure are reflected in Src functions.100 Despite this, Src kinase activity remains the pivotal signal for both types of structures where the vinculin ring partially overlaps with the F-actin core (Fig. 1B part c and d).
As stated above, invadopodia significantly differ, in many ways, from podosomes and this is also reflected in their regulation by RhoGTPases. The roles of Cdc42 and Rho appear more intricate here than in podosomal structures. On substrata that allow protrusion elongation, invadopodia are visualized as membrane protrusions into the ECM in aggressive tumor cells. Whereas the ECM and matrix receptors play a central role in podosome formation, the role of the matrix composition in invadopodia formation or the recruitment of integrins to the structure has not been investigated in detail.101 However, ECM rigidity is now known to directly increase both the number and activity of invadosomes in cancer102 or Src-transformed cells.103 The absence of a discernible ring around the actin core of invadopodia points to differences in the role of the adhesion proteins.11 Several stages in their formation have been reported, from instable invadopodia (nascent invadopodia) to more stable ones (mature degrading invadopodia), through cortactin phosphorylation and its subsequent regulation of the actin-severing activity of cofilin.104 Invadopodia are generated by formation of the Arp2/3 -dependent dendritic actin network105,106 but elongate as a result of the extension of bundled longitudinal actin fibers107 controlled by proteins such as VASP108 and mDia2.109 Thus, invadopodium formation appears to depend on both nucleation systems. Invadopodium elongation is accompanied by contact with microtubules and vimentin intermediate filaments, which both support the maturation of the structure.107 However and in contrast to spontaneous podosomes, microtubules are not required for invadopodium formation.110 The matrix degrading activity is acquired during the maturation step where actin-driven membrane protrusion and exocytosis cooperate for delivering and concentrating MT1-MMP. Under the control of Cdc42 and RhoA, the vesicle-tethering exocyst complex and IQGAP1 regulate the accumulation of cell surface MT1-MMP at invadopodia.111 Rho also promotes invadopodia stabilization by regulating the formation of a complex between fascin and LIMK that modulates the interaction of fascin with actin and regulates filament bundling.107,112 Mature invadopodia degrade the matrix, then eventually, one of the invadopodial protrusion enlarges to create a larger breach that ultimately allows the cell to penetrate the basement membrane and invade the surrounding tissue.107 Invadopodia formation is stimulated by cytokines such as TGFβ39 or CSF-1,38 an effect that might reflect stabilization of nascent invadopodia.
Intriguingly, cancer cell invadopodia are dependent on MMPs for their assembly113 whereas podosomes are not.114 The molecular mechanisms underlying this difference are not known yet. However, MMP activity is required for the formation of protrusive podosomes enabled on soft permissive substrata.115 This suggests that the architecture and functions of invadosomes are somehow linked. Alexander and colleagues indeed confirmed that ECM signals regulate both invadopodium architecture and functions.102 The fact that the latter are tightly integrated limits the use of conventional approaches based on GTPase inactivation or depletion to study the molecular mechanisms involved in these two aspects of invadopodia regulation. Invadopodia architecture closely resembles that of filopodia, and this is underscored by the requirement of filopodial proteins for invadopodia biogenesis, including Cdc42, VASP, IRSp53, and fascin.107,108,116
The dominant active mutant of Cdc42 promotes the formation of invadopodia and the contribution of Cdc42 is further illustrated by RNA interference in melanoma or carcinoma cells106,117 or the use of dominant negative mutant of the protein in Src-transformed cells.98 The membrane-deforming and curvature sensing IRSp53 filopodial protein is also involved in invadosome formation. In addition to Rac, IRSp53 also binds Cdc42 and N-WASp and may function with VASP to promote processive straight actin filament elongation by antagonizing the action of capping proteins.116 Likewise, activated Cdc42 and N-WASp associate with the scaffolding F-bar protein CIP4118 or FBP17119 at invadopodia. The depletion of these proteins also impairs invadopodium formation and decreases their invasive capacities.119 In prostate or breast cancer cells, Fgd1 has been identified as the GEF driving Cdc42-mediated invadopodia biogenesis and ECM degradation. Fgd1 is low or undetectable in the normal corresponding tissue. Since its expression level matches tumor aggressiveness, Fgd1 likely functions during tumorigenesis. Downstream of Cdc42, active N-WASp is visualized at the base of invadopodia, suggesting that Arp2/3-complex-mediated actin nucleation is confined to this area.120 Then actin bundles extend from the dendritic network and are thought to push at the membrane to form the invadopodial protrusion, a process resembling the formation of filopodia. The elongation of the actin filaments appears dependent on DRF/mDia family members.96,98
Oikawa et al., proposed a sequential mechanism for invadopodium formation in Src-transformed cells.121 The process is initiated at focal adhesions owing to changes in the phosphorylation status of focal adhesion proteins and in accumulation of PtdIns(3,4)P2 phosphoinositides at the plasma membrane. The onset of actin polymerization is then triggered by Tks5 recruitment and subsequent N-WASp accumulation, which requires Tks5 to interact with both phosphoinositides and the adaptor protein Grb2. In this mechanism, phosphoinositide 3-kinase (PI3K) plays an essential role.121 Kuroiwa and colleagues refined this scenario by identifying the missing link between Src and PI3K. Using an intact Src protein (c-src) and mass spectrometry, they showed that when Src is activated over a certain threshold at focal adhesions, it interacts with RhoGEFArhgef5, which in turn enhances Src activation through a positive-feedback mechanism. The activated Src and Arhgef5 recruit PI3K, to form a ternary complex where Arhgef5 is activated. PI3K products direct the recruitment of the Arhgef5 complex to invadopodium precursors. At the nascent structure, Arhgef5 activates RhoA, as well as Cdc42, thereby promoting cytoskeletal remodeling and subsequent invadosome maturation.98 Interestingly, the Src-Arhgef5-RhoA axis plays a triggering role in the pathways leading to invadopodium assembly in this model. Consistent with this, RhoA activation has been shown to precede that of Cdc42 and Rac under growth factor stimulation of cellular protrusions.122,123
The essential role of Rho and in particular, the spatially restricted Rho activity, was underscored when active Rho was shown to localize to invadosomes in Src-transformed cells.124 This was unexpected because the formation of invadosomes upon transformation by oncogenic Src is associated with the disruption of actin stress fibers. In these cells, inactivation of Rho by dominant negative RhoA or the Rho-specific inhibitor C3 toxin disrupts invadosomes and associated ECM degrading activities. Thus, Rho activity is confined to invadosome sites where it plays a key role in the biogenesis of the structures in subcellular regions devoid of stress fibers. The development of FRET-based biosensors for RhoGTPases allowed Rho protein activation in the structure to be studied. This showed that RhoC activity is spatially confined to the area surrounding invadopodium actin cores. The spatiotemporal restriction of RhoC activity is controlled by two regulatory elements: p190RhoGEF, which localizes around invadopodia to activate RhoC; and p190RhoGAP, which localizes inside invadopodia to deactivate the GTPase within the structure. Thus, RhoC activity is primarily excluded from the invadopodium core but is elevated in the areas surrounding the invadopodia, where it regulates cofilin phosphorylation through the ROCK/LIMK pathway. This focuses the severing activity of cofilin at the invadopodium core, to create free barbed ends for actin polymerization and filament turnover, which are required for the generation of protrusive force leading to invadopodial extension.125 In this context, it can be speculated that formins at the barbed ends of actin filaments connect onto the branched actin network to elongate actin filaments. When a RhoA biosensor was used, the signal showed random fluctuations both inside and surrounding the invadopodial structures with no defined patterns of RhoA activity during invadopodium formation.125 Since RhoA is also involved by regulating the delivery of MT1-MMP to the invadopodia,111,125 RhoC and RhoA appear to play distinct roles during invadopodium formation
Like podosomes, invadopodia may also act as microsensors, testing the matrix environment to seek out favorable routes of invasion. Transduction of ECM signals depends on the cellular contractile apparatus. The interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations in iDCs where mechanosensitive alterations in the structure of vinculin at the podosome ring transduce mechanical forces into signals.17 For invadopodia where vinculin may be less prominent, it seems that the mechano-signal transmission occurs mainly through p130Cas.102 Nevertheless, myosin IIA also contributes to invadopodium-sensing functions. It may be found localized around the invadopodial actin cores.102 Similar to the situation encountered with podosomes,126 ECM rigidity directly increases both the number and activity of invadopodia.102
The role of Rac has not been investigated in detail. Depletion of Rac inhibits invadopodium formation in NB19 glioblastoma cells.127 Of note that RhoG which may act upstream of Rac produces the same effect although to a lesser extent.127 In Src-transformed cells, Rac regulates cortactin localization and phosphorylation at invadosomes.128 However, a novel role for Rac at invadopodia has been revealed by studies performed on Tks5. Besides their roles in cytoskeletal organization Rac proteins are components of the NADPH oxidase complex. Tks5 and its close relative Tks4 recruit p67phox component of the complex at invadosomes and support the production of reactive oxygen species (ROS) in a Rac GTPase-dependent manner at these sites. The generation of ROS promotes the phosphorylation of Tks scaffolding proteins and associated proteins (N-WASp?), which contribute to invadopodia formation and function. Direct or indirect action of ROS on kinases or MMPs may also be involved.129,130
Conclusions
The invadosome family comprises at least four types of structures with distinct presentations, depending in the cell types in which they form (Fig. 1). The exact relationships between the various types of invadosomes are unclear and remain a matter of major debate. In this context and regarding RhoGTPase regulation, there is no doubt that subcellularly fine-tuned RhoGTPase activities are crucial for the regulation of all classes of invadosomes (Table 1; Fig. 3).
Table 1. Reported RhoGTPases involved in invadosome formation and function.
| Component (location) |
Consequence of knockdown, expression of DN mutant or bacterial toxin-mediated inhibition on invadosome formation | Consequence of DA mutant expression on invadosome formation** | Key references | |||||
|---|---|---|---|---|---|---|---|---|
| Spontaneous podosome |
Inducible podosome | Src-trans-formed cells | Invadopodia | Spontaneous podosome |
Inducible podosome | Invadopodia*** | ||
|
Cdc42 (core) |
Inhibitory or disruption of podosome organization (Macrophage, OC, iDC) |
Inhibitory (EC, BAEc, HUVECs) |
Inhibitory (Src-NIH-3T3) Inhibitory (Src-VSMC) |
Inhibitory (RPMI7951, melanoma; MTLn3, MDA-MB-231, adenocarcinoma) |
Disruption of podosome organization (Macrophage, iDC, OC) |
Stimulatory (EC, VSMC) Similar effect in arterial EC, with RhoJ (TC10) or RhoQ (TCL) |
Stimulation of matrix degradation (fibronectin) (RPMI7951, melanoma) |
31, 33, 34, 49, 85, 91, 98, 106, 111, 117, 121 |
|
Rac1 and/or Rac2 |
Inhibitory (OC, Rac1 and Rac2 are interchangeable) Rac1- podosomes devoid of rings (Macrophage) Rac2- complete podosome loss (Macrophage) |
No effect (in response to PMA in HUVEC) Inhibitory (in response to Src in VSMC) |
No effect (NIH3T3-Src) Inhibitory (Src-VSMC) |
Inhibitory (RPMI7951, melanoma; MTLn3, MDA-MB-231, adenocarcinoma) |
Disruption of podosome organization (Macrophage) No effect (iDC) |
Stimulation of matrix degradation (fibronectin) (VSMC) |
Stimulation of matrix degradation (fibronectin) (RPMI7951, melanoma) |
31, 33, 34, 98, 111, 117 |
| RhoA | Reduction of podosomes (Macrophage, OC, iDC) Disruption of podosome organization (Macrophage, OC, iDC) |
Inhibitory (in response to PMA in HUVEC) Inhibitory (in response to TGFβ in BAEc) No inhibition (in response to TGFβ in MCF10A non tumorigenic breast epithelial cells) |
Inhibitory (NIH3T3-Src) |
Inhibitory (MTLn3, MDA-MB-231, adenocarcinoma) |
No effect or slightly stimulatory (OC) Podosome dissolution (iDC) |
ND | ND | 31, 34, 49, 55, 56, 71, 85, 98, 111, 134 |
| RhoC | Not expressed | Inhibitory (in response to TGFβ in MCF10A non tumorigenic breast epithelial cells) | ND | No inhibition but unfocused actin polymerization and matrix degradation. Decrease in the length of invadopodial protrusion (MTLn3 adenocarcinoma) |
ND | ND | ND | 125, 134 |
|
RhoU (Wrch1) (ring) |
Disruption of podosome organization in rings/belt (on glass) but no structural or functional effect on the SZ (on bone) (OC) | *ND | ND | ND | Disruption of podosome organization in rings/belt (on glass) but no structural or functional effect on the SZ (on bone) (OC) | No effect (EC, PAEc) |
ND | 57, 83, 138 |
|
RhoE (Rnd3) (does not localize at invadosome) |
Disturbed podosome organization (OC) Reduces actin turnover |
ND | ND | ND | Expected to increase actin turnover (cofilin is dephosphorylated) | ND | ND | 60 |
ND, not done; **The effect of active RhoGTPases has not been tested in Src-transformed cells where active Src is the invadosome inducer; ***Most of the time, invadopodia formation is measured indirectly, being assessed by quantitating invadopodia-induced gelatin degradation
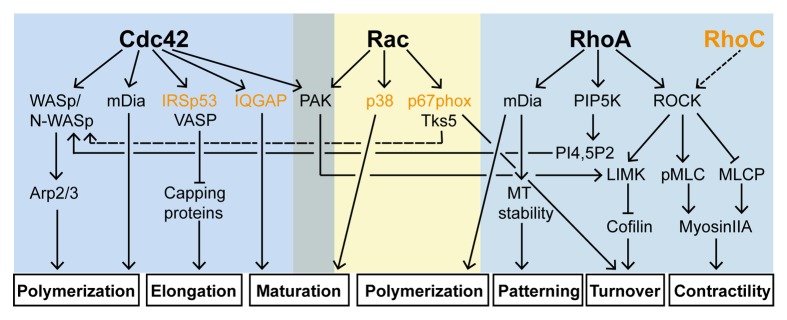
Figure 3. Cdc42, Rac, RhoA and their effector pathways in invadosome formation. In most cases, Cdc42, Rac and Rho participate in the construction of invadosomes. Cooperative and antagonist crosstalks exist between the pathways and at various levels. The contribution of each pathway to the formation of the structure depends on the cell type considered. Signaling elements in orange have so far been described at invadopodia only.
One can wonder whether the functions of individual GTPases are conserved across the respective manifestations of invadosomes: comparing their role in the formation and function of the structures reveals fundamental differences. Active Cdc42 disturbs the formation of spontaneous podosomes in myelomonocytic cells but induces the formation of podosomes in other cells, yet Cdc42 appears as the main GTPase involved in the elaboration of the structures. The Cdc42-WASp axis regulates the biogenesis of the actin core. WASP provides a platform where tyrosine-phosphorylated signaling molecules, Cdc42 and Arp2/3 complex are recruited. RhoA contributes to this process via the synthesis of PI(4,5)P2 which interacts with WASp49 but its instrumental role resides in podosome functioning where its spatiotemporal regulation holds the key to its function. The RhoA-myosin IIA pathway contributes to the mechanosensing activity that seems to predominate for this class of structures. RhoA has also a decisive role in podosome patterning, regulating the collective and dynamic behavior of podosome clusters. RhoA activity is regulated by matrix composition and stiffness which has a major impact on the architecture and lifetime of both individual podosomes and podosome superstructures. When RhoA is activated over a certain threshold, it provides a triggering signal for podosome dissolution. In contrast to Cdc42 and RhoA, the role of Rac is less understood. This arises, at least partly, from the fact that Rac1 and Cdc42 pathways share common components. In many cases they bind to the same effectors and they can be activated by the same GEFs.131 Nevertheless, Rac main effector PAK plays an important role in controlling F-actin levels at the structure and thereby regulates podosome size.
The contribution of RhoGTPases in invadopodia formation in cancer cells is more complex as the elaboration of the structure cannot be dissociated from its matrix degrading function. The structure develops from an N-WASp-dependent dendritic actin network, similarly to podosomes (N-WASp replaces WASp).106,111 However, the contribution of Cdc42 in the formation of the branched network is not always as clear as for podosomes. N-WASp activity in invadopodia is controlled by cortactin tyrosine phosphorylation via the recruitment of adaptors (Grb2121 or Nck1104). In addition, the construction of a functional matrix degrading invadopodium involves maturation and stabilization stages which relies on DRF/mDia proteins,109 fascin,107,112 myosin IIA,102 and MT1-MMP trafficking,111 all downstream of Rho. Tumor cells expressing a constitutively active RhoA mutant form more invadopodia than those expressing the wild type protein.43 By focusing actin polymerization within the assembling invadopodium, RhoC may promote efficient tumor cell invasion.125 Intriguingly, RhoC localizes at the perinuclear area where invadopodia are often found. This GTPase which is associated with aggressive cancer and metastasis132,133 may be specifically involved in invadopodium biogenesis as it is not involved in the formation of spontaneous podosomes.71 Consistent with this, RhoC is required for the formation of inducible podosomes which resemble invadopodia in TGFβ-treated epithelial cells.134 Collectively, these data indicate that, in the pathways leading to invadopodium assembly, Rho proteins play the prominent role.
As invadopodia are considered by many of us as the tumor cell counterpart of podosomes formed in normal cells, transformation of fibroblasts by oncogenic Src has often been used as a convenient model to generate the structures and study their regulation in the tumorigenic context. The involvement of RhoGTPases in this model may resemble the one elucidated downstream of Src in phorbol ester-treated non myeloid cells.85 However, it seems that invadosomes form here in response to a forced Src signal, not representative of that occurring in primary cancer cells; the invadosomes raised in these cells arise through pathways distinct from those of invadopodia and differ from them in their architecture, arrangement and properties. So, as a result of oncogenic Src expression, cells become transformed but Src signals to RhoGTPases promote the formation of invadosomes with podosome characteristics (including architecture and collective behavior).
One can speculate that extensive crosstalk between RhoGTPases, which depends on the cell specific expression levels of GEFs (such as Vav3 in OCs) and GAPs accounts for the differences observed between the four classes of invadosomes depicted herein. Mounting evidence exists to assign important roles to invadosomes in essential pathophysiological processes. Podosomes are thus considered as potential targets in the development of therapeutic strategies for osteoporosis. Likewise, preventing invadopodia formation represents a promising strategy to limit cancer cell invasion and metastasis. However RhoGTPases are not attractive pharmacological drug targets. Instead, GEFs, with their unique, context-specific RhoGTPase regulators, progressively emerge as candidates with a stronger potential. A number of them have now been identified (Table 2). Again, the osteoclast model provides a pioneering example of an inhibitory strategy targeting podosomes. A screen for DOCK5 has led to the selection of a small-molecule capable of hindering osteoclast-resorbing activity.64 This promising compound has the potential to treat osteoporosis.
Table 2. GEFs involved in invadosome assembly.
| GEF | Localization | GTPase | Consequences on invadosome formation | Cells | Type of invadosome | Key references | |
|---|---|---|---|---|---|---|---|
| GEF depletion (or expression of DN mutant) |
GEF overexpression (or expression of DA mutant) | ||||||
| Fgd1 | Core | Cdc42 | Inhibitory | Enhanced matrix degradation | EC, BAEc Adencarcinoma, MDA-MB-231 |
Inducible podosomes Invadopodia |
84 113 |
| FGD4 (frabin) | Core | Inhibitory | Enhanced matrix degradation | Melanoma, RPMI7951 | Invadopodia | 117 | |
| αPIX | Ring | Cdc42/Rac | No effect No effect on individual podosomes but podosomal belts (on glass) appear less compact |
Podosome clusters ND |
Macrophage OC |
Spontaneous podosomes |
75 139 |
| βPIX | Podosomes *ND Core |
Inhibitory No effect on individual podoomes but podosomal belts (on glass) appear less compact Inhibitory |
Increase in podosome size ND Increase in invadopodia formation and enhanced matrix degradation |
VSMC OC Melanoma,A375M Adenocarcinoma MDA-MB-231 |
Inducible podosomes Invadopodia |
93 139 140 |
|
| Vav1 | ND | Rac | Podosomes are larger compared with wt and aberrantly distributed | ND | iDC | Spontaneous podosomes |
141 |
| Vav3 | ND | No podosome belt but randomly distributed actin patches | ND | OC | Spontaneous podosomes |
63 | |
| Dock5 | Core | No or very few SZ on bone-like material | ND | 64 | |||
|
FARP2 (FRG) |
Core | No podosome belt on glass, no SZ on bone-like material | ND | 65 | |||
| Arhgef5 | Core | RhoA | Inhibitory | Induced formation of individual podosomes | NIH3T3 | Src-transformed cells | 98 |
| P190RhoGEF | Outer rim at the core | RhoC | ND | ND | Adenocarcinoma, MTLn3 |
Invadopodia | 125 |
Several open but fascinating points remain to be challenged. For instance, very little is known about podosome regulation by RhoGTPase in 3D, which is more representative of the in vivo context in most situations. Invadosomes have now been described in many other cells such as microglia,135 megakaryocytes,114 muscle cells136 or trophoblasts.137 A better understanding of the regulation of RhoGTPases in these models will bring new ideas for the design of efficient drugs to limit the processes in which these invadosome-forming cells are involved.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors acknowledge the contribution of a vast number of researchers to this field and apologize to the many scientists whose work could not be properly discussed and referenced owing to scope and space limitations. This work was supported by the ANR 2010 program [grant number BLAN1237], the European Union's Seventh Framework Programme (FP7/2007–2013; T3Net) [grant number FP7–237946], and the Ligue Nationale Contre Le Cancer.
Glossary
Abbreviations:
- EC
endothelial cells
- iDC
immature dendritic cell
- OC
osteoclast
- VSMC
vascular smooth muscle cell, GAP, GTPase activating protein
- GDI
guanine nucleotide-dissociation inhibitor
- GEF
guanine nucleotide-exchange factor
- LIMK
LIM kinase
- Ena/VASP
Enabled Vasodilatator stimulated phosphoprotein
- WASp/N-WASp
Wiskott-Aldrich syndrome proteins
- WIP
Wasp interacting protein
- ROCK
Rho-associated protein kinase
- MT1-MMP
membrane type-1 metalloprotease
- 2D
two-dimensional
- 3D
three-dimensional
- ECM
extracellular matrix
- PI3K
phosphoinositide 3-kinase
- DRF
Diaphaneous- related formin
References
- 1.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–55. doi: 10.1042/0264-6021:3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 3.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–57. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 4.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 5.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–57. doi: 10.1016/S0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 6.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–85. doi: 10.1016/S0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 8.Bhuwania R, Cornfine S, Fang Z, Krüger M, Luna EJ, Linder S. Supervillin couples myosin-dependent contractility to podosomes and enables their turnover. J Cell Sci. 2012;125:2300–14. doi: 10.1242/jcs.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu S, Biben T, Wang X, Jurdic P, Géminard JC. Internal dynamics of actin structures involved in the cell motility and adhesion: Modeling of the podosomes at the molecular level. J Theor Biol. 2011;270:25–30. doi: 10.1016/j.jtbi.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 10.van den Dries K, van Helden SF, te Riet J, Diez-Ahedo R, Manzo C, Oud MM, van Leeuwen FN, Brock R, Garcia-Parajo MF, Cambi A, et al. Geometry sensing by dendritic cells dictates spatial organization and PGE(2)-induced dissolution of podosomes. Cell Mol Life Sci. 2012;69:1889–901. doi: 10.1007/s00018-011-0908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branch KM, Hoshino D, Weaver AM. Adhesion rings surround invadopodia and promote maturation. Biol Open. 2012;1:711–22. doi: 10.1242/bio.20121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–41. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–85. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanjay A, Houghton A, Neff L, DiDomenico E, Bardelay C, Antoine E, Levy J, Gailit J, Bowtell D, Horne WC, et al. Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J Cell Biol. 2001;152:181–95. doi: 10.1083/jcb.152.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler AP, Smith SD, Ridley AJ. CSF-1 and PI 3-kinase regulate podosome distribution and assembly in macrophages. Cell Motil Cytoskeleton. 2006;63:132–40. doi: 10.1002/cm.20111. [DOI] [PubMed] [Google Scholar]
- 16.Labernadie A, Thibault C, Vieu C, Maridonneau-Parini I, Charrière GM. Dynamics of podosome stiffness revealed by atomic force microscopy. Proc Natl Acad Sci U S A. 2010;107:21016–21. doi: 10.1073/pnas.1007835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Dries K, Meddens MB, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, Cambi A. Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nat Commun. 2013;4:1412. doi: 10.1038/ncomms2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luxenburg C, Geblinger D, Klein E, Anderson K, Hanein D, Geiger B, Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS One. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson MW, Lye MH, Barthel SR, Duffy AK, Annis DS, Mosher DF. Eosinophils adhere to vascular cell adhesion molecule-1 via podosomes. Am J Respir Cell Mol Biol. 2004;31:413–22. doi: 10.1165/rcmb.2004-0099OC. [DOI] [PubMed] [Google Scholar]
- 20.Allavena P, Paganin C, Martin-Padura I, Peri G, Gaboli M, Dejana E, Marchisio PC, Mantovani A. Molecules and structures involved in the adhesion of natural killer cells to vascular endothelium. J Exp Med. 1991;173:439–48. doi: 10.1084/jem.173.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melder RJ, Walker ER, Herberman RB, Whiteside TL. Surface characteristics, morphology, and ultrastructure of human adherent lymphokine-activated killer cells. J Leukoc Biol. 1990;48:163–73. doi: 10.1002/jlb.48.2.163. [DOI] [PubMed] [Google Scholar]
- 22.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–97. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saltel F, Chabadel A, Bonnelye E, Jurdic P. Actin cytoskeletal organisation in osteoclasts: a model to decipher transmigration and matrix degradation. Eur J Cell Biol. 2008;87:459–68. doi: 10.1016/j.ejcb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Rottiers P, Saltel F, Daubon T, Chaigne-Delalande B, Tridon V, Billottet C, Reuzeau E, Génot E. TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels. J Cell Sci. 2009;122:4311–8. doi: 10.1242/jcs.057448. [DOI] [PubMed] [Google Scholar]
- 25.Seals DF, Azucena EF, Jr., Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–65. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 26.David-Pfeuty T, Singer SJ. Altered distributions of the cytoskeletal proteins vinculin and alpha-actinin in cultured fibroblasts transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980;77:6687–91. doi: 10.1073/pnas.77.11.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen WT, Chen JM, Parsons SJ, Parsons JT. Local degradation of fibronectin at sites of expression of the transforming gene product pp60src. Nature. 1985;316:156–8. doi: 10.1038/316156a0. [DOI] [PubMed] [Google Scholar]
- 28.Zambonin-Zallone A, Teti A, Carano A, Marchisio PC. The distribution of podosomes in osteoclasts cultured on bone laminae: effect of retinol. J Bone Miner Res. 1988;3:517–23. doi: 10.1002/jbmr.5650030507. [DOI] [PubMed] [Google Scholar]
- 29.Messier JM, Shaw LM, Chafel M, Matsudaira P, Mercurio AM. Fimbrin localized to an insoluble cytoskeletal fraction is constitutively phosphorylated on its headpiece domain in adherent macrophages. Cell Motil Cytoskeleton. 1993;25:223–33. doi: 10.1002/cm.970250303. [DOI] [PubMed] [Google Scholar]
- 30.Binks M, Jones GE, Brickell PM, Kinnon C, Katz DR, Thrasher AJ. Intrinsic dendritic cell abnormalities in Wiskott-Aldrich syndrome. Eur J Immunol. 1998;28:3259–67. doi: 10.1002/(SICI)1521-4141(199810)28:10<3259::AID-IMMU3259>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 31.Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–9. doi: 10.1182/blood.V98.4.1142. [DOI] [PubMed] [Google Scholar]
- 32.Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–53. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osiak AE, Zenner G, Linder S. Subconfluent endothelial cells form podosomes downstream of cytokine and RhoGTPase signaling. Exp Cell Res. 2005;307:342–53. doi: 10.1016/j.yexcr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 34.Varon C, Tatin F, Moreau V, Van Obberghen-Schilling E, Fernandez-Sauze S, Reuzeau E, Kramer I, Génot E. Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol. 2006;26:3582–94. doi: 10.1128/MCB.26.9.3582-3594.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabri S, Foudi A, Boukour S, Franc B, Charrier S, Jandrot-Perrus M, Farndale RW, Jalil A, Blundell MP, Cramer EM, et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood. 2006;108:134–40. doi: 10.1182/blood-2005-03-1219. [DOI] [PubMed] [Google Scholar]
- 36.VanWinkle WB, Snuggs M, Buja LM. Hypoxia-induced alterations in cytoskeleton coincide with collagenase expression in cultured neonatal rat cardiomyocytes. J Mol Cell Cardiol. 1995;27:2531–42. doi: 10.1006/jmcc.1995.0040. [DOI] [PubMed] [Google Scholar]
- 37.Veselý P, Blase C, Matouskova E, Bereiter-Hahn J. Arising podosomal structures are associated with neoplastic cell morphological phenotype induced by the microenvironment. Anticancer Res. 2006;26(2A):967–72. [PubMed] [Google Scholar]
- 38.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–83. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 39.Mandal S, Johnson KR, Wheelock MJ. TGF-beta induces formation of F-actin cores and matrix degradation in human breast cancer cells via distinct signaling pathways. Exp Cell Res. 2008;314:3478–93. doi: 10.1016/j.yexcr.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Monsky WL, Chen WT. Proteases of cell adhesion proteins in cancer. Semin Cancer Biol. 1993;4:251–8. [PubMed] [Google Scholar]
- 41.Han H, Kampik D, Grehn F, Schlunck G. TGF-β2-induced invadosomes in human trabecular meshwork cells. PLoS One. 2013;8:e70595. doi: 10.1371/journal.pone.0070595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu CH, Rafiq NB, Krishnasamy A, Hartman KL, Jones GE, Bershadsky AD, Sheetz MP. Integrin-Matrix Clusters Form Podosome-like Adhesions in the Absence of Traction Forces. Cell Rep 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L, Condeelis J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2013 doi: 10.1038/onc.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Zheng Y. Cell type-specific functions of Rho GTPases revealed by gene targeting in mice. Trends Cell Biol. 2007;17:58–64. doi: 10.1016/j.tcb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Wiesner C, Faix J, Himmel M, Bentzien F, Linder S. KIF5B and KIF3A/KIF3B kinesins drive MT1-MMP surface exposure, CD44 shedding, and extracellular matrix degradation in primary macrophages. Blood. 2010;116:1559–69. doi: 10.1182/blood-2009-12-257089. [DOI] [PubMed] [Google Scholar]
- 46.Väänänen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113:377–81. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 47.Destaing O, Saltel F, Géminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–16. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem. 2010;79:707–35. doi: 10.1146/annurev.biochem.77.060407.135452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chellaiah MA. Regulation of actin ring formation by rho GTPases in osteoclasts. J Biol Chem. 2005;280:32930–43. doi: 10.1074/jbc.M500154200. [DOI] [PubMed] [Google Scholar]
- 50.Ito Y, Teitelbaum SL, Zou W, Zheng Y, Johnson JF, Chappel J, Ross FP, Zhao H. Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J Clin Invest. 2010;120:1981–93. doi: 10.1172/JCI39650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calle Y, Carragher NO, Thrasher AJ, Jones GE. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J Cell Sci. 2006;119:2375–85. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- 52.Chabadel A, Bañon-Rodríguez I, Cluet D, Rudkin BB, Wehrle-Haller B, Genot E, Jurdic P, Anton IM, Saltel F. CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol Biol Cell. 2007;18:4899–910. doi: 10.1091/mbc.E07-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dovas A, Cox D. Signaling networks regulating leukocyte podosome dynamics and function. Cell Signal. 2011;23:1225–34. doi: 10.1016/j.cellsig.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang D, Udagawa N, Nakamura I, Murakami H, Saito S, Yamasaki K, Shibasaki Y, Morii N, Narumiya S, Takahashi N, et al. The small GTP-binding protein, rho p21, is involved in bone resorption by regulating cytoskeletal organization in osteoclasts. J Cell Sci. 1995;108:2285–92. doi: 10.1242/jcs.108.6.2285. [DOI] [PubMed] [Google Scholar]
- 55.Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci. 2005;118:2901–11. doi: 10.1242/jcs.02425. [DOI] [PubMed] [Google Scholar]
- 56.Chellaiah MA, Soga N, Swanson S, McAllister S, Alvarez U, Wang D, Dowdy SF, Hruska KA. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J Biol Chem. 2000;275:11993–2002. doi: 10.1074/jbc.275.16.11993. [DOI] [PubMed] [Google Scholar]
- 57.Ory S, Brazier H, Blangy A. Identification of a bipartite focal adhesion localization signal in RhoU/Wrch-1, a Rho family GTPase that regulates cell adhesion and migration. Biol Cell. 2007;99:701–16. doi: 10.1042/BC20070058. [DOI] [PubMed] [Google Scholar]
- 58.Aspenström P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J. 2004;377:327–37. doi: 10.1042/BJ20031041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saras J, Wollberg P, Aspenström P. Wrch1 is a GTPase-deficient Cdc42-like protein with unusual binding characteristics and cellular effects. Exp Cell Res. 2004;299:356–69. doi: 10.1016/j.yexcr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 60.Georgess D, Mazzorana M, Terrado J, Delprat C, Chamot C, Guasch RM, Pérez-Roger I, Jurdic P, Machuca-Gayet I. Comparative transcriptomics reveals RhoE as a novel regulator of actin dynamics in bone-resorbing osteoclasts. Mol Biol Cell. 2014;25:380–96. doi: 10.1091/mbc.E13-07-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Croke M, Ross FP, Korhonen M, Williams DA, Zou W, Teitelbaum SL. Rac deletion in osteoclasts causes severe osteopetrosis. J Cell Sci. 2011;124:3811–21. doi: 10.1242/jcs.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Razzouk S, Lieberherr M, Cournot G. Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur J Cell Biol. 1999;78:249–55. doi: 10.1016/S0171-9335(99)80058-2. [DOI] [PubMed] [Google Scholar]
- 63.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–90. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 64.Vives V, Laurin M, Cres G, Larrousse P, Morichaud Z, Noel D, Côté JF, Blangy A. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J Bone Miner Res. 2011;26:1099–110. doi: 10.1002/jbmr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takegahara N, Kang S, Nojima S, Takamatsu H, Okuno T, Kikutani H, Toyofuku T, Kumanogoh A. Integral roles of a guanine nucleotide exchange factor, FARP2, in osteoclast podosome rearrangements. FASEB J. 2010;24:4782–92. doi: 10.1096/fj.10-158212. [DOI] [PubMed] [Google Scholar]
- 66.Chou HC, Antón IM, Holt MR, Curcio C, Lanzardo S, Worth A, Burns S, Thrasher AJ, Jones GE, Calle Y. WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr Biol. 2006;16:2337–44. doi: 10.1016/j.cub.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsuboi S, Takada H, Hara T, Mochizuki N, Funyu T, Saitoh H, Terayama Y, Yamaya K, Ohyama C, Nonoyama S, et al. FBP17 Mediates a Common Molecular Step in the Formation of Podosomes and Phagocytic Cups in Macrophages. J Biol Chem. 2009;284:8548–56. doi: 10.1074/jbc.M805638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takano K, Toyooka K, Suetsugu S. EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization. EMBO J. 2008;27:2817–28. doi: 10.1038/emboj.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linder S, Hüfner K, Wintergerst U, Aepfelbacher M. Microtubule-dependent formation of podosomal adhesion structures in primary human macrophages. J Cell Sci. 2000;113:4165–76. doi: 10.1242/jcs.113.23.4165. [DOI] [PubMed] [Google Scholar]
- 70.Mersich AT, Miller MR, Chkourko H, Blystone SD. The formin FRL1 (FMNL1) is an essential component of macrophage podosomes. Cytoskeleton (Hoboken) 2010;67:573–85. doi: 10.1002/cm.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wheeler AP, Ridley AJ. RhoB affects macrophage adhesion, integrin expression and migration. Exp Cell Res. 2007;313:3505–16. doi: 10.1016/j.yexcr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 72.Abou-Kheir W, Isaac B, Yamaguchi H, Cox D. Membrane targeting of WAVE2 is not sufficient for WAVE2-dependent actin polymerization: a role for IRSp53 in mediating the interaction between Rac and WAVE2. J Cell Sci. 2008;121:379–90. doi: 10.1242/jcs.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baird D, Feng Q, Cerione RA. The Cool-2/alpha-Pix protein mediates a Cdc42-Rac signaling cascade. Curr Biol. 2005;15:1–10. doi: 10.1016/j.cub.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 74.Feng Q, Baird D, Cerione RA. Novel regulatory mechanisms for the Dbl family guanine nucleotide exchange factor Cool-2/alpha-Pix. EMBO J. 2004;23:3492–504. doi: 10.1038/sj.emboj.7600331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gringel A, Walz D, Rosenberger G, Minden A, Kutsche K, Kopp P, Linder S. PAK4 and alphaPIX determine podosome size and number in macrophages through localized actin regulation. J Cell Physiol. 2006;209:568–79. doi: 10.1002/jcp.20777. [DOI] [PubMed] [Google Scholar]
- 76.Destaing O, Sanjay A, Itzstein C, Horne WC, Toomre D, De Camilli P, Baron R. The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol Biol Cell. 2008;19:394–404. doi: 10.1091/mbc.E07-03-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Dries K, Schwartz SL, Byars J, Meddens MB, Bolomini-Vittori M, Lidke DS, Figdor CG, Lidke KA, Cambi A. Dual-color superresolution microscopy reveals nanoscale organization of mechanosensory podosomes. Mol Biol Cell. 2013;24:2112–23. doi: 10.1091/mbc.E12-12-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Helden SF, Oud MM, Joosten B, Peterse N, Figdor CG, van Leeuwen FN. PGE2-mediated podosome loss in dendritic cells is dependent on actomyosin contraction downstream of the RhoA-Rho-kinase axis. J Cell Sci. 2008;121:1096–106. doi: 10.1242/jcs.020289. [DOI] [PubMed] [Google Scholar]
- 79.van Helden SF, Krooshoop DJ, Broers KC, Raymakers RA, Figdor CG, van Leeuwen FN. A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J Immunol. 2006;177:1567–74. doi: 10.4049/jimmunol.177.3.1567. [DOI] [PubMed] [Google Scholar]
- 80.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–94. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dutartre H, Davoust J, Gorvel JP, Chavrier P. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J Cell Sci. 1996;109:367–77. doi: 10.1242/jcs.109.2.367. [DOI] [PubMed] [Google Scholar]
- 82.Moreau V, Tatin F, Varon C, Anies G, Savona-Baron C, Génot E. Cdc42-driven podosome formation in endothelial cells. Eur J Cell Biol. 2006;85:319–25. doi: 10.1016/j.ejcb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 83.Billottet C, Rottiers P, Tatin F, Varon C, Reuzeau E, Maître JL, Saltel F, Moreau V, Génot E. Regulatory signals for endothelial podosome formation. Eur J Cell Biol. 2008;87:543–54. doi: 10.1016/j.ejcb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Daubon T, Buccione R, Génot E. The Aarskog-Scott syndrome protein Fgd1 regulates podosome formation and extracellular matrix remodeling in transforming growth factor β-stimulated aortic endothelial cells. Mol Cell Biol. 2011;31:4430–41. doi: 10.1128/MCB.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tatin F, Varon C, Génot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci. 2006;119:769–81. doi: 10.1242/jcs.02787. [DOI] [PubMed] [Google Scholar]
- 86.Tatin F, Grise F, Reuzeau E, Genot E, Moreau V. Sodium fluoride induces podosome formation in endothelial cells. Biol Cell. 2010;102:489–98. doi: 10.1042/BC20100030. [DOI] [PubMed] [Google Scholar]
- 87.Guegan F, Tatin F, Leste-Lasserre T, Drutel G, Genot E, Moreau V. p190B RhoGAP regulates endothelial-cell-associated proteolysis through MT1-MMP and MMP2. J Cell Sci. 2008;121:2054–61. doi: 10.1242/jcs.025817. [DOI] [PubMed] [Google Scholar]
- 88.Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci. 2004;117:223–31. doi: 10.1242/jcs.00839. [DOI] [PubMed] [Google Scholar]
- 89.Burgstaller G, Gimona M. Podosome-mediated matrix resorption and cell motility in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;288:H3001–5. doi: 10.1152/ajpheart.01002.2004. [DOI] [PubMed] [Google Scholar]
- 90.Kaverina I, Stradal TE, Gimona M. Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J Cell Sci. 2003;116:4915–24. doi: 10.1242/jcs.00818. [DOI] [PubMed] [Google Scholar]
- 91.Furmaniak-Kazmierczak E, Crawley SW, Carter RL, Maurice DH, Côté GP. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ Res. 2007;100:1328–36. doi: 10.1161/CIRCRESAHA.106.147744. [DOI] [PubMed] [Google Scholar]
- 92.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Webb BA, Eves R, Crawley SW, Zhou S, Côté GP, Mak AS. PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a PAK-interacting exchange factor-dependent manner. Am J Physiol Cell Physiol. 2005;289:C898–907. doi: 10.1152/ajpcell.00095.2005. [DOI] [PubMed] [Google Scholar]
- 94.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brandt D, Gimona M, Hillmann M, Haller H, Mischak H. Protein kinase C induces actin reorganization via a Src- and Rho-dependent pathway. J Biol Chem. 2002;277:20903–10. doi: 10.1074/jbc.M200946200. [DOI] [PubMed] [Google Scholar]
- 96.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–85. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 97.Monsky WL, Lin CY, Aoyama A, Kelly T, Akiyama SK, Mueller SC, Chen WT. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994;54:5702–10. [PubMed] [Google Scholar]
- 98.Kuroiwa M, Oneyama C, Nada S, Okada M. The guanine nucleotide exchange factor Arhgef5 plays crucial roles in Src-induced podosome formation. J Cell Sci. 2011;124:1726–38. doi: 10.1242/jcs.080291. [DOI] [PubMed] [Google Scholar]
- 99.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–42. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 100.Oneyama C, Hikita T, Enya K, Dobenecker MW, Saito K, Nada S, Tarakhovsky A, Okada M. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol Cell. 2008;30:426–36. doi: 10.1016/j.molcel.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 101.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99:213–25. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 102.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–9. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collin O, Tracqui P, Stephanou A, Usson Y, Clément-Lacroix J, Planus E. Spatiotemporal dynamics of actin-rich adhesion microdomains: influence of substrate flexibility. J Cell Sci. 2006;119:1914–25. doi: 10.1242/jcs.02838. [DOI] [PubMed] [Google Scholar]
- 104.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–31. doi: 10.1016/j.ejcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 106.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–56. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–28. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lizárraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 2009;69:2792–800. doi: 10.1158/0008-5472.CAN-08-3709. [DOI] [PubMed] [Google Scholar]
- 110.Kikuchi K, Takahashi K. WAVE2- and microtubule-dependent formation of long protrusions and invasion of cancer cells cultured on three-dimensional extracellular matrices. Cancer Sci. 2008;99:2252–9. doi: 10.1111/j.1349-7006.2008.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D’Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–98. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–45. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tetè S, Luini A, Buccione R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–78. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 114.Schachtner H, Calaminus SD, Sinclair A, Monypenny J, Blundell MP, Leon C, Holyoake TL, Thrasher AJ, Michie AM, Vukovic M, et al. Megakaryocytes assemble podosomes that degrade matrix and protrude through basement membrane. Blood. 2013;121:2542–52. doi: 10.1182/blood-2012-07-443457. [DOI] [PubMed] [Google Scholar]
- 115.Gawden-Bone C, Zhou Z, King E, Prescott A, Watts C, Lucocq J. Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. J Cell Sci. 2010;123:1427–37. doi: 10.1242/jcs.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oikawa T, Okamura H, Dietrich F, Senju Y, Takenawa T, Suetsugu S. IRSp53 mediates podosome formation via VASP in NIH-Src cells. PLoS One. 2013;8:e60528. doi: 10.1371/journal.pone.0060528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells. 2003;8:1019–27. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 118.Pichot CS, Arvanitis C, Hartig SM, Jensen SA, Bechill J, Marzouk S, Yu J, Frost JA, Corey SJ. Cdc42-interacting protein 4 promotes breast cancer cell invasion and formation of invadopodia through activation of N-WASp. Cancer Res. 2010;70:8347–56. doi: 10.1158/0008-5472.CAN-09-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamamoto H, Sutoh M, Hatakeyama S, Hashimoto Y, Yoneyama T, Koie T, Saitoh H, Yamaya K, Funyu T, Nakamura T, et al. Requirement for FBP17 in invadopodia formation by invasive bladder tumor cells. J Urol. 2011;185:1930–8. doi: 10.1016/j.juro.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 120.Lorenz M, Yamaguchi H, Wang Y, Singer RH, Condeelis J. Imaging sites of N-wasp activity in lamellipodia and invadopodia of carcinoma cells. Curr Biol. 2004;14:697–703. doi: 10.1016/j.cub.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 121.Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–69. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Connor K, Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases. 2013;4:141–7. doi: 10.4161/sgtp.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilson CA, Tsuchida MA, Allen GM, Barnhart EL, Applegate KT, Yam PT, Ji L, Keren K, Danuser G, Theriot JA. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–7. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berdeaux RL, Díaz B, Kim L, Martin GS. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol. 2004;166:317–23. doi: 10.1083/jcb.200312168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21:635–44. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Juin A, Planus E, Guillemot F, Horakova P, Albiges-Rizo C, Génot E, Rosenbaum J, Moreau V, Saltel F. Extracellular matrix rigidity controls podosome induction in microvascular endothelial cells. Biol Cell. 2013;105:46–57. doi: 10.1111/boc.201200037. [DOI] [PubMed] [Google Scholar]
- 127.Kwiatkowska A, Didier S, Fortin S, Chuang Y, White T, Berens ME, Rushing E, Eschbacher J, Tran NL, Chan A, et al. The small GTPase RhoG mediates glioblastoma cell invasion. Mol Cancer. 2012;11:65. doi: 10.1186/1476-4598-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, Weed SA. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003;14:3216–29. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal. 2009;2:ra54. doi: 10.1126/scisignal.2000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karnoub AE, Symons M, Campbell SL, Der CJ. Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res Treat. 2004;84:61–71. doi: 10.1023/B:BREA.0000018427.84929.5c. [DOI] [PubMed] [Google Scholar]
- 132.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 133.Simpson KJ, Dugan AS, Mercurio AM. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 2004;64:8694–701. doi: 10.1158/0008-5472.CAN-04-2247. [DOI] [PubMed] [Google Scholar]
- 134.Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197:421–37. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Siddiqui TA, Lively S, Vincent C, Schlichter LC. Regulation of podosome formation, microglial migration and invasion by Ca(2+)-signaling molecules expressed in podosomes. J Neuroinflammation. 2012;9:250. doi: 10.1186/1742-2094-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Proszynski TJ, Gingras J, Valdez G, Krzewski K, Sanes JR. Podosomes are present in a postsynaptic apparatus and participate in its maturation. Proc Natl Acad Sci U S A. 2009;106:18373–8. doi: 10.1073/pnas.0910391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patel A, Dash PR. Formation of atypical podosomes in extravillous trophoblasts regulates extracellular matrix degradation. Eur J Cell Biol. 2012;91:171–9. doi: 10.1016/j.ejcb.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brazier H, Pawlak G, Vives V, Blangy A. The Rho GTPase Wrch1 regulates osteoclast precursor adhesion and migration. Int J Biochem Cell Biol. 2009;41:1391–401. doi: 10.1016/j.biocel.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 139.Heckel T, Czupalla C, Expirto Santo AI, Anitei M, Arantzazu Sanchez-Fernandez M, Mosch K, Krause E, Hoflack B. Src-dependent repression of ARF6 is required to maintain podosome-rich sealing zones in bone-digesting osteoclasts. Proc Natl Acad Sci U S A. 2009;106:1451–6. doi: 10.1073/pnas.0804464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Md Hashim NF, Nicholas NS, Dart AE, Kiriakidis S, Paleolog E, Wells CM. Hypoxia-induced invadopodia formation: a role for β-PIX. Open Biol. 2013;3:120159. doi: 10.1098/rsob.120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spurrell DR, Luckashenak NA, Minney DC, Chaplin A, Penninger JM, Liwski RS, Clements JL, West KA. Vav1 regulates the migration and adhesion of dendritic cells. J Immunol. 2009;183:310–8. doi: 10.4049/jimmunol.0802096. [DOI] [PubMed] [Google Scholar]