This study shows that two patterning pathways can operate during in vitro embryogenesis: a zygotic-like pathway driven by the suspensor, characterized by more regular cell division and a requirement for polar auxin transport to establish the embryo proper, and a suspensorless pathway, characterized by less regular cell division and no requirement for auxin transport.
Abstract
In Arabidopsis thaliana, zygotic embryo divisions are highly regular, but it is not clear how embryo patterning is established in species or culture systems with irregular cell divisions. We investigated this using the Brassica napus microspore embryogenesis system, where the male gametophyte is reprogrammed in vitro to form haploid embryos in the absence of exogenous growth regulators. Microspore embryos are formed via two pathways: a zygotic-like pathway, characterized by initial suspensor formation followed by embryo proper formation from the distal cell of the suspensor, and a pathway characterized by initially unorganized embryos lacking a suspensor. Using embryo fate and auxin markers, we show that the zygotic-like pathway requires polar auxin transport for embryo proper specification from the suspensor, while the suspensorless pathway is polar auxin transport independent and marked by an initial auxin maximum, suggesting early embryo proper establishment in the absence of a basal suspensor. Polarity establishment in this suspensorless pathway was triggered and guided by rupture of the pollen exine. Irregular division patterns did not affect cell fate establishment in either pathway. These results confirm the importance of the suspensor and suspensor-driven auxin transport in patterning, but also uncover a mechanism where cell patterning is less regular and independent of auxin transport.
INTRODUCTION
The basic body plan of a plant is established during embryogenesis by sequential divisions, which in most angiosperms start with the asymmetric division of the zygote. In the model plant Arabidopsis thaliana, the subsequent division planes of the embryo are highly regular, making it possible to follow the establishment of the different cell lineages from individual cells. The asymmetric first division of the zygote generates two cells with very different fates. The small apical cell gives rise to the embryo proper, while the larger basal cell divides transversally to produce the suspensor, a mostly extra-embryonic structure, that positions the embryo inside of the seed, provides nutrients and hormones to the embryo proper (Cionini et al., 1976; Nagl, 1990), and contributes to the root meristem (Berleth and Jürgens, 1993; Scheres et al., 1994). Embryos that lack a well-formed suspensor also show aberrant morphologies in the embryo proper (Lukowitz et al., 2004; Bayer et al., 2009; Ueda et al., 2011), while aberrant development of the embryo proper can trigger ectopic division and embryo formation in suspensor cells (Schwartz et al., 1994; Vernon and Meinke, 1994; Yadegari et al., 1994; Zhang and Somerville, 1997). These observations indicate that there is crosstalk between these two structures, in which the embryo proper inhibits embryo formation in suspensor cells and the suspensor supports growth and polarity establishment of the embryo proper.
Mutant analysis in Arabidopsis suggests that the asymmetric division that generates the initial apical-basal pattern of the embryo is important for subsequent cell fate establishment and morphogenesis, as mutants that fail to establish the correct division plane can show subsequent defects in embryo organization or even developmental arrest (Mayer et al., 1993; Breuninger et al., 2008; Ueda et al., 2011). Likewise, loss-of-function mutants of key suspensor genes in the YODA/GROUNDED pathway disrupt the elongation and first division of the zygote. These mutant embryos lack a well-defined suspensor and both the suspensor and the embryo proper show irregular cell divisions (Lukowitz et al., 2004; Bayer et al., 2009; Jeong et al., 2011).
The hormone auxin is a central regulator of cell division, differentiation, and growth and plays an important role in zygotic embryo patterning, as the majority of embryo pattern mutants studied in Arabidopsis are defective in auxin-related pathways. Auxin accumulation is regulated in part by the PIN family of auxin efflux carrier proteins (Křeček et al., 2009). Differential expression of PIN proteins combined with their polar localization on the cell membrane generates auxin gradients that specify cell and organ fates in a context-dependent manner. During embryo development, the PIN7-directed flow of auxin from the basal to the apical cell of the two-celled embryo establishes the embryo proper, while reversal of this flow at the globular stage by the polar localization of PIN1 and PIN4 toward the hypophysis establishes the basal embryo domain (Friml et al., 2003).
Polar auxin transport plays a major role in the establishment of the apical and basal embryo domains and bilateral symmetry in dicot embryos (Liu et al., 1993; Friml et al., 2003). Auxin transport also provides robustness to the embryo pattern, as it buffers auxin distribution in the embryo to local changes in auxin homeostasis (Weijers et al., 2005). Due to functional redundancy among PIN family members, single pin mutants only show weakly penetrant embryo patterning phenotypes (Friml et al., 2003); however, quadruple pin mutants (pin2 pin3 pin4 pin7 and pin1 pin3 pin4 pin7) show strong and highly penetrant apical-basal patterning defects that are associated with mislocalization of apical and basal stem cell regulators (Blilou et al., 2005). Global changes in PIN polarity, as in the gnom or the RPS5A:PINOID mutant backgrounds, also lead to strong-apical basal patterning defects (Steinmann et al., 1999; Friml et al., 2004).
Many of the Arabidopsis embryo mutants that initially show patterning defects often recover and are able to develop into functional seedlings. This, combined with the large amount of variation in the organization of the embryo proper and suspensor in species other than Arabidopsis (Rutishauser, 1969; Johri et al., 1992; Yeung and Meinke, 1993; Kaplan and Cooke, 1997; Madrid and Friedman, 2010; Guillon et al., 2012), as well as the observation that many embryos produced in vitro do not show early morphological signs of patterning (Mordhorst et al., 1997; Raghavan, 2004; Bassuner et al., 2007), indicates that ordered cell divisions are not required for embryo patterning and functionality.
Here, we used the in vitro Brassica napus microspore embryogenesis system to understand how nonregular patterns of cell division influence the formation of a functional embryo. In this system, embryogenesis can be induced from isolated microspores and bicellular pollen grains by a short heat stress treatment (Custers et al., 1994). B. napus zygotic embryos, like those of Arabidopsis, undergo highly regular cell divisions (Tykarska, 1976, 1979). In contrast, the first division during B. napus microspore embryogenesis is usually symmetric, and embryo development does not proceed via a regular pattern of cell division. Suspensor development is also not a prerequisite for B. napus microspore-derived embryo formation, although suspensor-like structures with varying degrees of organization are observed and can be induced at a high frequency in some genotypes (Hause et al., 1994; Yeung et al., 1996; Ilić-Grubor et al., 1998; Supena et al., 2008).
In this study, we show that the two different pathways of haploid in vitro embryo development, with and without initial suspensor formation, are marked by differences in auxin response and transport. Both pathways are highly flexible in that they are not compromised by irregular cell divisions. Our data provide insight into how embryo cell fate and patterning are established in the absence of highly regular cell divisions.
RESULTS
Haploid Embryo Development in the Absence of a Suspensor
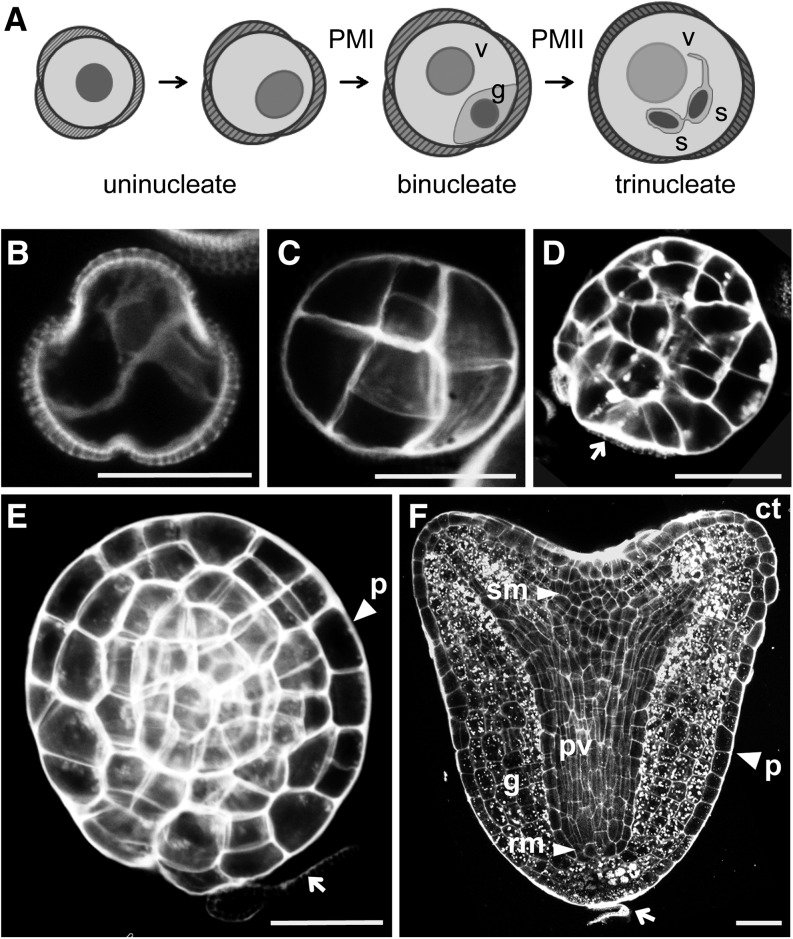
In B. napus, male gametophyte development starts with the single-celled microspore and after two mitotic divisions (pollen mitosis I and pollen mitosis II) results in the formation of a trinucleate pollen grain (Figure 1A). Heat stress induces a sporophytic program in cultured microspores and binucleate pollen that leads to the formation of haploid embryos. Many correlative cell biology observations have suggested that the first characteristic of sporophytic development in microspore culture is the symmetric division of the uninucleate microspore or vegetative cell of the pollen grain, which can be distinguished from the first asymmetric division that characterizes pollen development (Figure 1B) (Fan et al., 1988; Zaki and Dickinson, 1990). In embryos that develop without a suspensor, this initial division is followed by a series of randomly oriented divisions in which the different cell layers, normally present in zygotic embryos, cannot be recognized (Figure 1C). Further random division of these structures stretches the surrounding exine (pollen coat) until one of the exine locules breaks, releasing a globular-shaped structure (Figure 1D). The embryonic epidermis, the protoderm, forms at this time (Figure 1E) and is followed by histodifferentiation of the major tissue types and organs of the embryo (Figure 1F).
Figure 1.
Developmental Pathways in Microspore Culture.
(A) Pollen development
(B) to (F) Histodifferentiated embryo production.
(B) Symmetrically divided microspore.
(C) Proembryo enclosed in the exine.
(D) Globular embryo released from the exine.
(E) Globular embryo with a well-formed protoderm layer.
(F) Heart stage embryo.
Arrow, exine remnants; c, cell wall; ct, cotyledon; g, ground tissue; p, protoderm; pv, provascular tissue, rm, root meristem; sm, shoot meristem; s, spermatid; v, vegetative cell. Bars = 20 μm.
Establishment of Embryo Identity Does Not Require Microspore Division
The irregular cell divisions observed during microspore embryogenesis make it difficult to determine morphologically when a structure first becomes embryogenic. To more precisely define when cultured microspores become committed to the embryo development pathway, we generated B. napus lines carrying the GLYCINE-RICH PROTEIN (proGRP:GFP-GUS) reporter. This reporter is expressed in B. napus at the zygote stage (Li et al., 2014), in both the apical and basal cells of the embryo (Supplemental Figures 1A to 1C). Its expression gradually becomes restricted to the suspensor and basal cells of the embryo proper and finally to the cells that form the columella root cap of the embryo proper. The GRP reporter is not expressed during in planta pollen development (Supplemental Figures 1D to 1H). The percentage of GRP-positive structures at day 2 of culture was highly correlated with the number of embryos formed (Supplemental Figure 1I). GRP expression is therefore a suitable marker for following the establishment of embryo cell fate in microspore cultures.
We examined GRP expression in combination with 4′,6-diamidino-2-phenylindole (DAPI) staining to define the developmental stage at which the embryogenic program is established in microspore culture. GRP reporter expression could be observed as early as 1 d after the start of culture, in ∼0.2% of the microspores, and this percentage rapidly increased by the third day of culture to up to 4% of the total population.
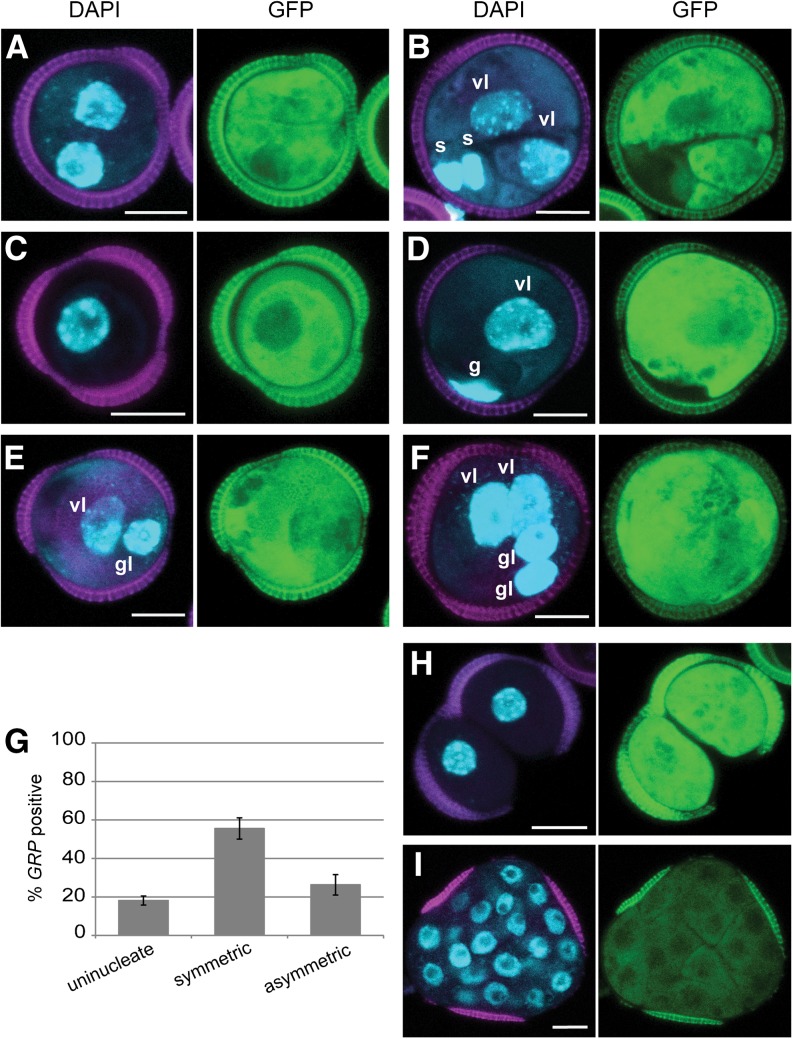
Symmetrically divided microspores and the symmetrically divided vegetative cell of binucleate pollen are reported to form haploid embryos in B. napus (Fan et al., 1988; Zaki and Dickinson, 1990). We observed GRP-driven GFP expression in these symmetrically divided cell types (Figures 2A and 2B), which represented almost 60% of the GRP-positive structures (Figure 2G). Notably, GRP expression was absent in ∼40% of the symmetrically divided cells (Supplemental Figures 1J and 1K) (Li et al., 2014), suggesting that symmetric division is not an absolute marker for the change in cell fate from pollen to embryo development.
Figure 2.
Embryo Identity in Microspore Culture Is Not Dependent on Cell Division or Division Symmetry.
(A) to (F) proGRP:GUS-GFP embryo marker expression after 3 d of culture. DAPI staining (blue), autofluorescence (magenta), and GFP expression (green).
(A) Symmetrically divided microspore showing GRP expression in both cells.
(B) GRP expression in the two symmetrically divided vegetative-like cells, but not the smaller generative/sperm-like cells.
(C) GRP-positive microspore
(D) GRP expression in the vegetative-like cell, but not in the smaller generative-like cell.
(E) and (F) GRP expression in both the vegetative- and generative-like cells. Note that the generative-like nuclei in (E) and (F) are larger and the DNA is less condensed than in (D).
(E) Asymmetrically divided bicellular structure.
(F) Multinucleate structures.
(G) Proportion of microspores and symmetrically and asymmetrically divided structures that express proGRP:GFP-GUS at day 3 of culture. Structures where GRP was only expressed in the vegetative cell of binucleate (D) were not included in the graph.
(H) and (I) GRP-positive structures found at 6 d of culture.
(H) Sporophytic structure prematurely released from the exine.
(I) Proembryo enclosed in the exine. Note that GFP fluorescence was weaker in multinucleate proembryos than in few celled embryos ([A] to [F]).
Error bars in (G) indicate the se of three replicates. vl, vegetative-like nucleus; gl, generative-like nucleus. Bars = 10 μm.
We also observed strong GFP expression in three other types of structures that were not considered in the literature to be embryogenic. These comprised microspores (Figures 2C and 2G) and pollen vegetative cells (Figure 2D), asymmetrically divided structures with large and small GFP-positive cells (Figures 2E to 2G), and loosely connected cells that had burst prematurely out of the exine (Figure 2H). In asymmetrically divided structures, the nucleus of the smaller GFP-positive cell resembled a pollen generative cell, but did not show the typical lens shape and DNA compaction found in the generative nuclei of (GFP-negative) pollen (compare Figure 2D with Figures 2E and 2F). The loosely connected GFP-positive cells were described previously as “nonembryogenic” based on their lack of histodifferentiation (Fan et al., 1988; Telmer et al., 1995; Ilić-Grubor et al., 1998). These clusters increased in cell number during the culture period, forming unorganized masses of round, expanded cells, but eventually died.
Our data suggest that many microspores are initially programmed to develop as embryos and that this initial switch in developmental pathways can occur in the absence of cell division and independent of the initial division symmetry. However, only ∼0.5% of the sporophytic structures will eventually produce the compact structures that form histodifferentiated embryos (Figure 2I) (Li et al., 2014), suggesting that additional signaling events are required to ensure further embryo growth and differentiation.
Auxin Signaling Marks Embryogenic Microspores
In view of the important role of auxin in zygotic embryo development, we examined the timing and spatial distribution of the auxin response reporter proDR5:GFP and the Arabidopsis proPIN1:PIN1-GFP and proPIN7:PIN7-GFP reporters to gain insight into auxin dynamics during the initiation of B. napus microspore embryo development. The expression patterns of these reporters are conserved between Arabidopsis and B. napus zygotic embryos (Supplemental Figures 2A to 2C), except that in B. napus proDR5:GFP expression was only observed in the embryo proper from the octant stage of development onward, whereas in Arabidopsis, DR5 expression is first detected in the embryo proper at the one-cell stage (Friml et al., 2003). In B. napus, neither the PIN1-GFP protein nor the PIN7-GFP protein was observed during male gametophyte development in planta. proDR5:GFP expression was observed during male gametophyte development in planta, but at an earlier stage than is used for microspore culture (Supplemental Figure 2D). In conclusion, none of the auxin reporters were expressed in any of the gametophytic structures that are present in microspore cultures.
During the first 3 to 5 d of microspore culture, neither PIN1-GFP nor PIN7-GFP was observed in gametophytic structures or randomly divided, suspensorless sporophytic structures. These data suggest that suspensorless proembryos growing inside the exine do not show the same early apical-basal specification that occurs in zygotic embryos.
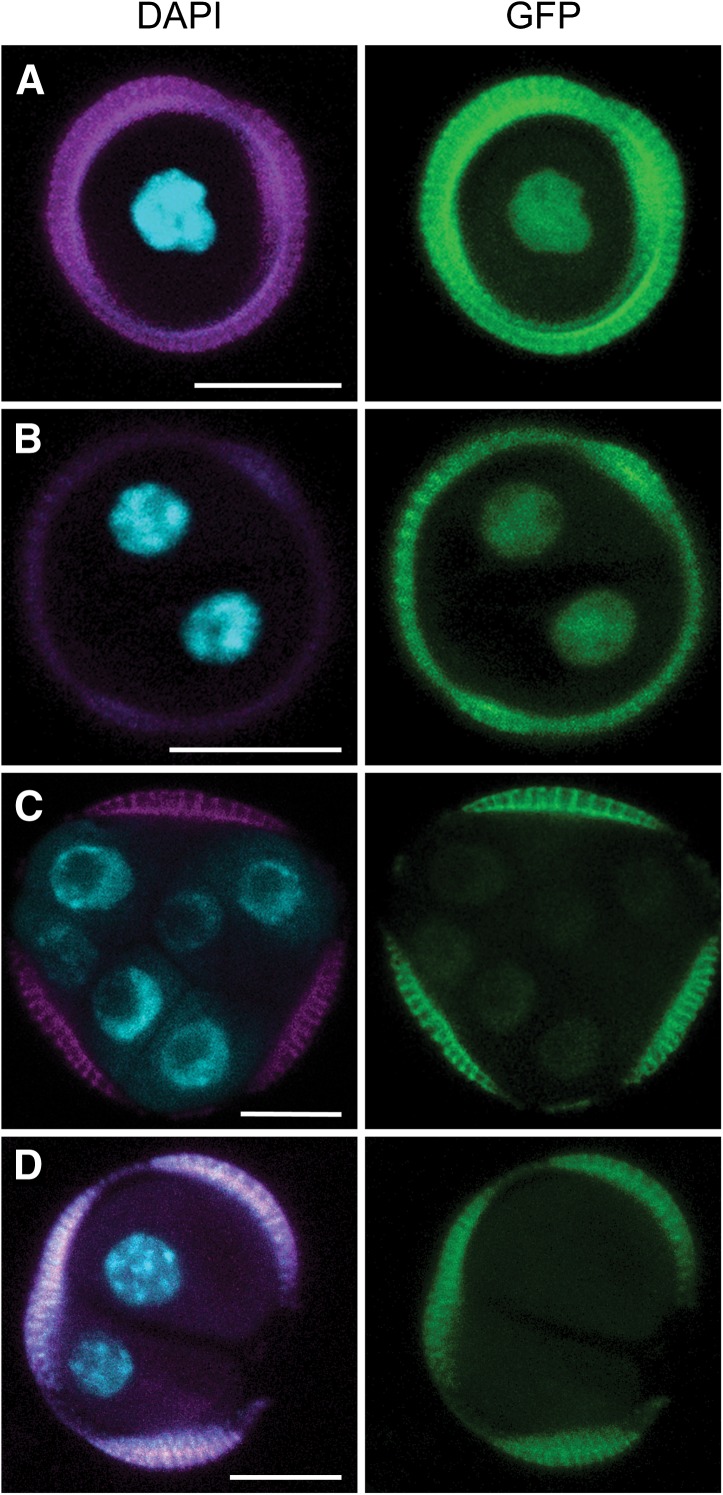
Weak DR5 expression was first observed around the third day of culture in up to 0.2% of the microspore population. These GFP-positive structures were either uninucleate or had divided symmetrically (Figures 3A and 3B). Notably, DR5 expression was very weak and could not be detected in compact embryo structures after the two-celled stage (Figure 3C). The proportion of proDR5:GFP-expressing cells was always lower than the ∼4% marked by GRP, but in line with the final embryo yield (Supplemental Figure 1I). Unlike GRP expression, DR5 expression did not mark the asymmetric pollen-like structures, nor did it mark the loosely connected cell clusters that released prematurely from the exine (Figure 3D). These results show that a transient auxin response marks a subset of embryogenic cells. In analogy with the DR5 maximum in the embryo proper of zygotic embryos (Friml et al., 2003), DR5 expression in embryogenic microspores suggests that these cells are initially programmed as an embryo proper. The failure of certain GRP-marked embryogenic structures (i.e., asymmetrically divided microspores and callus-like cells) to establish an auxin response suggests that these structures are associated with a different pathway of haploid embryo development in which the formation of an embryo proper is not initiated or is delayed.
Figure 3.
DR5 Expression Marks Embryogenic Cells.
(A) Microspore-like structure
(B) Symmetrically divided microspore.
(C) Compact multinucleate structures.
(D) DR5 is not expressed in multinucleate structures that emerged prematurely from the exine
DAPI staining (blue), autofluorescence (magenta), and GFP fluorescence (green). Bars = 10 μm.
Embryo Polarization Follows Exine Rupture
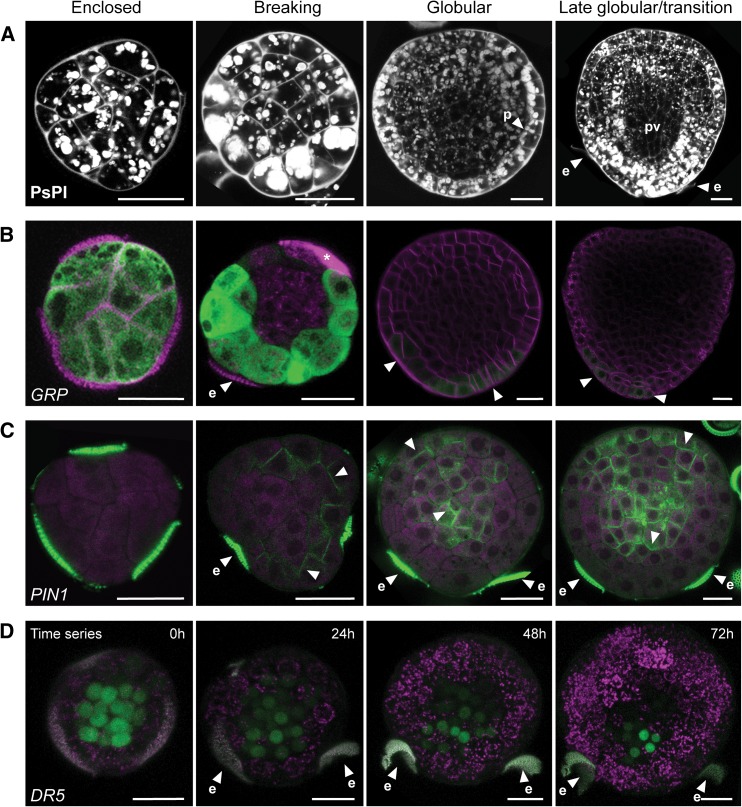
Early growth in suspensorless embryos causes stretching of the exine, which eventually breaks. After exine rupture, the apical and basal poles of the haploid embryo become morphologically apparent through the formation of cotyledons and elongation of the embryo axis (Yeung et al., 1996). The position of the remaining exine pieces highly correlated with the basal region of the embryo (Figure 1E) (Ilić-Grubor et al., 1998; Supena et al., 2008; Tang et al., 2013) and is therefore a much earlier marker for polarity establishment than apical growth and axis elongation. This polarization was also marked by the accumulation of large starch granules at the future basal pole of the embryo. Starch grains were abundant throughout the haploid embryo prior to exine rupture, but upon exine rupture they accumulated predominately in the cells that are located away from the site of exine rupture (i.e., the future basal pole of the embryo) (Figure 4A) (Hause et al., 1994).
Figure 4.
Embryo Polarization Occurs after Exine Rupture.
(A) to (C) Four successive stages of microspore embryo development are shown from left to right: embryos still enclosed in the exine, embryos shortly after the exine has broken at one of the locules, globular stage embryos released from the exine, and late globular to transition stage embryos.
(A) Starch grain accumulation (white globules) and cell walls (white) visualized by Pseudo-Schiff-propidium iodide (PsPI) staining.
(B) proGRP:GUS-GFP expression. Membrane staining by FM4-64 (magenta) and GFP fluorescence (green).
(C) proPIN1:PIN1-GFP expression. Propidium iodide counterstain (magenta) and GFP fluorescence (green).
(D) Time-lapse image of proDR5:GFP expression at the time of exine rupture. Exine and chloroplast autofluorescence (magenta) and GFP fluorescence (green).
Arrowheads, GFP localization; e, exine; p, protoderm; pv, provascular tissue. The asterisk marks cellular debris stained by FM4-64. Bars = 20 μm.
Unlike the early stages of suspensorless microspore embryo development, the morphological changes that took place after release of the embryo from the exine were similar to those that take place during zygotic embryogenesis. First, the protoderm was established (Figure 4A), followed by a triangle/transition stage in which the area surrounding the apical pole grew, giving rise to a bilaterally polarized embryo (Figure 4A). The provasculature in triangle/transition stage embryos was free of starch, while the basal pole was marked by the accumulation of large starch grains (Figure 4A). Later, outgrowth of the cotyledons marked the establishment of bilateral symmetry (Figure 1E). The root meristem was not as well defined in microspore embryos as in zygotic embryos, but could be recognized at the early heart stage as a group of isodiametric cells below the provasculature that were devoid of starch and that were often larger than neighboring cells (Figure 1E) (Yeung et al., 1996).
We followed the GRP, PIN, and DR5 reporters in microspore embryos as they emerged from the exine and underwent histodifferentiation. GRP was initially expressed throughout the embryo clusters while they were still enclosed by the exine (Figure 4B). GRP expression at this stage was much weaker than in few-celled embryos. After exine rupture, GRP expression disappeared from the cells at the rupture site and from the inner cells of the embryo, remaining localized at the site of the presumptive basal pole (Figure 4B). Later, at the globular and transition stages, GRP expression was very weak and marked the basal cell tiers of the embryo and the future columella (Figure 4B). At this stage, GRP expression was highly correlated with the presence of pollen wall remnants on the surface of the embryo (Supplemental Figure 3A).
During zygotic embryo development, PIN1 is expressed in the embryo proper from the eight-cell stage embryo onward (Friml et al., 2003). PIN1-GFP was not observed prior to exine rupture in microspore embryos lacking suspensors. Rather, PIN1-GFP was first observed in cells that protruded through the pollen pores at the time that the exine started to break (Figure 4C). In embryos that had completely burst free of the exine, PIN1-GFP expression was strong on the outer cell layer in the apical region and in the inner cells, with no clear cellular polarization, and gradually marked the formation of provascular strands at the late globular/transition stage (Figure 4C). At the heart stage, proPIN1:PIN1-GFP was expressed in the same pattern in microspore and zygotic embryos, in the epidermal cell layer, where the fusion protein showed clear apical polarization, and in the provascular tissue, where the fusion protein showed a rather basally oriented polarity (Supplemental Figures 2A and 3B). In contrast to PIN1, proPIN7:PIN7-GFP expression was not observed in suspensorless embryos.
Together, these data suggest that in suspensorless embryos, PIN1 and GRP expression mark the formation of the apical and basal domains of the embryo, respectively, that apical-basal axis determination takes place late in embryo development, after exine rupture, and that PIN7 expression does not mark the basal cell lineage.
These data were supported by time-lapse imaging of the proDR5:GFP reporter during exine rupture (Figure 4D). As noted above, DR5 expression was very weak or could not be detected in compact embryo structures after the two-celled stage, but became strongly expressed in the inner cells of embryos comprising 15 to 17 cells that showed stretching of the exine (Figure 4D, 0 h). The site of DR5 expression did not change when the exine broke, but rather became weaker (Figure 4D, 24 h), disappeared from the site where the pollen wall broke (Figure 4D, 48 h), and eventually became stronger again and restricted to a few cells that mark the site of presumptive root meristem (Figure 4D, 72 h). These results show that in microspore embryos that lack a suspensor, a basal auxin maximum is established after exine rupture, in the same spatial pattern as the GRP reporter. However, polarization of DR5 expression to the basal pole occurs slowly, while GRP expression disappears more rapidly from the inner and apical cells.
Exine Rupture and Apical-Basal Polarity Establishment Are Independent of Polar Auxin Transport
PIN1-GFP was localized to the sites of pollen wall rupture and later marked the apical pole of the embryo. This observation, together with the basal auxin maximum established after exine rupture, suggests that PIN1 directs auxin away from the apical region of the embryo. However, it is not clear whether (PIN-directed) polar auxin transport (PAT) is the driving force behind exine rupture. We used the PAT inhibitor, N-1-naphthylphthalamidic acid (NPA) to understand the relationship between PAT and exine rupture in suspensorless microspore embryo development.
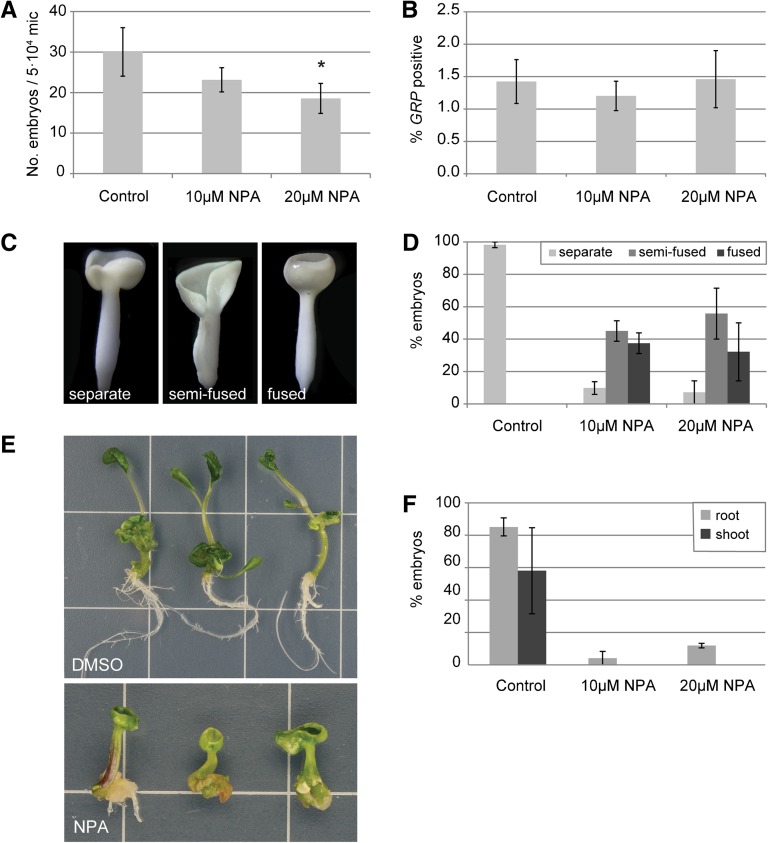
Continuous treatment of cultured DH12075 microspores with NPA consistently reduced the proportion of cells that developed into embryos, although this trend was only statistically significant in 20 µM NPA treatments (Figure 5A). Application of NPA to microspore cultures did not change the proportion of GRP-positive microspores at day 3 of culture (Figure 5B), suggesting that the effect of NPA on embryo yield occurs later in development. As previously reported, NPA induced the formation of morphologically defective cup-shaped embryos or embryos with fused cotyledons (Figures 5C and 5D) (Hadfi et al., 1998; Friml et al., 2003; Hakman et al., 2009). Despite these defects, almost all NPA-treated embryos had clear root and shoot poles (Figures 5C and 5D), but the function of the meristems was compromised: <10% of the embryos that developed in the presence of NPA produced roots after 5 d on regeneration medium, while roots were produced in more than 80% of the control embryos (Figures 5E and 5F). After 25 d on regeneration medium, some NPA-treated embryos had formed roots indirectly, either from the basal part of the hypocotyl, or from callus tissue in the basal region of the embryo. None of the embryos from NPA-treated cultures produced a shoot from the apical meristem, compared with 60% in the control cultures (Figures 5E and 5F).
Figure 5.
Inhibition of Polar Auxin Transport Induces Defects in Embryo Development but Not Embryo Initiation.
(A) Effect of continuous treatment with NPA on embryo yield. Cultures were scored at day 25.
(B) Percentage of proGRP:GUS-GFP expressing microspores at day 3 of culture.
(C) Cotyledon morphologies: left, embryos with two separated cotyledons; center, embryos with semifused cotyledons in which only one cotyledon boundary is observed and; right, embryos with completely fused or collar-shaped cotyledons.
(D) Relative abundance of the different embryo morphologies shown in (C) after treatment with NPA.
(E) Control embryos gave rise to a high frequency of plantlets containing both a root and a shoot (upper panel), while NPA-treated embryos showed reduced root growth and failed to form shoots (lower panel).
(F) The relative frequency of root (light-gray bars) and shoot (dark-gray bars) formation in control and NPA-treated embryos in a germination assay.
Error bars indicate the se of 3 to 11 replicates. Asterisk indicates statistically significant difference with control P < 0.05 (ANOVA).
[See online article for color version of this figure.]
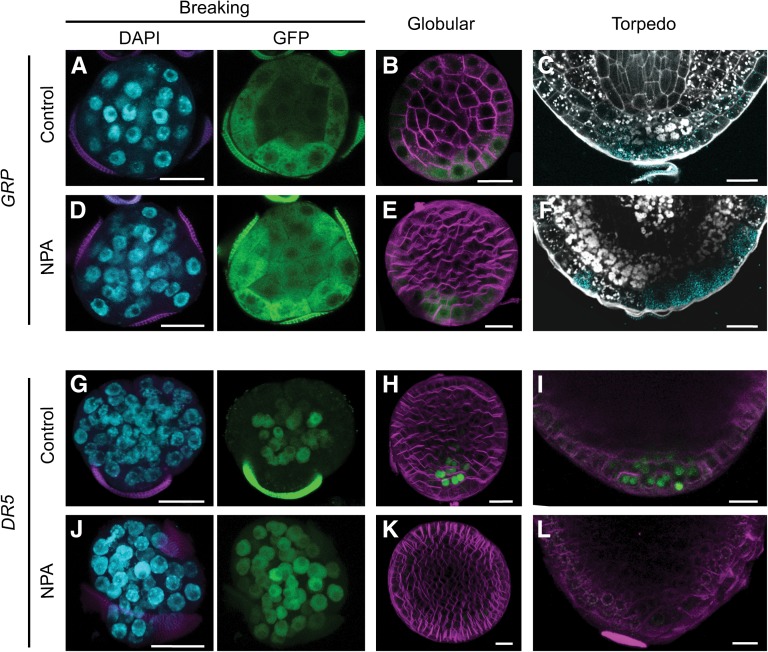
Exine rupture in NPA-treated cultures was similar to that in control cultures. GRP expression and starch accumulation were also properly localized to the basal pole after exine rupture in NPA-treated embryos (Figures 6A to 6F). However, starch also accumulated in the inner cells at the basal side of the provascular tissue, adjacent to the root pole, suggesting differentiation of vascular stem cells (Figure 6F). In contrast to GRP expression, DR5 expression was perturbed in embryo cultures treated with NPA. At the time of exine rupture, DR5 was expressed throughout the embryo rather than being localized to the inner cells of embryos, as in the control cultures (Figures 6G and 6J). After exine rupture, DR5 expression was extremely weak and randomly localized throughout the embryo, rather than strongly expressed at the basal pole, as in control embryos (Figures 6H and 6K). In NPA-treated tube-shaped embryos, DR5 expression was localized to a broad region of the basal area and then eventually disappeared (Figures 6I and 6L). These data, together with our morphological observations, suggest that PAT does not direct exine rupture nor does it play a role in the initial establishment of apical-basal polarity in suspensorless embryos. Rather, PAT appears to be required to focus the initial auxin maximum at the basal pole, and this seems to be important for the transition from radial to bilateral symmetry and for the establishment of well-defined poles with functional shoot and root meristems.
Figure 6.
Auxin Polar Transport Is Not Required for Microspore Embryo Polarization.
(A) to (F) Establishment of the embryo basal domain, marked by proGRP:GFP-GUS.
(G) to (L) Localization of auxin response maxima by proDR5:GFP expression.
Comparison of control and NPA-treated embryos at the stage where the exine starts to break ([A], [D], [G], and [J]), at the globular stage after release from the exine ([B], [E], [H], and [K]), and in the root pole at the torpedo stage at the torpedo stage ([C], [F], [I], and [L]). DAPI staining of the nuclei (blue) and GFP expression (green) are shown separately. FM4-64 (magenta) was used to stain membranes in globular stage embryos. GUS staining of proGRP:GFP-GUS lines (light blue) was combined with starch and cell wall localization by Pseudo-Schiff-propidium iodide staining (white). Bars = 20 μm.
Suspensor-Derived Embryogenesis Is PAT Dependent
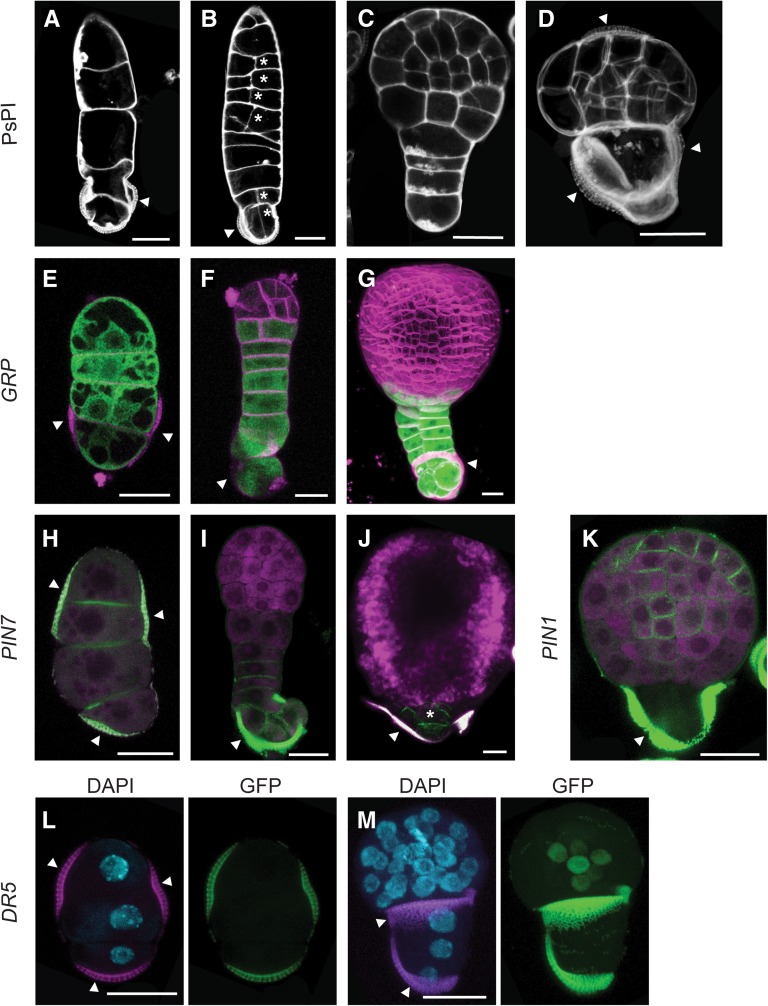
The efficiency of suspensor-bearing embryo production in microspore culture is genotype dependent. Suspensor-bearing embryos are efficiently induced in the model B. napus line Topas DH4079 (Joosen et al., 2007; Supena et al., 2008) by exposing the microspores to a shorter heat stress treatment. In this genotype, a long uniseriate cell filament emerges through a pore in the exine wall. The embryo proper is initiated by a transverse division of the suspensor cell that is most distal to the exine remnants and divides in a more ordered pattern, similar to the zygotic embryo. A wide range of abnormal suspensor morphologies are also observed, ranging from multiple files of cells to small protrusions (Supena et al., 2008). Unlike the model line Topas DH4079, suspensor-bearing embryos are only found in a low percentage of the population in the DH12075 line used to generate the reporter lines (∼1.5% of the embryos) and were more often short and contained additional cell files or longitudinal divisions (Figures 7A to 7D). These abnormally formed suspensors have been associated with a poorly organized embryo proper (Supena et al., 2008).
Figure 7.
Domain Specification in Suspensor-Bearing Microspore Embryos Is Similar to Zygotic Embryos.
(A) to (D) Suspensor morphologies observed in microspore culture.
(A) Uniseriate suspensor.
(B) Suspensor with aberrant longitudinal divisions (asterisk).
(C) Microspore embryo with suspensor and embryo proper.
(D) Microspore embryo with two domains, enclosed in the exine. Arrowheads point to the three exine locules enclosing the proembryo.
(E) to (G) proGRP:GFP-GUS expression. GFP is shown in green and FM4-64–stained membranes in magenta.
(E) Uniseriate suspensor.
(F) Suspensor-bearing embryo at the early globular stage.
(G) Transition-stage suspensor-bearing embryo, with two cell files.
(H) to (J) proPIN7:PIN7-GFP expression (green) in the suspensor. Propidium iodide stain or autofluorescence (magenta)
(H) Uniseriate suspensor.
(I) Globular stage embryo with suspensor
(J) Transition stage embryo with a rudimentary suspensor.
(K) proPIN1:PIN1-GFP expression (green) in a globular stage suspensor-bearing embryo.
(L) and (M) proDR5:GFP expression (green) in the embryo proper. DAPI nuclear stain (blue) and autofluorescence (magenta).
(L) DR5 is not expressed in the suspensor.
(M) Suspensor-bearing embryo at the globular stage showing DR5 expression in the inner cells at the basal region of the embryo proper.
Arrowhead, exine. Bars = 20 μm.
We used the embryo and auxin reporter lines to follow the establishment of the apical (PIN1) and basal (PIN7, DR5, and GRP) tiers and histodifferentiation events in suspensor-bearing microspore-derived embryos. As in the zygotic embryo, GRP was expressed in all cells of the suspensor (Figure 7E) and was confined to the basal-most tier of the embryo proper from the early globular stage onward (Figures 7F and 7G). The expression of the DR5 and PIN reporters in suspensor-bearing embryos was similar to their expression in zygotic embryos, with proPIN7:PIN7-GFP expressed in the suspensor (Figures 7H to 7J) and DR5 and proPIN1:PIN1-GFP expressed in the embryo proper (Figures 7K to 7M). These data provide further support that suspensor-bearing embryos follow a zygotic embryo-like program, even though the embryo proper is derived from the apical cell of a multicellular suspensor, rather than from an asymmetric division of the zygote.
We also detected PIN7-GFP in embryos that contained a small suspensor-like protrusion at the basal end of the embryo, rather than a file of cells (Figures 7D and 7J). These embryos comprised ∼10% of the embryo population and were characterized by two heterogeneous domains, one comprising larger vacuolated cells (Figure 7D), in which PIN7-GFP was observed (Figure 7J), and one comprising smaller and more compact cells (Figure 7D), in which PIN1-GFP was observed (Figure 7K). PIN1-GFP and PIN7-GFP could be observed occasionally in a localized region in embryos that were still enclosed in the exine (Supplemental Figure 4). These two domains are reminiscent of the smaller embryo proper cells and the larger suspensor-like cells. PIN7 expression in the larger and more vacuolated basal cells suggests that these cells function as a suspensor, despite being more rudimentary in structure, and by extension, that unlike zygotic embryo development, suspensor establishment during haploid embryo development does not rely on a highly regular pattern of cell divisions.
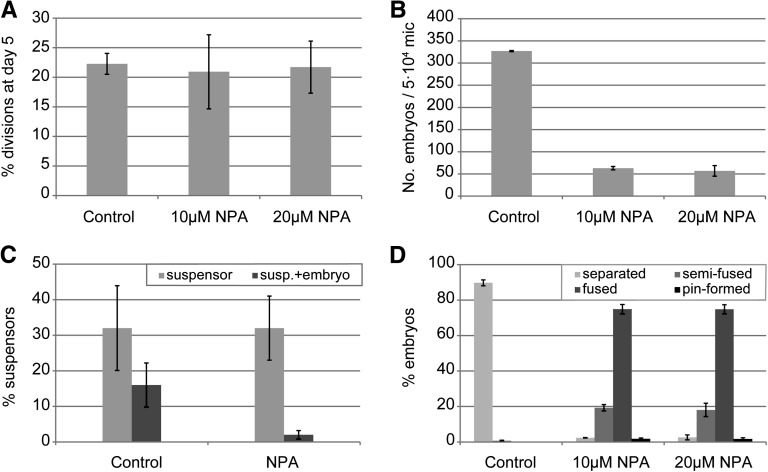
The presence of PIN7-GFP in suspensors suggests that PAT plays an important role in this developmental pathway. Addition of NPA to microspore cultures of genotype DH4079, which shows high percentage of suspensor formation, did not have a significant effect on the proportion of initial sporophytic divisions or suspensor development (Figure 8A). However, after 20 d of culture, NPA treatment significantly reduced the final embryo yield in cultures with a large proportion of suspensors (Figure 8B). Interestingly, NPA did not affect the ability of DH4079 microspores to develop suspensors, but rather it specifically affected the ability of the distal cell of the suspensor to form the embryo proper, as shown by the reduction in the number of embryos with a suspensor (Figure 8C). At later stages, a high proportion of embryos with cup-shaped or fused cotyledons was observed after NPA treatment, as in line DH12075, although the effect of NPA on line DH4079 was more pronounced, with a higher proportion of completely cup-shaped cotyledons, as well as the appearance of pin-shaped embryos (Figure 8D).
Figure 8.
Polar Auxin Transport Is Required for Embryo Proper Formation in Suspensor-Bearing Embryos.
(A) Percentage of sporophytic divisions at day 5 of culture in control and NPA-treated cultures.
(B) Number of embryos formed in control and NPA-treated cultures at day 25 of culture.
(C) Percentage of structures with only a suspensor or with both a suspensor and an embryo proper in control and NPA-treated cultures. The data were calculated relative to the total number of sporophytic structures.
(D) Relative abundance of cotyledon morphologies after treatment with NPA. The morphologies are as in Figure 5C: embryos with two separated cotyledons, with semifused cotyledons in which only one boundary is observed, with completely fused or collar-shaped cotyledons, and embryos lacking cotyledons (pin-formed).
Error bars indicate the se of three replicates.
Our data suggest that PAT is not required for suspensor formation, but rather, for the establishment of the embryo proper from the suspensor. This observation, combined with our data on development of embryos lacking a suspensor, suggest that differences in auxin response and transport mark the two different pathways of microspore embryo development.
DISCUSSION
Microspore-derived embryos can be obtained via two pathways, one pathway where embryo development occurs in the absence of a suspensor and a second pathway that starts with the development of a suspensor that eventually forms an embryo proper. Here, we show that in both pathways, the regular pattern of cell division seen in zygotic embryos is not required to establish embryo identity nor is it required later to establish the apical and basal domains of the haploid embryo. More importantly, we show that the two pathways are marked by differences in auxin response and transport. Embryogenic microspores that develop without a suspensor are marked by an initial auxin response and do not require PAT for embryo induction but do require PAT later for meristem formation and positioning of the cotyledon primordia. In contrast, in the other pathway, suspensor development is not preceded by an auxin response, and PAT is required for embryo proper formation at the distal end of the suspensor. Our observations suggest that plant embryo developmental pathways can be highly flexible and driven by different mechanisms within the same species.
Auxin Response Reports Embryogenesis-Competent Microspores
An auxin response is first observed at the single celled stage in the suspensorless embryo pathway. This developmental program is different from the Arabidopsis (Friml et al., 2003) or B. napus zygotic embryogenesis program and from the pathway in which haploid embryos develop with suspensors, where an auxin response is only observed in the embryo proper. This suggests that microspore-derived embryos that develop without a suspensor are already programmed as an embryo proper. Auxin is not added to the tissue culture medium, implying that these haploid embryos accumulate auxin, either through de novo biosynthesis or through deconjugation of existing auxin pools (Rosquete et al., 2012; Korasick et al., 2013).
The auxin response marked by DR5 expression was absent in the asymmetrically divided cells and the loose, callus-like structures that fail to develop into differentiated embryos, although these structures expressed the GRP embryo reporter, as well as LEAFY COTYLEDON1, a key regulator of embryo growth and maturation (Li et al., 2014). Thus, DR5 expression appears to mark a subset of embryogenic cells that enter a specific developmental pathway. The lack of auxin response in asymmetrically divided and callus-like embryogenic structures suggests that, in addition to a switch to embryonic cell fate, an initial auxin response is required to instruct the future differentiation of the embryo.
Polarity Establishment following Exine Rupture
The site of exine rupture plays an important role in polarity establishment in microspore-derived embryos. Cell elongation and apical-basal cell fates in microspore embryos can be induced by early, controlled exine rupture, supporting the role of external positional cues in cell fate establishment (Tang et al., 2013). We have shown that PIN1 first accumulates on the cell membrane at the sites of exine rupture. Recently, it was shown that PIN1 expression and membrane localization can be directed by mechanical signals (Heisler et al., 2010; Nakayama et al., 2012), which are transduced by changes in microtubule orientation and plasma membrane properties. PIN1 membrane localization increases in tomato (Solanum lycopersicum) shoot apex cells when cell turgor and membrane tension are raised (Nakayama et al., 2012). In line with this, during microspore embryogenesis, PIN1-GFP is not observed in the tightly contained and growth-constrained multicellular structures enclosed in the exine, but only becomes apparent at the time of rupture, when the external pressure from the exine is released and the embryo cells expand.
The polar secretion and localization of different membrane or cell wall components might also be involved in early embryo polarization. In the brown algae Fucus, embryo polarization starts with labile polarization of calcium channels and is stabilized by polarized secretion, producing regions with distinct cell wall properties that are determinants for cell fate specification (Berger et al., 1994; Shaw and Quatrano, 1996). Differential membrane and cell wall properties induced by exine rupture could also give rise to initial positional and polarization cues.
Research on mechanical, cell wall, and auxin signaling processes during microspore embryogenesis can shed light on how mechanical stimuli are perceived in plants and their relevance in zygotic embryo patterning. The difference in timing between the establishment of embryonic fate and histodifferentiation in microspore embryos offers the opportunity to characterize these processes independently from each other.
Differential PAT Dependency of the Two Haploid Embryogenesis Programs
PAT is a major regulator of apical-basal axis establishment during zygotic embryogenesis. Inhibition of PAT by NPA in Arabidopsis and Brassica juncea zygotic embryos causes defects in apical-basal axis establishment, as well as radial patterning and cotyledon formation (Liu et al., 1993; Hadfi et al., 1998; Friml et al., 2003; Weijers et al., 2005). NPA treatment of cultured B. napus microspores indicated that PAT was required to produce functional meristems in suspensorless embryos but was not required to establish the embryonic apical-basal axis; apical and basal poles were recognizable and basal pole markers (GRP and starch accumulation) were not disrupted. By contrast, PINs and PAT are required to specify the shoot and root pole in Arabidopsis zygotic embryos (Friml et al., 2003; Blilou et al., 2005; Robert et al., 2013). Our results point to an alternative mechanism of cell fate determination in the embryo that is independent of PAT.
In contrast to suspensorless embryos, suspensor-bearing microspore embryos develop in a similar fashion to zygotic embryos with respect to PIN and DR5 expression. In zygotic embryos, an apical, PIN7-directed flow of auxin is required for the formation of the embryo proper (Friml et al., 2003). NPA inhibition of PAT has not been applied to preglobular stage zygotic embryos, but when applied at later stages of development (globular or transition), NPA induces severe defects in cotyledon outgrowth and mild defects in shoot and root regeneration (Liu et al., 1993; Hadfi et al., 1998). However, single and higher order pin mutants do show early apical and/or basal embryo division defects (Friml et al., 2003; Blilou et al., 2005; Vieten et al., 2005), indicating an important role for PAT in early zygotic embryo patterning. Inhibition of PAT in suspensor-bearing microspore embryos did not interfere with the initiation and specification of suspensor cell fate, but rather inhibited the ability of the distal cell of the suspensor to form the embryo proper. This phenotype is strikingly similar to that of early pin7 mutant embryos, which frequently lack an embryo proper and/or an apical DR5 maximum, but eventually become rescued by the onset of PIN1 and PIN4 expression (Friml et al., 2003). Our results support previous observations in Arabidopsis zygotic embryos that suggest that the PIN7-directed flow of auxin from the suspensor to the apical cell is important for specification of the embryo proper (Friml et al., 2003; Robert et al., 2013).
Intriguingly, the DR5 and PIN1 expression patterns are maintained in microspore embryos even when the cellular organization of the suspensor or the embryo proper is aberrant. Cell identities are clearly specified in suspensor-bearing embryos regardless of the division pattern. Inhibition of auxin response in zygotic embryos causes excessive divisions in suspensor cells and ectopic expression of genes that are normally restricted to the embryo proper, suggesting that a specific auxin response maintains suspensor cell identity in zygotic embryos (Schlereth et al., 2010; Rademacher et al., 2012).
Conclusion
It is well known that in vitro–formed embryos initially show highly variable cell division planes and initially lack clear morphological signs of cell patterning and polar organization (Mordhorst et al., 1997). At which point, these structures become embryogenic, and how they differentiate is a major question in plant biology, with implications for the normal development of zygotic embryos. We addressed this question using embryo and auxin cell fate markers in B. napus microspore culture. Our marker data show that, unlike zygotic embryos, irregular cell division patterns or the absence of a suspensor are not necessarily detrimental to microspore embryo development and differentiation. We also show that establishment of the canonical auxin maxima observed in zygotic embryos is not required to establish apical-basal polarity in microspore culture, but rather, is required to establish embryo proper cell fate from the suspensor and to produce functional meristems. If cell fate and patterning during in vitro embryogenesis are flexible processes, then why do many Arabidopsis zygotic embryo patterning mutants fail to develop properly? Embryos that develop in microspore culture are autonomous units, while the growth and differentiation of zygotic embryos needs to be coordinated with that of the surrounding seed tissues, the seed coat and the endosperm. Our results raise the possibility that arrest or abortion of many Arabidopsis patterning mutants is due to failure to coordinate the temporal development of the embryo with that of the maternal and filial tissues. Alternatively, many of the Arabidopsis patterning phenotypes might be the result of secondary gene effects on development, rather than the result of defects in cell division patterns per se.
METHODS
Plant Material and Microspore Culture
Plants of Brassica napus cv Topas lines DH4079 and DH12075 were grown according to and cultured as described by Supena et al. (2008) with a few modifications. DH12075 microspores were cultured at a higher density (50,000 microspores/mL), and microspores were incubated for 1 to 3 d, at 32°C for line DH4079 and at 33.5°C for line DH12075. After the heat stress treatment, microspore cultures were transferred to 25°C for further culture. NPA (Sigma-Aldrich PS343) was dissolved in DMSO and added to NLN-13 medium at the beginning of the culture. The same volume of DMSO was added to control cultures. The plates were refilled with an equal volume of media after 15 d. For regeneration of plants from haploid embryos, 4- to 5-week-old embryos (21 per treatment, three replicates) were transferred to solid B5 medium containing 1% sucrose and cultured at 21°C under a 16-h-light:8-h-dark photoperiod.
Reporter Lines
The proGRP:GUS-GFP reporter line has been previously described (Li et al., 2014). The proDR5:GFP construct (GIIK DR5rev:SV40:33GFP; Weijers et al., 2006) and the Arabidopsis thaliana proPIN1:PIN1-GFP (Benková et al., 2003) and proPIN7:PIN7-GFP (Blilou et al., 2005) constructs were transformed to Agrobacterium tumefaciens strain C58C1 pMP90 and then to B. napus DH12075 (Moloney et al., 1989). All reporter lines showed wild-type phenotypes and embryo yields.
GUS Analysis
GUS staining was performed in GUS staining solution (50 mM sodium phosphate buffer, pH 7.2, containing 10 mM EDTA, 0.1% [v/v] Triton X-100, 2.5 mM potassium ferri- and ferrocyanide, and 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-glucuronic acid; Duchefa) for 2 to 4 h at 37°C. Samples were cleared in solution containing water (30 mL):chloral hydrate (80 g):glycerol (10 mL) and observed using differential interference contrast microscopy (Nikon OPTIPHOT).
Pseudo-Schiff-Propidium Iodide Staining
Modified Pseudo-Schiff-propidium iodide staining was performed as described by Truernit et al. (2008) with modifications. Wild-type or GUS-stained samples were fixed as described, but the incubation step in 80% ethanol at 80°C was omitted. After fixation, samples were rinsed in water and embedded in 0.9% Sea Plaque Agarose (Duchefa). The samples were then treated overnight with α-amylase (0.3 mg/mL) at 37°C. After three washes with water, the samples were incubated in 1% periodic acid for 40 min and then processed further as described (Truernit et al., 2008).
Confocal Microscopy
When indicated, membranes of live material were stained with 10 µg/mL of FM4-64, which was added to the culture medium. Samples were also observed after fixation to allow combined GFP and DAPI imaging. Samples were fixed in MTSB buffer, 4% PFA (Sigma-Aldrich), and 0.1% Triton X-100 at 4°C for at least 24 h, rinsed several times with MTSB buffer diluted 1:10 in water, counterstained with DAPI (1 µg/mL) or propidium iodide (10 µg/mL), and then mounted in Vectashield (Vector Laboratories).
Fluorescence was observed using a Leica DM5500 confocal microscope. FM4-64 and propidium iodide were exited with the 532-nm laser line and fluorescence emission detected in the 617- and 655-nm light band. GFP was excited with the 488-nm laser line and light emission detected between 510 and 530 nm. DAPI was excited with the 405-nm laser line and detected between 458 and 487 nm. Autofluorescence was detected between 659 and 784 nm in samples counterstained with DAPI and between 672 and 713 nm for samples counterstained with propidium iodide or FM4-64.
Time-Lapse Imaging
Microspores were embedded at the start of the culture in SeaPlaque agarose (Duchefa) as follows. Freshly isolated microspores were resuspended at a density of 200,000 microspores/mL in NLN-13 medium. One volume of microspores was mixed with two volumes of a 1:1 mixture of melted 1.8% (w/v) agarose and two-times concentrated NLN-13 medium that was kept at 33°C, and then plated in a thin layer onto gridded µ-Dishes (Ibidi) on a prewarmed electric plate at 33°C. The agarose was allowed to solidify for 10 min at room temperature and then the dishes were filled with 300 μL of semisolid 0.45% Sea Plaque agarose in NLN-13. The plates were inverted for imaging.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL databases under the following accession numbers: GRP, At2g30560; PIN1, At1g73590; and PIN7, At1g23080.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. proGRP:GFP-GUS Expression.
Supplemental Figure 2. Expression of Auxin and Embryo Reporters in B. napus Embryos and Pollen.
Supplemental Figure 3. GRP and PIN1 Expression in Microspore Embryos after Exine Rupture.
Supplemental Figure 4. Expression of proPIN1:PIN1-GFP and proPIN7:PIN7-GFP in Two-Domain, Exine-Enclosed Embryos.
Supplementary Material
Acknowledgments
We thank Mieke Weemen and Tjitske Riksen for technical assistance, Ginette Seguin Schwartz (Agriculture and Agri-Food Canada) for the DH12075 seeds, J. Friml and D. Weijers for the DR5 and PIN reporter constructs, respectively, and J. Hammerlindl for help with the B. napus transformation protocol. This work was funded by grants to K.B. from the Centre for BioSystems Genomics. H.L. was supported by a China Scholarship Council fellowship. The support of COST Action FA0903 “Harnessing Plant Reproduction for Crop Improvement” is acknowledged.
AUTHOR CONTRIBUTIONS
M.S., G.C.A., C.J., R.O., and K.B. designed the research. M.S., H.L., C.J., and J.K. performed research. M.S., G.C.A., R.O., and K.B. wrote the article.
Glossary
- DAPI
4′,6-diamidino-2-phenylindole
- NPA
N-1-naphthylphthalamidic acid
- PAT
polar auxin transport
Footnotes
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Bassuner B.M., Lam R., Lukowitz W., Yeung E.C. (2007). Auxin and root initiation in somatic embryos of Arabidopsis. Plant Cell Rep. 26: 1–11. [DOI] [PubMed] [Google Scholar]
- Bayer M., Nawy T., Giglione C., Galli M., Meinnel T., Lukowitz W. (2009). Paternal control of embryonic patterning in Arabidopsis thaliana. Science 323: 1485–1488. [DOI] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. [DOI] [PubMed] [Google Scholar]
- Berger F., Taylor A., Brownlee C. (1994). Cell fate determination by the cell wall in early fucus development. Science 263: 1421–1423. [DOI] [PubMed] [Google Scholar]
- Berleth T., Jürgens G. (1993). The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118: 575–587. [Google Scholar]
- Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44. [DOI] [PubMed] [Google Scholar]
- Breuninger H., Rikirsch E., Hermann M., Ueda M., Laux T. (2008). Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev. Cell 14: 867–876. [DOI] [PubMed] [Google Scholar]
- Cionini P.G., Bennici A., Alpi A., D’Amato F. (1976). Suspensor, gibberellin and in vitro development of Phaseolus coccineus embryos. Planta 131: 115–117. [DOI] [PubMed] [Google Scholar]
- Custers J.B.M., Cordewener J.H.G., Nöllen Y., Dons H.J.M., Van Lockeren Campagne M.M. (1994). Temperature controls both gametophytic and sporophytic development in microspore cultures of Brassica napus. Plant Cell Rep. 13: 267–271. [DOI] [PubMed] [Google Scholar]
- Fan Z., Armstrong K.C., Keller W.A. (1988). Development of microspores in vivo and in vitro in Brassica napus L. Protoplasma 147: 191–199. [Google Scholar]
- Friml J., Vieten A., Sauer M., Weijers D., Schwarz H., Hamann T., Offringa R., Jürgens G. (2003). Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153. [DOI] [PubMed] [Google Scholar]
- Friml J., et al. (2004). A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306: 862–865. [DOI] [PubMed] [Google Scholar]
- Guillon F., Larré C., Petipas F., Berger A., Moussawi J., Rogniaux H., Santoni A., Saulnier L., Jamme F., Miquel M., Lepiniec L., Dubreucq B. (2012). A comprehensive overview of grain development in Brachypodium distachyon variety Bd21. J. Exp. Bot. 63: 739–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfi K., Speth V., Neuhaus G. (1998). Auxin-induced developmental patterns in Brassica juncea embryos. Development 125: 879–887. [DOI] [PubMed] [Google Scholar]
- Hakman I., Hallberg H., Palovaara J. (2009). The polar auxin transport inhibitor NPA impairs embryo morphology and increases the expression of an auxin efflux facilitator protein PIN during Picea abies somatic embryo development. Tree Physiol. 29: 483–496. [DOI] [PubMed] [Google Scholar]
- Hause B., Vanveenendaal W.L.H., Hause G., Vanlammeren A.A.M. (1994). Expression of polarity during early development of microspore-derived and zygotic embryos of Brassica napus L. cv. Topas. Bot. Acta 107: 407–415. [Google Scholar]
- Heisler M.G., Hamant O., Krupinski P., Uyttewaal M., Ohno C., Jönsson H., Traas J., Meyerowitz E.M. (2010). Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 8: e1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić-Grubor K., Attree S.M., Fowke L.C. (1998). Comparative morphological study of zygotic and microspore-derived embryos of Brassica napus L. as revealed by scanning electron microscopy. Ann. Bot. (Lond.) 82: 157–165. [Google Scholar]
- Jeong S., Palmer T.M., Lukowitz W. (2011). The RWP-RK factor GROUNDED promotes embryonic polarity by facilitating YODA MAP kinase signaling. Curr. Biol. 21: 1268–1276. [DOI] [PubMed] [Google Scholar]
- Johri, B.M., Ambegaokar, K.B., and Srivastava, P.S. (1992). Comparative Embryology of Angiosperms. (Berlin: Springer). [Google Scholar]
- Joosen R., Cordewener J., Supena E.D.J., Vorst O., Lammers M., Maliepaard C., Zeilmaker T., Miki B., America T., Custers J., Boutilier K. (2007). Combined transcriptome and proteome analysis identifies pathways and markers associated with the establishment of rapeseed microspore-derived embryo development. Plant Physiol. 144: 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.R., Cooke T.J. (1997). Fundamental concepts in the embryogenesis of dicotyledons: A morphological interpretation of embryo mutants. Plant Cell 9: 1903–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korasick D.A., Enders T.A., Strader L.C. (2013). Auxin biosynthesis and storage forms. J. Exp. Bot. 64: 2541–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Křeček P., Skůpa P., Libus J., Naramoto S., Tejos R., Friml J., Zažímalová E. (2009). The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 10: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Soriano M., Cordewener J., Muiño J.M., Riksen T., Fukuoka H., Angenent G.C., Boutilier K. (2014). The histone deacetylase inhibitor trichostatin a promotes totipotency in the male gametophyte. Plant Cell 26: 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Xu Z., Chua N.H. (1993). Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W., Roeder A., Parmenter D., Somerville C. (2004). A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell 116: 109–119. [DOI] [PubMed] [Google Scholar]
- Madrid E.N., Friedman W.E. (2010). Female gametophyte and early seed development in Peperomia (Piperaceae). Am. J. Bot. 97: 1–14. [DOI] [PubMed] [Google Scholar]
- Mayer U., Buttner G., Jurgens G. (1993). Apical-basal pattern formation in the Arabidopsis embryo: studies on the role of the gnom gene. Development 117: 149–162. [Google Scholar]
- Moloney M.M., Walker J.M., Sharma K.K. (1989). High efficiency transformation of Brassica napus usingAgrobacterium vectors. Plant Cell Rep. 8: 238–242. [DOI] [PubMed] [Google Scholar]
- Mordhorst A.P., Toonen M.A.J., de Vries S.C., Meinke D. (1997). Plant embryogenesis. Crit. Rev. Plant Sci. 16: 535–576. [Google Scholar]
- Nagl W. (1990). Translocation of putrescine in the ovule, suspensor and embryo of Phaseolus coccineus. J. Plant Physiol. 136: 587–591. [Google Scholar]
- Nakayama N., Smith R.S., Mandel T., Robinson S., Kimura S., Boudaoud A., Kuhlemeier C. (2012). Mechanical regulation of auxin-mediated growth. Curr. Biol. 22: 1468–1476. [DOI] [PubMed] [Google Scholar]
- Rademacher E.H., Lokerse A.S., Schlereth A., Llavata-Peris C.I., Bayer M., Kientz M., Freire Rios A., Borst J.W., Lukowitz W., Jürgens G., Weijers D. (2012). Different auxin response machineries control distinct cell fates in the early plant embryo. Dev. Cell 22: 211–222. [DOI] [PubMed] [Google Scholar]
- Raghavan V. (2004). Role of 2,4-dichlorophenoxyacetic acid (2,4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2,4-D. Am. J. Bot. 91: 1743–1756. [DOI] [PubMed] [Google Scholar]
- Robert H.S., Grones P., Stepanova A.N., Robles L.M., Lokerse A.S., Alonso J.M., Weijers D., Friml J. (2013). Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr. Biol. 23: 2506–2512. [DOI] [PubMed] [Google Scholar]
- Rosquete M.R., Barbez E., Kleine-Vehn J. (2012). Cellular auxin homeostasis: gatekeeping is housekeeping. Mol. Plant 5: 772–786. [DOI] [PubMed] [Google Scholar]
- Rutishauser, A. (1969). Embryology and Reproduction Biology in the Angiosperms. An Introduction. (Vienna, Austria: Springer-Verlag). [Google Scholar]
- Scheres B., Wolkenfelt H., Willemsen V., Terlouw M., Lawson E., Dean C., Weisbeek P. (1994). Embryo origin of the Arabidopsis primary root and root meristem initials. Development 120: 2475–2487. [Google Scholar]
- Schlereth A., Möller B., Liu W., Kientz M., Flipse J., Rademacher E.H., Schmid M., Jürgens G., Weijers D. (2010). MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916. [DOI] [PubMed] [Google Scholar]
- Schwartz B.W., Yeung E.C., Meinke D.W. (1994). Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development 120: 3235–3245. [DOI] [PubMed] [Google Scholar]
- Shaw S.L., Quatrano R.S. (1996). The role of targeted secretion in the establishment of cell polarity and the orientation of the division plane in Fucus zygotes. Development 122: 2623–2630. [DOI] [PubMed] [Google Scholar]
- Steinmann T., Geldner N., Grebe M., Mangold S., Jackson C.L., Paris S., Gälweiler L., Palme K., Jürgens G. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318. [DOI] [PubMed] [Google Scholar]
- Supena E.D.J., Winarto B., Riksen T., Dubas E., van Lammeren A., Offringa R., Boutilier K., Custers J. (2008). Regeneration of zygotic-like microspore-derived embryos suggests an important role for the suspensor in early embryo patterning. J. Exp. Bot. 59: 803–814. [DOI] [PubMed] [Google Scholar]
- Tang X., Liu Y., He Y., Ma L., Sun M.X. (2013). Exine dehiscing induces rape microspore polarity, which results in different daughter cell fate and fixes the apical-basal axis of the embryo. J. Exp. Bot. 64: 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telmer C.A., Newcomb W., Simmonds D.H. (1995). Cellular changes during heat shock induction and embryo development of cultured microspores of Brassica napus cv. Topas. Protoplasma 185: 106–112. [Google Scholar]
- Truernit E., Bauby H., Dubreucq B., Grandjean O., Runions J., Barthélémy J., Palauqui J.-C. (2008). High-resolution whole-mount imaging of three-dimensional tissue organization and gene expression enables the study of phloem development and structure in Arabidopsis. Plant Cell 20: 1494–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykarska T. (1976). Rape embryogenesis. I. The proembryo development. Acta Soc. Bot. Pol. 45: 3–16. [Google Scholar]
- Tykarska T. (1979). Rape embryogenesis. II. Development of embryo proper. Acta Soc. Bot. Pol. 49: 391–421. [Google Scholar]
- Ueda M., Zhang Z., Laux T. (2011). Transcriptional activation of Arabidopsis axis patterning genes WOX8/9 links zygote polarity to embryo development. Dev. Cell 20: 264–270. [DOI] [PubMed] [Google Scholar]
- Vernon D.M., Meinke D.W. (1994). Embryogenic transformation of the suspensor in twin, a polyembryonic mutant of Arabidopsis. Dev. Biol. 165: 566–573. [DOI] [PubMed] [Google Scholar]
- Vieten A., Vanneste S., Wiśniewska J., Benková E., Benjamins R., Beeckman T., Luschnig C., Friml J. (2005). Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531. [DOI] [PubMed] [Google Scholar]
- Weijers D., Sauer M., Meurette O., Friml J., Ljung K., Sandberg G., Hooykaas P., Offringa R. (2005). Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell 17: 2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D., Schlereth A., Ehrismann J.S., Schwank G., Kientz M., Jürgens G. (2006). Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev. Cell 10: 265–270. [DOI] [PubMed] [Google Scholar]
- Yadegari R., Paiva G., Laux T., Koltunow A.M., Apuya N., Zimmerman J.L., Fischer R.L., Harada J.J., Goldberg R.B. (1994). Cell differentiation and morphogenesis are uncoupled in Arabidopsis raspberry embryos. Plant Cell 6: 1713–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E.C., Meinke D.W. (1993). Embryogenesis in angiosperms: development of the suspensor. Plant Cell 5: 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E.C., Rahman M.H., Thorpe T.A. (1996). Comparative development of zygotic and microspore-derived embryos in Brassica napus L cv Topas. I. Histodifferentiation. Int. J. Plant Sci. 157: 27–39. [Google Scholar]
- Zaki M.A.M., Dickinson H.G. (1990). Structural changes during the first divisions of embryos resulting from anther and free microspore culture in Brassica napus. Protoplasma 156: 149–162. [Google Scholar]
- Zhang J.Z., Somerville C.R. (1997). Suspensor-derived polyembryony caused by altered expression of valyl-tRNA synthetase in the twn2 mutant of Arabidopsis. Proc. Natl. Acad. Sci. USA 94: 7349–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.