Abstract
In several mammalian species, lactating females show blunted neural, hormonal, and behavioral responses to stressors. It is not known whether new fathers also show stress hyporesponsiveness in species in which males provide infant care. To test this possibility, we determined the effects of male and female reproductive status on stress responsiveness in the biparental, monogamous California mouse (Peromyscus californicus).Breeding (N=8 females, 8 males), nonbreeding (N=10 females, 10 males) and virgin mice (N=12 females, 9 males) were exposed to a 5-min predator-urine stressor at two time points, corresponding to the early postpartum (5–7 days postpartum) and mid/late postpartum (19–21 days postpartum) phases, and blood samples were collected immediately afterwards. Baseline blood samples were obtained 2 days prior to each stress test. Baseline plasma corticosterone (CORT) concentrations did not differ among male or female groups. CORT responses to the stressor did not differ among female reproductive groups, and all three groups showed distinct behavioral responses to predator urine. Virgin males tended to increase their CORT response from the first to the second stress test, while breeding and nonbreeding males did not. Moreover, virgin and nonbreeding males showed significant behavioral changes in response to predator urine, whereas breeding males did not. These results suggest that adrenocortical responses to a repeated stressor in male California mice may be modulated by cohabitation with a female, whereas behavioral responses to stress may be blunted by parental status.
Keywords: Corticosterone, Lactation, Maternal care, Paternal care, Biparental care, Stress, HPA reactivity, Monogamy
Introduction
Lactational hyporesponsiveness, or blunted responsiveness to stress in lactating females, has been described in a number of mammalian species, including sheep (Ovis aries), flying foxes (Pteropus hypomelanus), Columbian ground squirrels (Spermophilus columbianus), Norway rats (Rattus norvegicus), and humans (Deschamps et al., 2003; Hubbs et al., 2000; Lightman, 1992; Reeder et al., 2004; Shanks et al., 1997; Tilbrook et al., 2006; Tu et al., 2005; Windle et al., 1997a). This phenomenon has been characterized most thoroughly in rats. Compared to virgin females, lactating female rats have been found to exhibit reduced adrenocorticotropic hormone (ACTH) and corticosterone (CORT) responses to a variety of stressors, including immobilization, endotoxins, acoustic startle, predator urine, and open-field tests (Deschamps et al., 2003; Lightman, 1992; Shanks et al., 1997; Toufexis et al., 1999a; Windle et al., 1997a). Lactating rats also exhibit reduced anxiety-like and stress-related behavior in open-field, elevated plus maze, noise stress, and acoustic startle response tests, compared to virgin females (Fleming and Luebke, 1981; Lonstein, 2005; Toufexis et al., 1999b; Windle et al., 1997a).
At the central level, lactating female rats show reduced stress-induced activation of afferent projections to the paraventricular nucleus of the hypothalamus (PVN), which activates the hypothalamic-pituitary-adrenal (HPA) axis through the release of the ACTH secretagogues corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) (da Costa et al., 2001; Toufexis and Walker, 1996; Toufexis et al., 1998). In addition, baseline CRH mRNA levels are lower, and AVP mRNA levels higher, in the PVN of lactating compared to non-lactating female rats (Fischer et al., 1995; Walker et al., 2001), while lactating rats express lower CRH mRNA levels in the PVN in response to hypertonic saline and restraint stress, compared to virgin females (da Costa et al., 2001; Lightman and Young, 1989). Lactating rats have also been found to exhibit reduced pituitary sensitivity to CRH compared to virgin females, thus partly explaining their lower CORT responses to stress (Toufexis et al., 1999a).
The functional significance of neuroendocrine hyporesponsiveness to stress during lactation is unknown. One possibility is that it could protect infants from high glucocorticoid concentrations in the mother's milk (Lightman et al., 2001; Slattery and Neumann, 2008). CORT treatment of rat dams during lactation has long-term effects on HPA activity and anxiety-related behavior in their offspring (Brummelte et al., 2006; Casolini et al., 1997; Catalani et al., 1993, 2000). Another possibility is that reduced peripartum stress responsiveness functions to preserve energy essential for lactation (Numan and Insel, 2003), or to buffer parental behavior from disruption by stressors, as hormones of the HPA axis are thought to suppress parental behavior (Brunton et al., 2008; Carter et al., 2001; Lightman et al., 2001; Rivier and Rivest, 1991; Saltzman and Abbott, 2009; Wingfield and Sapolsky, 2003).
In approximately 6% of mammalian species, fathers, in addition to mothers, engage in parental care (Kleiman and Malcolm, 1981). The neuroendocrine basis of paternal behavior in rodents is unclear, and the role of hormones in the regulation of paternal behavior is currently under debate (Schradin, 2007; Wynne-Edwards and Timonin, 2007). Moreover, little is known of the neuroendocrine and affective consequences for fathers of engaging in paternal behavior. If, as described above, lactational hyporesponsiveness evolved to buffer parental behavior from stress, a reasonable hypothesis is that fathers of dependent offspring, in addition to lactating females, exhibit reduced neuroendocrine and behavioral responses to stress in biparental species.
We tested this hypothesis in a biparental rodent, the California mouse (Peromyscus californicus). California mice are socially and genetically monogamous, and males engage in extensive parental behavior upon the birth of their pups (Gubernick and Alberts, 1987; Ribble, 1991). The presence of the father accelerates pup development and increases pup survival, especially under energetically challenging conditions (Cantoni and Brown, 1997; Dudley, 1974; Gubernick et al., 1993; Gubernick and Teferi, 2000; Wright and Brown, 2002). Importantly, both circulating prolactin concentrations and AVP-ir within the brain have been shown to correlate with paternal behaviors in California mouse fathers (Bester-Meredith and Marler, 2003; Gubernick and Nelson, 1989). Given that both of these peptides are involved in the modulation of stress responsiveness, it is plausible that they may play a dual role in facilitating paternal behavior and ameliorating stress-reactivity in new fathers (Appenrodt et al., 1998; Bales et al., 2004; Everts and Koolhaas, 1999;Landgraf, 2006;Parker and Lee, 2001;Torner et al., 2002).
We characterized behavioral and CORT responses to predator-odor stress in breeding male and female California mice as compared to (1) virgin males and virgin females housed in same-sex pairs, and (2) nonbreeding (vasectomized) males and their female pairmates housed in heterosexual pairs. We chose to use predator urine as a stressor because it is ecologically relevant and because predator odors are known to be potent stressors in a variety of mammalian species (Figueiredo et al., 2003; Kavaliers et al., 2001; Ward et al., 1996; Zhang et al., 2003). Specifically, we used urine from bobcats and coyotes, two predators that are sympatric with California mice through at least part of the latter’s range (including the region from which our colony was derived, the Santa Monica Mountains in southern California), and that prey heavily on rodents (Fedriani et al., 2000). We tested the breeding animals in the presence of their pups to avoid separation-induced stress (Lonstein, 2005); however, conflicting data describe the effect of the infants' presence during exposure to a stressor as either eliminating (rats: Deschamps et al., 2003) or further accentuating (sheep: Tilbrook et al., 2006) neuroendocrine hyporesponsiveness in mothers.
Previous studies of stress hyporesponsiveness in other species focused on females that were either pregnant or lactating, but not both (Lightman and Young, 1989; Shanks et al., 1999; Walker et al., 1992, 1995). In California mice and many other rodents, however, females commonly undergo a postpartum estrus and conceive shortly after giving birth, so that pregnancy and lactation coincide (Gubernick, 1988). Therefore, we characterized stress responsiveness in female California mice that were concurrently pregnant and lactating, in order to study the animals under more naturalistic reproductive conditions. In rats, the stage of lactation affects females' HPA responses to stress; early lactating females (≤1 week postpartum) display higher CORT and ACTH responses to stress than mid-lactating females (approximately 2 weeks postpartum; Deschamps et al., 2003; Walker et al., 1995). We therefore tested breeding pairs during both the females' early (5–7 days) and mid/late (19–21 days) postpartum periods, which coincided with early- and mid-pregnancy.
Materials and methods
Animals
We used male and female California mice purchased as adults from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC, USA). Mice were maintained as described previously (de Jong et al., 2009, 2010). Briefly, animals were housed in transparent, 44×24×20 cm polycarbonate cages, with aspen shavings as bedding, cotton wool as nesting material, and food (Purina Rodent Chow 5001) and water available ad libitum. Lights were on from 0500 h to 1900 h (14:10), and room temperature and humidity were maintained at approximately 18–26 °C and 60–70%, respectively. Animals were inspected daily and weighed twice per week to monitor health and pregnancies, and cages and water bottles were changed once per week. Mice had been weaned at 25–35 days of age, initially housed in same-sex groups of 2–3 animals, and then housed in same-sex pairs upon their arrival in our lab until the start of the study. Animals had been ear-punched at weaning, and were marked with non-toxic hair color (Bigen, Hoyu Co. Ltd., Nagoya, Japan) at the start of the study for rapid identification. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of California, Riverside (UCR) IACUC. UCR is fully accredited by AAALAC.
Design
Male and female mice were randomly assigned to three reproductive groups (same-sex siblings were assigned to different conditions): breeding pairs, nonbreeding pairs, and virgins. Breeding pairs (N=8) comprised a female (breeding females) and sham-vasectomized male (breeding males). Nonbreeding pairs (N=10) comprised a female (nonbreeding females) and vasectomized male (nonbreeding males). Virgin males (sham-vasectomized; N=9) and virgin females (N=12) were maintained in unrelated same-sex pairs from the time of arrival in our laboratory and throughout the experiment.
Each pair of mice underwent two stress tests (see below) separated by 14 days, with each test immediately followed by collection of a blood sample; a baseline blood sample was collected two days before each stress test. Breeding males and breeding females underwent their first baseline blood sample 2–5 days after the birth of their first litter of pups. Breeding females gave birth to their first litter 42.3±6.3 days after pair formation (mean±SE), and all but two gave birth to their second litter 14–23 (16.2±3.0) days after the second stress test. The schedule of baseline blood samples and stress tests for nonbreeding males, nonbreeding females, virgin males and virgin females was established by matching each nonbreeding or virgin pair to specific breeding pairs. Cohorts of animals containing mice from each reproductive group were tested concurrently. Time from pair formation (first day of pair-housing) to the first baseline blood sample did not differ between breeding (48.8±6.2 days) and nonbreeding (44.1±2.5 days) pairs (P=0.463).
Mean age of males (381.4±16.1 days) at the time of the first stress test did not differ significantly among the three reproductive groups (P=0.259), and there were no significant differences in the mean length of time between surgery and the first stress test (61.4±7.4 days) across male reproductive groups (P=0.147). Mean age of females, however, differed among breeding (385.1±20.4 days), nonbreeding (312.9±12.0 days), and virgin (341.4±12.9 days) females (F[2,29]= 5.375, P=0.011). Breeding females were significantly older than nonbreeding females (P=0.009), while virgin females did not differ significantly from the other two groups.
Surgeries
Vasectomies and sham vasectomies were performed using sterile conditions and standard surgical procedures. Mice were anesthetized with isoflurane, a ventral midline incision (approximately 1 cm) was made, the vas deferens was sutured and cut, and the incision was closed with tissue glue. Ketoprofen (5 mg/kg, s.c.) was administered at the completion of the surgery to provide analgesia. Following one week of recovery, animals were reunited with their cagemates.
Stress tests
Data collection was divided into two phases, with phase 1 corresponding to the early postpartum period in breeding pairs and phase 2 corresponding to the mid/late postpartum period, two weeks later. In each of the two phases, a baseline blood sample was obtained, a stress test was performed, and a post-stress blood sample was collected from each mouse. Animals in all reproductive groups were tested with their pairmate present, and breeding males and breeding females were tested with their pups also present (1–4 pups per litter). For each stress test, the pair (or family) of mice was placed in a clean cage with bedding, cotton, and a plastic cup (diameter: 4 cm×height: 2 cm) containing a clean cotton ball, and taken to an unfamiliar room containing no other animals. Animals were allowed to acclimate to the environment for 5 min, after which two trained observers (inter-observer reliability scores >90%) each scored the behavior of one focal animal for 5 min on laptop computers using the JWatcher event-recorder program (Blumstein and Daniel, 2007). The cup containing a clean cotton ball was then replaced with an identical cup containing a cotton ball soaked with 1 ml predator urine (see below), and behavioral observations were continued for an additional 5 min. Duration of time that mice spent within 3 cm of the plastic cup (distance measured with reference to a 3 cm ruler), auto-grooming, grooming its cagemate, and freezing, as well as number of jumps, was scored continuously for all focal animals before and during presentation of predator urine. In addition, every 30 s, upon an audible signal from a timer, the observers recorded whether or not each test subject was in proximity to its cagemate, and whether it was immobile or locomoting. The length of the cage was demarcated into three equal zones, and the zone in which each subject was located (zone containing the cup, zone furthest from the cup, or intermediate zone) was scored on every 30-s scan. For breeding animals, duration of time spent licking, in proximity to (within 3 cm), and nursing the pups was also scored.
Bobcat and coyote urine (Predatorpee.com, Lexington Outdoors, Robbinston, ME, USA) were used as predator odors in a stress-test protocol modified from that of Deschamps et al. (2003). The urine was collected through floor drains from bobcats and coyotes housed in game farms and refuges, and following collection, was filtered and stored at room temperature. Each pair of mice was exposed to one odor in its first stress test and the other odor in its second stress test. The order of odor presentations was randomly balanced across pairs within each reproductive group. We wiped down the testing area with 70% alcohol immediately following each test; nonetheless, we cannot rule out the possibility that animals may have been exposed to lingering predator urine scents from previous tests. All procedures were carried out between 0900 h and 1100 h, when baseline plasma CORT levels in our mice are low and incremental responses to stress are pronounced (unpub. data).
Blood sampling
Mice were anesthetized with isoflurane, and blood samples of up to 140 µl were collected from the retro-orbital sinus into heparinized microhematocrit tubes. All blood samples, except one, were obtained within 3 min of initial disturbance to the animals (baseline samples) or within 3 min following the end of a stress test (post-test samples) to prevent the capture procedures from influencing CORT levels (Good et al., 2003). The remaining blood sample was obtained in 3 min 40 s following the end of a stress test; however, the CORT concentration of this sample fell within the range of other post-stress blood samples in this study. Blood was centrifuged at 13,200 rpm for 12 min at 4 °C, and plasma was aspirated and stored at −80 °C until assayed.
Corticosterone assays
Corticosterone concentrations in plasma (100 µl of a 1:400 dilution) were determined using a double-antibody 125I-radioimmunoassay kit (MP Biomedicals, Costa Mesa, CA, USA). All samples were assayed in duplicate at the recommended volume. The kit standards generate a curve adequate to measure CORT concentrations between 25 and 1000 ng/ml. Preliminary data indicated that plasma CORT concentrations range from 40 to 2000 ng/ml in the California mouse. To accommodate this range we diluted plasma 1:400 and extended the lower end of the standard curve to 12.5 ng/ml by diluting the provided 25 ng/ml standard by half.
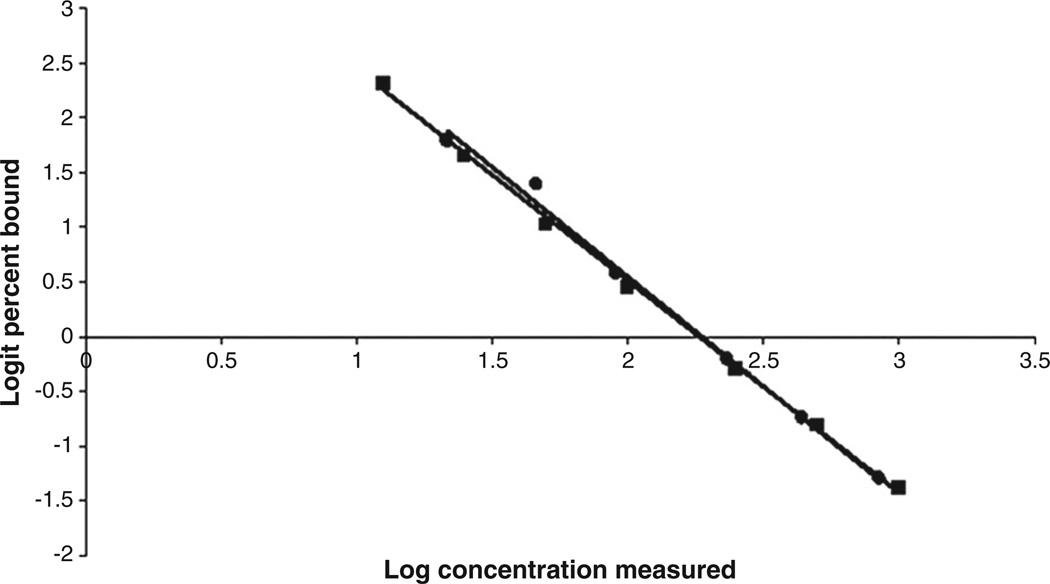
To assess the biochemical validity of the kit with California mouse plasma, we performed three validation procedures, parallelism, accuracy, and precision, following the methods of Harper and Austad (2000) and Good et al. (2003). In brief, parallelism was determined by comparing the log-logit slope of serially diluted California mouse plasma to the log-logit slope of the standard curve (see Fig. 1). Slope equality was determined using linear regression in GraphPad Prism (version 5, GraphPad Software, Inc., La Jolla, CA, USA). The log-logit-transformed slope of the standard curve was statistically indistinguishable (P=0.363) from the log-logit-transformed slope of serially diluted California mouse plasma. Accuracy samples were generated by adding 10 µl of California mouse plasma with a known CORT concentration to each point on the standard curve. Accuracy was determined by comparing the observed CORT concentration in each sample to the expected concentration. Average assay accuracy was 99.1%, and the difference between the observed and expected CORT concentrations per tube was statistically indistinguishable from zero (P=0.620). Assay precision was determined by calculating intra-assay variation as the coefficient of variation (CV) between replicate samples in an assay. Inter-assay variation was determined by calculating the CV for the average CORT levels in a laboratory-maintained California mouse plasma pool. The intra- and inter-assay CVs were 4.2% and 5.1%, respectively. All samples from each animal were run in the same assay, and each assay included samples from at least one animal from each sex and reproductive condition.
Fig. 1.
Parallelism of corticosterone levels from serial dilutions of California mouse plasma (circles) and standards from a commercially available double-antibody 125I-radioimmunoassay kit (squares). Data were transformed using the log-logit method. California mouse plasma: y=−1.9966x+4.5394. Standard curve: y=−1.9243x+4.3507. Difference between slopes: P=0.363.
Data analysis
CORT data were log-transformed to achieve normality and analyzed by 3-way repeated-measures ANOVA for each sex separately, with reproductive group (breeding, nonbreeding, virgin) as a between-groups factor, and phase (early postpartum [phase 1], mid/late postpartum [phase 2]) and baseline vs. post-test (basal_test) as within-groups factors. We report both main effects and interactions from each ANOVA in the Results section. CORT data were tested for outliers (Sokal and Rohlf, 1995), and test subjects with outlying or missing hormonal data were removed from the analysis. Final numbers of animals used in CORT analyses were 8 breeding males, 9 nonbreeding males, 5 virgin males, 7 breeding females, 9 nonbreeding females, and 11 virgin females.
All behavioral scores, except jumping, are expressed as percentages of total observation duration or percentages of total number of 30-s instantaneous scans; jumps are expressed as total number of occurrences within each 5-min testing period before or during predator-urine exposure. Behavioral data were compared among groups nonparametrically, using the Kruskal–Wallis test; significant overall effects were followed by nonparametric pairwise post-hoc comparisons (Siegel and Castellan, 1988). Within reproductive groups, behavior was compared between phases and before vs. during predator-urine exposure using Wilcoxon Signed Ranks tests. Statistical analyses were performed using SPSS (IBM Corporation, Somers, NY, USA) and Systat (Systat Software, Inc., Chicago, IL, USA), and were evaluated at the 0.05 level (2-tailed).
Results
Males
Corticosterone
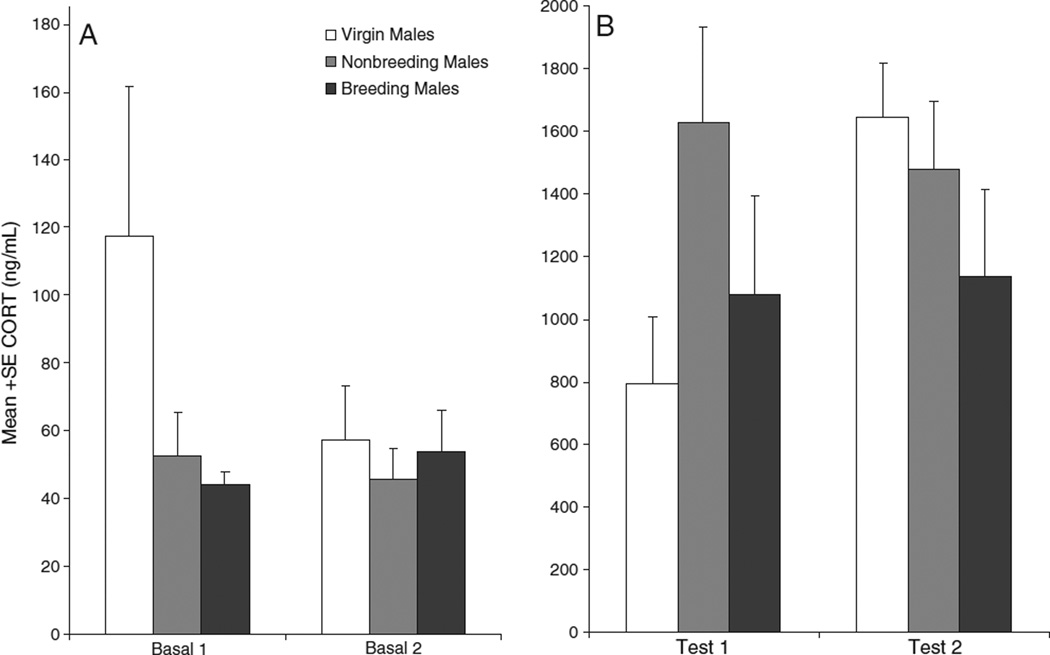
Initial comparisons of baseline plasma CORT concentrations revealed that baseline levels did not differ among breeding, nonbreeding, and virgin males (main effect of group: F[2,19]=1.463, P=0.256) or between phase 1 and phase 2 (main effect of phase: F[1,19]=3.956, P=0.061; group×phase interaction: F[2,19]=1.984, P=0.165; Fig. 2A). Predator-urine tests significantly elevated plasma CORT above basal levels in male California mice (main effect of basal_test: F[1,19]= 405.649, P<0.001; compare Figs. 2A and B; note different y-axes). The magnitude of the CORT response to stress relative to basal levels differed between phase 1 and phase 2 (basal_test ×phase interaction: F[1,19]= 10.635, P=0.004), and this effect differed among the three groups of males (group×basal_test×phase interaction: F[2,17] =3.901, P=0.031). Virgin males tended to increase the magnitude of their CORT response to stress from phase 1 to phase 2 (F[1,4]=5.712, P=0.075), while breeding and nonbreeding males did not (breeding males: F[1,7]=0.118, P=0.745; nonbreeding males: F[1,8]=1.485, P =0.258; Fig. 2B).
Fig. 2.
Plasma corticosterone concentrations of breeding, nonbreeding and virgin male California mice in phase 1 and phase 2. A: Baseline CORT concentrations; B: CORT concentrations immediately following exposure to predator urine. CORT levels were significantly higher after predator-urine exposure than under baseline conditions (P<0.001). A group×phase×basal_test interaction (P=0.031) revealed that the change in CORT responses to stress from test 1 to test 2 differed among groups. Virgin males tended to increase their CORT response to stress from phase 1 to phase 2 (P=0.075), whereas breeding and nonbreeding males did not. Note difference between graphs in y-axis scale.
Planned comparisons revealed that virgin males had a significantly greater increase in the magnitude of the stress response from phase 1to phase 2, as compared to nonbreeding males (group×basal_test×phase interaction: F[1,12]=6.412, P=0.026), and tended to have a greater increase in the magnitude of the stress response as compared to breeding males (group×basal_test×phase interaction: F[1,11]=4.620, P=0.055).
Behavior
Locomotion, autogrooming, approaches to within 3 cm of the cup (containing a clean or predator-urine-soaked cotton ball), proximity to cagemate, grooming cagemate, and freezing behavior did not differ among groups of males in either phase 1 or phase 2, either before or during predator-urine exposure (Table 1). To determine whether repetition of the stressor or exposure to predator urine affected behavior, we compared behavior in the 5 min before vs. the 5 min during predator-urine exposure, as well as in phase 1 vs. phase 2, using Wilcoxon tests for each group of males. Phase did not significantly influence behavior either during or before urine exposure in any of the groups of males (see Table 1). Predator urine did, however, affect behavior in the nonbreeding and virgin male groups. During predator-urine exposure, as compared to the 5 min prior, virgin and nonbreeding males spent significantly less time in proximity to the cup (nonbreeding males, phase 1: Z=−2.380, P=0.017, N=10; nonbreeding males, phase 2: Z=−2.547, P=0.011, N=9; virgin males, phase 2: Z= −2.201, P=0.028, N=8), and less time autogrooming (virgin males, phase 1: Z=2.366, P=0.018, N=9; nonbreeding males, phase 2: Z= −2.521, P=0.012, N=9). In contrast, no differences were found between behaviors before and during stressor exposure in breeding males. Breeding males did not differ in the duration of time spent licking their pup(s) or in proximity to their pup(s) before vs. during predator-urine exposure (Table 3).
Table 1.
Behavior (median, range) before and during exposure to predator urine in breeding (N=8), nonbreeding (N=9–10), and virgin (N=8–9) male California mice
| Behavior | Breeding males |
Nonbreeding males |
Virgin males |
Kruskal–Wallis, χ2 |
P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | |
| Within 3 cm of cupa | ||||||||||
| Pre-stress | 2.2 (0.6, 18.5) | 7.0 (0.4, 17.1) | 6.0 (0.0, 47.4) | 5.2 (0.78, 14.0) | 2.6 (0.0, 50.6) | 1.5 (0.0, 14.6) | 0.065 | 3.707 | 0.968 | 0.157 |
| During stress | 4.0 (0.0, 15.2) | 1.6 (0.0, 12.5) | 0.7b (0.0, 19.7) | 0.4b (0.0, 3.4) | 1.5 (0.0, 11.3) | 0.2b (0.0, 4.4) | 2.396 | 1.783 | 0.302 | 0.410 |
| Autogrooma | ||||||||||
| Pre-stress | 0.9 (0.0, 7.0) | 1.5 (0.0, 22.1) | 1.9 (0.0, 7.2) | 3.6 (0.0, 21.0) | 2.8 (0.0, 18.9) | 1.7 (0.0, 17.8) | 2.864 | 2.332 | 0.239 | 0.312 |
| During stress | 0.4 (0.0, 7.5) | 0.1 (0.0, 3.9) | 0.0 (0.0, 2.9) | 0.0b (0.0, 0.0) | 0.0b (0.0, 4.3) | 0.0 (0.0, 4.0) | 3.142 | 5.248 | 0.208 | 0.073 |
| Groom partnera | ||||||||||
| Pre-stress | 0.0 (0.0, 0.9) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 1.9) | 0.0 (0.0, 0.0) | 2.368 | 0.000 | 0.306 | 1.000 |
| During stress | 0.0 (0.0, 2.8) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 2.375 | 0.000 | 0.305 | 1.000 |
| Freezea | ||||||||||
| Pre-stress | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.3) | 0.0 (0.0, 1.7) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.6) | 2.817 | 1.895 | 0.244 | 0.388 |
| During stress | 0.2 (0.0, 32.6) | 0.0 (0.0, 2.8) | 0.0 (0.0, 21.5) | 0.3 (0.0, 13.6) | 0.0 (0.0, 2.9) | 0.6 (0.0, 26.1) | 1.503 | 1.342 | 0.472 | 0.511 |
| Jumpc | ||||||||||
| Pre-stress | 29.0 (0.0, 93.0) | 19.0 (2.0, 86.0) | 29.5 (0.0, 52.0) | 25.0 (3.0, 86.0) | 12.0 (0.0, 83.0) | 22.5 (0.0, 134.0) | 3.605 | 0.051 | 0.165 | 0.975 |
| During stress | 40.5 (0.0, 99.0) | 15.5 (0.0, 63.0) | 35.0 (0.0, 139.0) | 20.0 (0.0, 258.0) | 15.0 (0.0, 88.0) | 5.5 (0.0, 100.0) | 0.590 | 0.151 | 0.745 | 0.927 |
| Locomotiond | ||||||||||
| Pre-stress | 40.0 (0.0, 60.0) | 40.0 (0.0, 70.0) | 30.0 (0.0, 60.0) | 30.0 (0.0, 70.0) | 10.0 (0.0, 100.0) | 5.0 (0.0, 40.0) | 1.840 | 5.303 | 0.398 | 0.071 |
| During stress | 8.9 (0.0, 100.0) | 11.1 (0.0, 66.7) | 50.0 (0.0, 66.7) | 22.2 (0.0, 66.7) | 11.1 (0.0, 88.9) | 0 (0.0, 66.7) | 2.279 | 1.865 | 0.320 | 0.394 |
| Proximity to partnerd | ||||||||||
| Pre-stress | 80.0 (10.0, 90.0) | 75.0 (40.0, 100.0) | 60.0 (0.0, 90.0) | 70.0 (20.0, 100.0) | 60.0 (40.0, 100.0) | 85.0 (30.0, 90.0) | 0.368 | 0.501 | 0.832 | 0.779 |
| During stress | 72.3 (0.0, 100.0) | 66.7 (0.0, 88.9) | 72.3 (0.0, 100.0) | 77.8 (0.0, 100.0) | 66.7 (33.3, 88.9) | 61.1 (11.1, 100.0) | 0.116 | 0.721 | 0.944 | 0.697 |
| In cage zone furthest from predator urined | ||||||||||
| Pre-stress | 40.0 (10.0, 90.0) | 50.0 (20.0, 70.0) | 40.0 (10.0, 100.0) | 60.0 (0.0, 100.0) | 60.0 (0.0, 100.0) | 70.0 (20.0, 100.0) | 0.324 | 1.434 | 0.850 | 0.488 |
| During stress | 38.9 (0.0, 100.0) | 66.7 (11.1, 100.0) | 33.3 (11.1, 100.0) | 44.4b (11.1, 100.0) | 66.7 (33.3, 100.0) | 94.5 (11.1, 100.0) | 3.543 | 1.313 | 0.170 | 0.519 |
Percent of time during the 5-min period before or during predator-urine exposure.
Significantly different from pre-stress scores for the same group of males (P<0.05).
Total number of occurrences.
Percent of 30-s instantaneous scans during the 5-min period before or during predator-urine exposure.
Table 3.
Parental behavior (median, range) before and during exposure to predator urine in breeding males (N=8) and breeding females (N=8).
| Behavior | Breeding males |
Breeding females |
||
|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 1 | Phase 2 | |
| Lick pup(s)a | ||||
| Pre-stress | 0.0 (0.0, 6.7) | 0.0 (0.0, 2.4) | 6.3 (0.0, 35.1) | 3.1 (0.0, 59.3) |
| During stress | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 1.8 (0.0, 36.5) | 0.2 (0.0, 44.19) |
| Nursinga | ||||
| Pre-stress | – | – | 99.7 (98.6, 99.8) | 99.7 (98.0, 99.7) |
| During stress | – | – | 99.7 (99.1, 100.0) | 99.7 (99.6, 100.0) |
| Proximity to pup (s)b | ||||
| Pre-stress | 25.0 (0.0, 100.0) | 30.0 (0.0, 70.0) | 100.0 (90.0, 100.0) | 95.0 (40.0, 100.0) |
| During stress | 27.8 (0.0, 100.0) | 33.3 (0.0, 100.0) | 100.0 (100.0, 100.0) | 100.0 (88.9, 100.0) |
Percent of time during the 5-min period before or during predator-urine exposure.
Percent of 30-s instantaneous scans during the 5-min period before or during predator-urine exposure.
Females
Corticosterone
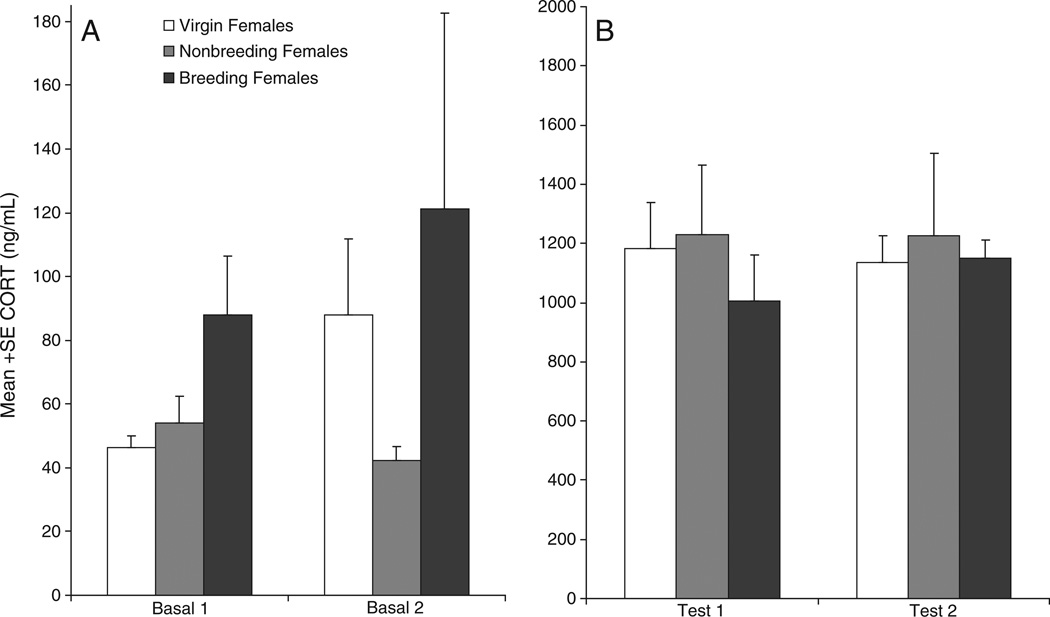
Initial comparisons of baseline plasma CORT concentrations revealed that CORT levels did not differ among breeding, nonbreeding, and virgin females (main effect of group: F[2,23] = 0.446, P = 0.645) or between phase 1 and phase 2 (main effect of phase: F[1,23] = 0.746, P = 0.397; group × phase interaction: F[2,23] = 1.417, P = 0.263; Fig. 3A). Exposure to predator urine significantly raised females' plasma CORT concentrations above basal levels (main effect of basal_test: F[1,23] = 9.277, P<0.001) (compare Figs. 3A and B; note different y-axes). However, neither overall CORT levels nor the difference between basal and post-stress CORT levels differed among breeding, nonbreeding, and virgin females (main effect of group: F[2,23] = 0.871, P = 0.432; group × basal_test interaction: F[2,23] = 0.181, P =0.836) or between phases (main effect of phase: F[1,23]= 0.086, P=0.771; phase×basal_test interaction: F[1,23]=0.071, P=0.291) (Fig. 3B). Because female groups differed significantly in age (see Materials and methods), age was used as a covariate in the analyses; however, no significant effects of age were found.
Fig. 3.
Plasma corticosterone concentrations of breeding, nonbreeding and virgin female California mice in phase 1 and phase 2. A: Baseline CORT concentrations; B: CORT concentrations immediately following exposure to predator urine. CORT levels were significantly higher after predator-urine stress exposure than under baseline conditions (P<0.001) but did not differ between phases or among groups. Note difference between graphs in y-axis scale.
Behavior
Locomotion, approaches to within 3 cm of the cup, proximity to cagemate, grooming cagemate, and freezing behavior did not differ among groups of females in either phase 1 or phase 2, either before or during predator-urine exposure (Table 2). >During phase 1, however, female reproductive groups differed in the amount of time spent autogrooming during stressor exposure (χ2 = 6.022, P=0.049). Pairwise post-hoc comparisons revealed that breeding females engaged in more autogrooming than virgin females (Z= −2.007, P=0.045), while nonbreeding females did not differ from either of these groups.
Table 2.
Behavior (median, range) before and during exposure to predator urine in breeding (N=8), nonbreeding (N=9–10), and virgin (N=12) female California mice.
| Behavior | Breeding females |
Nonbreeding females |
Virgin females |
Kruskal–Wallis, χ2 |
P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | Phase 1 | Phase 2 | |
| Within 3 cm of cupa | ||||||||||
| Pre-stress | 7.6 (0.0, 40.8) | 5.7 (0.0, 18.8) | 4.9 (0.0, 14.1) | 2.3 (0.0, 8.4) | 3.3 (0.0, 32.8) | 5.1 (0.3, 22.1) | 0.669 | 4.756 | 0.716 | 0.093 |
| During stress | 2.5 (0.0, 59.4) | 0.1 (0.0, 5.0) | 0.5 (0.0, 7.8) | 0.8 (0.0, 4.9) | 2.7 (0.0, 17.3) | 0.9b (0.0, 20.1) | 1.679 | 0.988 | 0.432 | 0.610 |
| Autogrooma | ||||||||||
| Pre-stress | 2.0 (0.0, 8.8) | 1.4 (0.0, 6.0) | 3.6 (0.0, 8.8) | 2.8 (0.0, 17.9) | 1.2 (0.0, 4.9) | 2.1 (0.0, 6.6) | 0.510 | 0.484 | 0.775 | 0.785 |
| During stress | 1.1 (0.0, 6.2) | 0.0 (0.0, 46.5) | 0.0b (0.0, 5.2) | 0.0 (0.0, 20.1) | 0.0 (0.0, 4.4) | 0.2 (0.0, 5.2) | 6.022 | 0.188 | 0.049 | 0.910 |
| Groom partnera | ||||||||||
| Pre-stress | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 1.3) | 0.0 (0.0, 10.7) | 0.0 (0.0, 4.9) | 0.0 (0.0, 0.0) | 0.762 | 2.222 | 0.683 | 0.329 |
| During stress | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 5.2) | 0.0 (0.0, 16.9) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 2.000 | 2.222 | 0.368 | 0.329 |
| Freezea | ||||||||||
| Pre-stress | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.3) | 0.0 (0.0, 0.8) | 0.0 (0.0, 16.9) | 0.0 (0.0, 5.9) | 0.0 (0.0, 0.2) | 0.224 | 1.126 | 0.894 | 0.569 |
| During stress | 1.0 (0.0, 18.2) | 0.4 (0.0, 27.9) | 0.2 (0.0, 37.6) | 1.0b (0.0, 25.6) | 0.0 (0.0, 0.6) | 0.1b (0.0, 5.5) | 2.021 | 0.544 | 0.364 | 0.762 |
| Jumpc | ||||||||||
| Pre-stress | 0.0 (0.0, 50.0) | 2.0 (0.0, 91.0) | 4.5 (0.0, 81.0) | 7.0 (0.0, 119.0) | 13.5 (0.0, 84.0) | 5.0 (0.0, 120.0) | 3.894 | 1.137 | 0.143 | 0.566 |
| During stress | 0.0 (0.0, 137.0) | 0.0 (0.0, 31.0) | 19.5 (0.0, 110.0) | 25.0 (0.0, 83.0) | 2.0 (0.0, 92.0) | 3.0 (0.0, 241.0) | 2.872 | 4.341 | 0.238 | 0.114 |
| Locomotiond | ||||||||||
| Pre-stress | 20.0 (0.0, 60.0) | 35.0 (0.0, 70.0) | 30.0 (0.0, 70.0) | 30.0 (0.0, 50.0) | 40.0 (0.0, 100.0) | 20.0 (0.0, 70.0) | 2.217 | 0.705 | 0.330 | 0.703 |
| During stress | 0.0 (0.0, 77.8) | 5.6 (0.0, 55.6) | 22.2 (0.0, 77.8) | 22.2 (0.0, 55.6) | 33.3b (0.0, 88.9) | 5.6e (0.0, 77.8) | 0.920 | 0.634 | 0.631 | 0.728 |
| Proximity to partnerd | ||||||||||
| Pre-stress | 80.0 (10.0, 90.0) | 75.0 (40.0, 100.0) | 60.0 (0.0, 90.0) | 70.0 (20.0, 100.0) | 90.0 (40.0, 100.0) | 75.0 (30.0, 90.0) | 2.235 | 0.471 | 0.327 | 0.790 |
| During stress | 72.3 (0.0, 100.0) | 66.7 (0.0, 88.9) | 72.3 (0.0, 100.0) | 77.8 (0.0, 100.0) | 77.8 (11.1, 100.0) | 66.7 (0.0, 88.9) | 0.143 | 1.023 | 0.931 | 0.600 |
| In cage zone furthest from predator urined | ||||||||||
| Pre-stress | 45.0 (0.0, 100.0) | 30.0 (0.0, 80.0) | 55.0 (20.0, 100.0) | 60.0 (10.0, 100.0) | 50.0 (0.0, 100.0) | 50.0 (10.0, 90.0) | 0.088 | 2.534 | 0.957 | 0.282 |
| During stress | 66.7 (22.2, 100.0) | 72.3b (33.3, 100.0) | 44.5 (0.0, 100.0) | 66.7 (0.0, 100.0) | 55.6 (11.1, 100.0) | 83.4e (0.0, 100.0) | 0.390 | 0.158 | 0.823 | 0.924 |
Bold indicates statistically significant among-groups comparisons.
Percent of time during the 5-min period before or during predator-urine exposure.
Significantly different from pre-stress scores for the same group of females (P<0.05).
Total number of occurrences.
Percent of 30-s instantaneous scans during the 5-min period before or during predator-urine exposure.
Significantly different from phase 1 scores for the same group of females (P<0.05).
Phase did not significantly influence behavior in breeding and nonbreeding females. In contrast, virgin females engaged in more locomotor activity during stressor exposure in phase 1 than in phase 2 (Z=−2.588, P=0.010, N=12), and spent more time in the cage zone furthest from the predator urine during stressor exposure in phase 2 than in phase 1 (Z=−2.106, P=0.035, N=12). Acute exposure to predator urine altered behavior in all three groups of females. During predator-urine exposure, as compared to the preceding 5 min, virgin females significantly reduced their locomotor activity (phase 1: Z=−2.296, P=0.022, N=12) and proximity to the cup (phase 2: Z=−2.667, P=0.008, N=12), nonbreeding females decreased the amount of time spent autogrooming (phase 1: Z=−1.992, P=0.046, N=10), and both virgin and nonbreeding females increased the amount of time spent freezing compared to their behavior before stressor exposure (phase 2, virgin females: Z=−2.197, P=0.028, N=12; phase 2, nonbreeding females: Z=−1.992, P=0.046, N=9). Breeding females spent more time in the cage zone furthest from the cup during predator-urine exposure, compared to the preceding 5 min (phase 2: Z=−2.240, P=0.025, N=8; see Table 2), but maternal behaviors (licking the pup(s), proximity to the pup(s), and nursing) were not affected by predator-urine exposure (Table 3).
Discussion
The present study is among the first to investigate the effects of reproductive state on responses to acute stress in males of a biparental mammalian species. We predicted that we would find blunted CORT and behavioral responses to a predator-urine stressor in new fathers and mothers, based on the hypothesis that reduced stress responsiveness found in lactating mammals may have evolved to protect parental behavior from disruption by stressors. We found partial support for this hypothesis: virgin males tended to increase their CORT response from the first to the second stress test, while breeding and nonbreeding males did not. In addition, virgin and nonbreeding males showed significant behavioral changes in response to predator urine, whereas breeding males did not. In contrast, no differences were found in CORT responses to stress in females, and all female groups showed behavioral changes in response to predator urine. Our findings point to a role of reproductive status in the modulation of behavioral and endocrine stress responsiveness in males, but do not provide evidence for an effect of reproductive state on stress responsiveness in female California mice.
Baseline corticosterone levels
Initially, we compared baseline CORT levels among reproductive groups within each sex. Breeding females tended to have higher mean baseline CORT concentrations than virgin and nonbreeding females during both the early lactational period and the late lactational period, but these differences did not reach statistical significance. Elevated baseline levels of CORT have been found in lactating female rats (Fischer et al., 1995; Stern et al., 1973; Walker et al., 1995) and have been attributed to the high energetic costs of lactation (Walker et al., 1992). The breeding females in the current study were simultaneously lactating and pregnant, however, and in other rodents, early-pregnant females have low baseline CORT levels (Ogle and Kitay, 1977; Stefanski et al., 2005; Waddell and Atkinson, 1994). Thus, our use of breeding females that were simultaneously pregnant and lactating could have been responsible for the lack of significant differences in baseline CORT among female groups in the current study.
Baseline plasma CORT concentrations did not differ among breeding, nonbreeding and virgin males. This finding suggests that fatherhood, pair bonds, and same-sex relationships do not influence baseline CORT differentially in male California mice. This is similar to findings in prairie voles (Microtus ochrogaster), in which baseline CORT levels did not differ between isolated males and males housed with a female pairmate (DeVries et al., 1997), but contrasts with findings from Glasper and DeVries (2005) in which male California mice paired with an ovariectomized female had significantly lower baseline CORT than isolated males. In that study, social isolation, rather than the absence of a female pairmate, might have resulted in elevated baseline CORT levels (McCormick et al., 2006; Ruscio et al., 2007). In biparental cotton-top tamarins (Saguinus oedipus), baseline urinary cortisol levels were not found to differ between new fathers and males paired with a female (Ziegler et al., 1996), although in biparental black tufted-ear marmosets (Callithrix kuhlii), males' baseline urinary cortisol levels increased after the birth of their first litter (Nunes et al., 2001). Altogether, these findings suggest that being a father does not influence baseline CORT levels consistently across species, and that paternal behavior may be relatively disengaged from hormones of the HPA axis, as has been proposed previously (Wynne-Edwards and Timonin, 2007).
Baseline CORT concentrations in California mice in this study were considerably lower than those in other studies of the same species (Glasper and Devries, 2005; Oyegbile and Marler, 2006; Trainor et al., 2010). Differences in methodology might explain this disparity. In the Glasper and Devries (2005) study, baseline plasma samples were obtained from animals that had previously been subject to wounding procedures; however, the times of day at which wounding and blood collection were performed, as well as the latency from disturbance to blood collection, were not specified. Without this information, it is difficult to compare baseline CORT measures in the two studies. Predictably high baseline CORT levels were found by Oyegbile and Marler (2006) and Trainor et al. (2010) shortly after lights out, corresponding to the rise in CORT around the time of waking in nocturnal rodent species (Dallman et al., 1993; Malisch et al., 2008; unpub. data). Furthermore, Trainor et al. (2010) obtained baseline plasma samples >7 min following initial disturbance to the animals. In contrast, baseline blood samples in the present study were collected several hours after lights-on, near the nadir of the daily CORT rhythm (Dallman et al., 1993; Malisch et al., 2008; unpub. data), and were collected within 3 min of initial disturbance to the animals.
Predator-urine stressor
Predator odor is an ecologically relevant stressor that represents a threat to the individual mouse and its pups, and that elicits a conserved and stereotyped neuroendocrine response across rodent species (Deschamps et al., 2003; Kavaliers et al., 2001; Masini et al., 2005; Perrot-Sinal et al., 1999). Among female California mice in the current study, CORT levels were markedly increased following predator-urine stress tests in all reproductive groups. No differences were found in the magnitude of the CORT response to stress among breeding, nonbreeding, and virgin females. These results contrast with numerous findings in rats, in which lactating dams were shown to have a reduced CORT response to noise, endotoxin, elevated plus maze, and light/dark box tests compared to virgin females (Lightman, 1992; Shanks et al., 1997; Toufexis et al., 1999b; Windle et al., 1997a). Again, our findings might reflect the fact that the breeding females in the present study were concurrently pregnant and lactating, unlike the lactating females used in previous studies of other rodent species. In rats, stress responsiveness differs between lactating and pregnant females: pronounced CORT responses to stress, similar to those seen in virgins, have been found in early-pregnant rats (Nakamura et al., 1997; Neumann et al., 1998), while blunted CORT responses to stress, similar to those of lactating females, have been found in late-pregnant rats (Brunton et al., 2008).
Another difference between the present study and most previous studies of lactational hyporesponsiveness in rodents is that breeding females in our experiment were exposed to the predator-urine stressor while in the presence of their pups (and mate), which might have sensitized the lactating females' CORT response to stress, possibly circumventing any adaptations for stress hyporesponsiveness. Deschamps et al. (2003) found that early lactating rats tested in the presence of their pups had a significantly higher CORT response to predator urine than early lactating females separated from their pups. In sheep, however, the lambs’ presence and suckling greatly attenuated their mothers' cortisol responses to isolation and restraint stress, compared to ewes tested in the absence of their lambs or with their lambs present but unable to suckle (Tilbrook et al., 2006). Finally, the differences among studies may also be due to the nature of the stressor. For example, while the CORT response to repeated predator odor stressors is often sustained or sensitized in rodents (Blanchard et al., 1998; Figueiredo et al., 2003; Plata-Salaman et al., 2000), the response to other stressors such as chronic restraint or isolation typically habituates (Cole et al., 2000; Fernandes et al., 2002; Girotti et al., 2006; McCormick et al., 2006). As so few studies on lactational hyporesponsiveness to stress have utilized predator odor as a stressor, however, comparisons have to be made with studies applying different types of stress paradigms.
Studies of several other species have failed to detect reduced glucocorticoid responses to stressors in lactating as compared to non-lactating females (mice: Douglas et al., 2003; rhesus macaques, Macaca mulatta: Maestripieri et al., 2008; humans: Altemus et al., 2001; Kaye et al., 2004). (Note that Douglas et al. (2003) found that lactating mice had a significantly reduced ACTH response, but not CORT response, to forced swimming compared to virgin females). This, in addition to a number of studies in which blunted anxiety and fear were not found in lactating rats (Sibolboro-Mezzacappa et al., 2003; Silva et al., 1997; Young and Cook, 2004; Zuluaga et al., 2005), suggests that the occurrence of lactational stress hyporesponsiveness is highly variable and dependent on experimental design, as well as species.
In the present study, the inclusion of nonbreeding pairs of California mice (i.e., vasectomized males and their female mates) allowed us to evaluate possible effects of a pair bond, in addition to parenthood, on stress reactivity. Being a parent and engaging in a pair bond did not differentially modulate stress responsiveness in females, as no differences were found in the stress reactivity of breeding females compared to nonbreeding and virgin females. Social isolation has been found to greatly sensitize adrenocortical responses to stress in female prairie voles, Siberian hamsters (Phodopus sungorus), and black tufted-ear marmosets, in comparison to females housed with a male or female pairmate (Detillion et al., 2004; Grippo et al., 2007; Smith et al., 1998). Few studies, however, have attempted to characterize differential effects of male–female pair bonds, same-sex cagemates, and parental status on stress responsiveness. Importantly, the nonbreeding females in our study may have become pseudopregnant following copulation (Kenney et al., 1977). Elevated prolactin levels commonly found in pseudopregnant females could have dampened the nonbreeding females' neuroendocrine response to stress, similar to what was expected in breeding females. As neither breeding nor nonbreeding females differed from virgin females in their behavioral or hormonal responses to stress, however, this scenario seems unlikely.
We found that the change in males' CORT responses to predator urine from phase 1 to phase 2 differed among reproductive groups. Virgin males tended to become sensitized to the stressor, with an increased CORT response in phase 2 compared to phase 1, while males pair-housed with a female (either breeding or nonbreeding) responded similarly in the two phases. These data suggest an effect of mating or male–female pair bonding in modulating the response to repeated predator-urine stress in males. As described above, social support in the form of a male–female pair bond or same-sex social bond typically reduces the CORT response to acute stress in male prairie voles in comparison to socially isolated males (Carter, 1998; DeVries et al., 1997). Oxytocin released within the brain during mating and/or pair formation in prairie voles (Cho et al., 1999; Waldherr and Neumann, 2007) has been implicated in the social buffering and modulation of stress responsiveness (DeVries et al., 1997; Petersson et al., 1999; Windle et al., 1997b), and this might be the mechanism through which breeding and nonbreeding male California mice avoided sensitization to the predator-urine stressor. Furthermore, central AVP associated with the facilitation and maintenance of pair bonds (Cho et al., 1999; Liu et al., 2001; Winslow et al., 1993) may have modulated the stress response in breeding and nonbreeding males, although it is unclear whether it would play a stimulatory or inhibitory role (Landgraf, 2006). Finally, exposure to estrous, pseudopregnant, or pregnant females could have played a part in dampening the CORT responses to repeated predator-urine stress in breeding and nonbreeding males. Such an effect has previously been found in male mice (Mus musculus), whereby brief exposure to odors from an estrous female facilitated the dampening of CORT responses to predator odor (Kavaliers et al., 2001).
Reproductive status also modulated behavioral responses to the predator-urine stressor in male, but not female, California mice. Predator urine affected behavior in nonbreeding and virgin males, causing acute reductions in time spent in proximity to the cup and in time spent autogrooming. In contrast, breeding males showed no significant behavioral changes in response to predator urine. These results, along with recent findings showing that paternal experience correlates with altered behavioral responses to novelty, but not fear, in pup-exposed California mice (Bardi et al., 2011), suggest that fatherhood, engaging in paternal behavior, or exposure to a pregnant female blunts the effects of stress on behavior in males.
Neuroendocrine hyporesponsiveness to stress in lactating female mammals is thought to have evolved as a mechanism to protect parental behavior from disruption in the face of stressors (Altemus et al., 1995; Brunton et al., 2008; Carter et al., 2001; Lightman et al., 2001; Wingfield and Sapolsky, 2003). If this hypothesis is correct, we would expect fathers in biparental species to exhibit reduced stress responsiveness compared to non-fathers. The results of this study suggest that fatherhood, or engaging in paternal behavior, does buffer the behavioral response to stress, but that this effect is not clearly associated with, or mediated by, diminished glucocorticoid responses to stress, at least in this biparental species. The reduction in anxiety-related behavior found in lactating females of other species is likely mediated by a combination of maternal hormones and contact with their pups (Figueira et al., 2008; Lonstein, 2005). Therefore, it is likely that stimuli from and interaction with the pups may have contributed to breeding males' reduced stress-related behavior in the current study. These findings suggest that differential mechanisms underlie the lactation-induced suppression of behavioral and neuroendocrine responses to stressors in parents, with parental behavior or stimuli from pups possibly mediating an altered behavioral response, but not an altered adrenocortical response to stress.
Conclusion
In summary, the results of this study suggest that parenthood does not modulate corticosterone responses to an acute stressor in male and female California mice. Cohabitation with a female pairmate, however, does appear to reduce the adrenocortical response to repeated stress in males, while fatherhood appears to mitigate the behavioral response to stress. Ongoing studies further characterizing HPA function, stress-induced neural activation, and anxiety-related behavior in California mouse fathers and non-fathers are being conducted to more fully ascertain the extent to which fatherhood may influence stress responsiveness and emotionality in this monogamous, biparental mammal.
Acknowledgments
This research was supported by NIH grant R21MH087806 and by funds from the University of California, Riverside. The authors would like to thank Leslie Karpinski, Jim Sinclair, and John Kitasako for their excellent caretaking of the mice, Dr. Akiko Sato for veterinary care, Dr. Manuela Martins-Green for use of her environmental chamber, and Joseph Chang, Julia Cho, Breanna Harris, Giancarlo Lembo, Hoa Nguyen, Amit Parikh, and Juan Pablo Perea-Rodriguez for their assistance in the lab.
References
- Altemus M, Duester PA, Galliven E, Carter CS, Gold PW. Suppression of hypothalamic-pituitary-adrenal axis responses to stress in lactating women. J. Clin. Endocrinol. Metab. 1995;80:2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- Altemus M, Redwine LS, Leong YM, Frye CA, Porges SW, Carter CS. Responses to laboratory psychosocial stress in postpartum women. Psychosom. Med. 2001;63:814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- Appenrodt E, Schnabel R, Schwarzberg H. Vasopressin administration modulates anxiety-related behavior in rats. Physiol. Behav. 1998;64:543–547. doi: 10.1016/s0031-9384(98)00119-x. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm. Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bardi M, Franssen CL, Hampton JE, Shea EA, Fanean AP, Lambert KG. Paternal experience and stress responses in California mice (Peromyscus californicus) Comp. Med. 2011;61:20–30. [PMC free article] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav. Neurosci. 2003;117(3):455–463. doi: 10.1037/0735-7044.117.3.455. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Nikulina JN, Sakal RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol. Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC. Quantifying Behavior the J Watcher Way. Sunderland, MA, USA: Sinauer Associates, Inc.; 2007. [Google Scholar]
- Brummelte S, Pawluski JL, Galea LAM. High post-partum levels of corticosterone given to dams influence postnatal hippocampal cell proliferation and behavior of offspring: a model of post-partum stress and possible depression. Horm. Behav. 2006;50:370–382. doi: 10.1016/j.yhbeh.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, Douglas AJ. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J. Neuroendocrinol. 2008;20:764–776. doi: 10.1111/j.1365-2826.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- Cantoni D, Brown RE. Male influence on interbirth interval in the monogamous California mouse when required to forage for food. Ann. N.Y. Acad. Sci. 1997;807:486–489. doi: 10.1111/j.1749-6632.1997.tb51946.x. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog. Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- Casolini P, Cigliana G, Alemà GS, Ruggieri V, Angelucci L, Catalani A. Effect of increased maternal corticosterone during lactation on hippocampal corticosteroid receptors, stress response and learning in offspring in the early stages of life. Neuroscience. 1997;79:1005–1012. doi: 10.1016/s0306-4522(96)00668-9. [DOI] [PubMed] [Google Scholar]
- Catalani A, Marinelli M, Scaccianoce S, Nicolai R, Muscolo LAA, Porcu A, Korányi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Res. 1993;624:209–215. doi: 10.1016/0006-8993(93)90079-3. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav. Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J. Neuroendocrinol. 2000;12:1034–1042. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- da Costa APC, Ma X, Ingram CD, Lightman SL, Aguilera G. Hypothalamic and amygdaloid corticotropin-releasing hormone (CRH) and CRH receptor-1 mRNA expression in the stress-hyporesponsive late pregnant and early lactating rat. Mol. Brain Res. 2001;91:119–130. doi: 10.1016/s0169-328x(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front. Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm. Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Measor KR, Chauke M, Harris BN, Saltzman W. Brief pup exposure induces Fos expression in the lateral habenula and serotonergic caudal dorsal raphe nucleus of paternally experienced male California mice (Peromyscus californicus) Neuroscience. 2010;169:1094–1104. doi: 10.1016/j.neuroscience.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Woodside B, Walker CD. Pups’ presence eliminates the stress hyporesponsiveness of early lactating females to a psychological stress representing a threat to the pups. J. Neuroendocrinol. 2003;15:486–497. doi: 10.1046/j.1365-2826.2003.01022.x. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TKS, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Taymans SE, Carter CS. Social modulation of corticosteroid responses in male prairie voles. Ann. N.Y. Acad. Sci. 1997;807:494–497. doi: 10.1111/j.1749-6632.1997.tb51949.x. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Brunton PJ, Bosch OJ, Russell JA, Neumann ID. Neuroendocrine responses to stress in mice: hyporesponsiveness in pregnancy and parturition. Endocrinology. 2003;144:5268–5276. doi: 10.1210/en.2003-0461. [DOI] [PubMed] [Google Scholar]
- Dudley D. Paternal behavior in the California mouse, Peromyscus californicus . Behav. Biol. 1974;11:247–252. doi: 10.1016/s0091-6773(74)90433-7. [DOI] [PubMed] [Google Scholar]
- Everts HGJ, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial-learning, social recognition, and anxiety-related behaviors in rats. Behav. Brain. Res. 1999;99:7–16. doi: 10.1016/s0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Fedriani JM, Fuller TK, Sauvajot RM, York EC. Competition and intraguild predation among three sympatric carnivores. Oecologia. 2000;125:258–270. doi: 10.1007/s004420000448. [DOI] [PubMed] [Google Scholar]
- Fernandes GA, Perks P, Cox NK, Lightman SL, Ingram CD, Shanks N. Habituation and cross-sensitization of stress-induced hypothalamic-pituitary-adrenal activity: effect of lesions in the paraventricular nucleus of the thalamus or bed nuclei of the stria terminalis. J. Neuroendocrinol. 2002;14:593–602. doi: 10.1046/j.1365-2826.2002.00819.x. [DOI] [PubMed] [Google Scholar]
- Figueira RJ, Peabody MF, Lonstein JL. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behav. Neurosci. 2008;122:618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- Fischer D, Patchev VK, Hellbach S, Hassan AHS, Almeida OFX. Lactation as a model of naturally reversible hypercorticalism plasticity in the mechanisms governing hypothalamo-pituitary-adrenocortical activity in rats. J. Clin. Invest. 1995;96:1208–1215. doi: 10.1172/JCI118153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Luebke C. Timidity prevents the virgin female rat from being a good mother: emotionality differences between nulliparous and parturient females. Physiol. Behav. 1981;27:863–868. doi: 10.1016/0031-9384(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TWW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Glasper ER, DeVries AC. Social structure influences effects of pair-housing on wound healing. Brain. Behav. Immun. 2005;19:61–68. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Good T, Khan MZ, Lynch JW. Biochemical and physiological validation of a corticosteroid radioimmunoassay for plasma and fecal samples in oldfield mice (Peromyscus polionotus) Physiol. Behav. 2003;80:405–411. doi: 10.1016/j.physbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Sah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernick DJ, Alberts JR. The biparental care system of the California mouse, Peromyscus californicus . J. Comp. Psychol. 1987;101:169–177. [PubMed] [Google Scholar]
- Gubernick DJ. Reproduction in the California mouse, Peromyscus californicus . J. Mammal. 1988;69:857–860. [Google Scholar]
- Gubernick DJ, Nelson RJ. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus . Horm. Behav. 1989;23:203–210. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Wright SL, Brown RE. The significance of father’s presence for offspring survival in the monogamous California mouse, Peromyscus californicus . Anim. Behav. 1993;46:539–546. [Google Scholar]
- Gubernick DJ, Teferi T. Adaptive significance of male parental care in a monogamous mammal. Proc. R. Soc. Lond. B. Biol. Sci. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Austad SN. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol. Biochem. Zool. 2000;73:12–22. doi: 10.1086/316721. [DOI] [PubMed] [Google Scholar]
- Hubbs AH, Millar JS, Wiebe P. Effect of brief exposure to a potential predator on cortisol concentrations in female Columbian ground squirrels (Spermophilus columbianus) Can. J. Zool. 2000;78:578–587. [Google Scholar]
- Kaye J, Soothill P, Hunt M, Lightman S. Responses to the 35% CO2 challenge in postpartum women. Clin. Endocrinol. (Oxf) 2004;61:582–588. doi: 10.1111/j.1365-2265.2004.02133.x. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Colwell DD. Brief exposure to female odors ‘emboldens’ male mice by reducing predator-induced behavioral and hormonal responses. Horm. Behav. 2001;40:497–509. doi: 10.1006/hbeh.2001.1714. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Lanier DL, Dewsbury DA. Effects of vaginal-cervical stimulation in seven species of muroid rodents. J. Reprod. Fert. 1977;49:305–309. doi: 10.1530/jrf.0.0490305. [DOI] [PubMed] [Google Scholar]
- Kleiman DG, Malcolm J. The evolution of male parental investment in mammals. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. New York: Plenum Press; 1981. pp. 347–387. [Google Scholar]
- Landgraf R. The involvement of the vasopressin system in stress-related disorders. CNS Neurol Disord Drug Targets. 2006;5:167–179. doi: 10.2174/187152706776359664. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., III Lactation inhibits stress-mediated secretion of corticosterone and oxytocin and hypothalamic accumulation of corticotrophin-releasing factor and enkephalin messenger ribonucleic acids. Endocrinology. 1989;124:2358–2364. doi: 10.1210/endo-124-5-2358. [DOI] [PubMed] [Google Scholar]
- Lightman SL. Alterations in hypothalamic-pituitary responsiveness during lactation. Ann. N.Y. Acad. Sci. 1992;652:340–346. doi: 10.1111/j.1749-6632.1992.tb34365.x. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog. Brain Res. 2001;133:111–129. doi: 10.1016/s0079-6123(01)33009-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav. Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm. Behav. 2005;47:241–255. doi: 10.1016/j.yhbeh.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Fulks R, Gerald MS. Plasma cortisol responses to stress in lactating and nonlactating female rhesus macaques. Horm. Behav. 2008;53:170–176. doi: 10.1016/j.yhbeh.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr. Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen. Comp. Endocrinol. 2008;156:210–217. doi: 10.1016/j.ygcen.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav. Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Merrick A, Secen J, Helmreich DL. Social instability in adolescence alters the central and peripheral hypothalamic-pituitary-adrenal responses to a repeated homotypic stressor in male and female rats. J. Neuroendocrinol. 2006;19:116–126. doi: 10.1111/j.1365-2826.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Seto T, Nagase H, Yoshida M, Dan S, Ogino K. Inhibitory effect of pregnancy on stress-induced immunosuppression through corticotropin releasing hormone (CRH) and dopaminergic systems. J. Neuroimmunol. 1997;75:1–8. doi: 10.1016/s0165-5728(96)00232-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J. Physiol. 1998;508:289–300. doi: 10.1111/j.1469-7793.1998.289br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Nunes S, Fite JE, Patera J, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii) Horm. Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Ogle TF, Kitay JI. Ovarian and adrenal steroids during pregnancy and the oestrous cycle in the rat. J. Endocrinol. 1977;74:89–98. doi: 10.1677/joe.0.0740089. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Marler CA. Weak winner effect in a less aggressive mammal: correlations with corticosterone but not testosterone. Physiol. Behav. 2006;89:171–179. doi: 10.1016/j.physbeh.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in Microtus pennsylvanicus (meadow voles) Horm. Behav. 2001;39:285–294. doi: 10.1006/hbeh.2001.1655. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Ossenkopp KP, Kavaliers M. Brief predator odour exposure activates the HPA axis independent of locomotor changes. Neuroreport. 1999;10:775–780. doi: 10.1097/00001756-199903170-00021. [DOI] [PubMed] [Google Scholar]
- Petersson M, Hulting AL, Uvnas-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neurosci. Lett. 1999;264:41–44. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, Merali Z, Anisman H. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1β. system, tumor necrosis factor-α, transforming growth factor-β.1, and neuropeptide mRNAs in specific brain regions. Brain Res. Bull. 2000;51:187–193. doi: 10.1016/s0361-9230(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Reeder DM, Kunz TH, Widmaier EP. Baseline and stress-induced glucocorticoids during reproduction in the variably flying fox Pteropus hypomelanus (Chiroptera: Pteropodidae) J. Exp. Zool. A. Comp. Exp. Biol. 2004;301(8):682–690. doi: 10.1002/jez.a.58. [DOI] [PubMed] [Google Scholar]
- Ribble DO. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav. Ecol. Sociobiol. 1991;29:161–166. [Google Scholar]
- Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol. Reprod. 1991;45:523–532. doi: 10.1095/biolreprod45.4.523. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm. Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Abbott DH. Effects of elevated circulating cortisol concentrations on maternal behavior in common marmoset monkeys (Callithrix jacchus) Psychoneuroendocrinology. 2009;34:1222–1234. doi: 10.1016/j.psyneuen.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schradin C. Comments to K.E. Wynne-Edwards and M.E. Timonin 2007. Paternal care in rodents: weakening support of hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care, Horm & Behav 52: 114–121. Horm. Behav. 2007;52:557–559. [Google Scholar]
- Shanks N, Kusnecov A, Pezzone M, Berkun J, Rabin BS. Lactation alters the effects of conditioned stress on immune function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;41:R16–R25. doi: 10.1152/ajpregu.1997.272.1.R16. [DOI] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Perks P, Wood S, Ingram CD, Lightman SL. The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J. Neuroendocrinol. 1999;11:857–865. doi: 10.1046/j.1365-2826.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Sibolboro-Mezzacappa E, Tu AY, Myers MM. Lactation and weaning effects on physiological and behavioral response to stressors. Physiol. Behav. 2003;78:1–9. doi: 10.1016/s0031-9384(02)00889-2. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ., Jr. Nonparametric Statistics for the Behavioral Sciences. Second ed. New York: McGraw-Hill Book Company; 1988. [Google Scholar]
- Silva R, Bernardi MM, Nasello AG, Felicio LF. Influence of lactation on motor activity and elevated plus-maze behavior. Braz. J. Med. Biol. Res. 1997;30:241–244. doi: 10.1590/s0100-879x1997000200013. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J. Physiol. 2008;586.2:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhlii) Horm. Behav. 1998;34:211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ, editors. Biometry. 3rd ed. New York: W.H. Freeman and Co.; 1995. [Google Scholar]
- Stefanski V, Raabe C, Schulte M. Pregnancy and social stress in female rats: influences on blood leukocytes and corticosterone concentrations. J. Neuroimmunol. 2005;162:81–88. doi: 10.1016/j.jneuroim.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Stern JM, Goldman L, Levine S. Pituitary-adrenal responsiveness during lactation in rats. Neuroendocrinology. 1973;12:179–191. doi: 10.1159/000122167. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Ibbott MD, Clarke IJ. Activation of the hypothalamo-pituitary-adrenal axis by isolation and restraint stress during lactation in ewes: effect of the presence of the lamb and suckling. Endocrinology. 2006;147:3501–3509. doi: 10.1210/en.2005-1632. [DOI] [PubMed] [Google Scholar]
- Torner L, Toschi N, Nava G, Clapp C, Neumann ID. Increased hypothalamic expression of prolactin in lactation: involvement in behavioural and neuroendocrine stress responses. Eur. J. Neurosci. 2002;15:1381–1389. doi: 10.1046/j.1460-9568.2002.01965.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Walker CD. Noradrenergic facilitation of the adrenocorticotropin response to stress is absent during lactation in the rat. Brain Res. 1996;737:71–77. doi: 10.1016/0006-8993(96)00627-0. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Silva AL, Crean KK, Hostetler C. Sex differences in hormonal responses to social conflict in the monogamous California mouse. Horm. Behav. 2010;58:506–512. doi: 10.1016/j.yhbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis DJ, Thrivikraman KV, Plotsky PM, Morilak DA, Huang N, Walker CD. Reduced noradrenergic tone to the hypothalamic paraventricular nucleus contributes to the stress hyporesponsiveness of lactation. J. Neuroendocrinol. 1998;10:417–427. doi: 10.1046/j.1365-2826.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Tesolin S, Huang N, Walker CD. Altered pituitary sensitivity to corticotropin-releasing factor and arginine vasopressin participates in the stress hyporesponsiveness of lactation in the rat. J. Neuroendocrinol. 1999a;11:757–764. doi: 10.1046/j.1365-2826.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Rochford J, Walker CD. Lactation-induced reduction in rats’ acoustic startle is associated with changes in noradrenergic neurotransmission. Behav. Neurosci. 1999b;113:176–184. doi: 10.1037//0735-7044.113.1.176. [DOI] [PubMed] [Google Scholar]
- Tu MT, Lupien SJ, Walker CD. Measuring stress responses in postpartum mothers: perspectives from studies in human and animal populations. Stress. 2005;8(1):19–34. doi: 10.1080/10253890500103806. [DOI] [PubMed] [Google Scholar]
- Waddell BJ, Atkinson HC. Production rate, metabolic clearance rate and uterine extraction of corticosterone during rat pregnancy. J. Endocrinol. 1994;143:183–190. doi: 10.1677/joe.0.1430183. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C-D, Lightman SL, Steele MK, Dallman MF. Suckling is a persistent stimulus to the adrenocortical system of the rat. Endocrinology. 1992;130:115–125. doi: 10.1210/endo.130.1.1309321. [DOI] [PubMed] [Google Scholar]
- Walker C-D, Trottier G, Rochford J, Lavallee D. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. J. Neuroendocrinol. 1995;7:615–622. doi: 10.1111/j.1365-2826.1995.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Walker C-D, Tilders FJH, Burlet A. Increased colocalization of corticotropin-releasing factor and arginine vasopressin in paraventricular neurones of the hypothalamus in lactating rats: evidence from immunotargeted lesions and immunohistochemistry. J. Neuroendocrinol. 2001;13:74–85. doi: 10.1046/j.1365-2826.2001.00589.x. [DOI] [PubMed] [Google Scholar]
- Ward JF, MacDonald DW, Doncaster CP, Mauget C. Physiological response of the European hedgehog to predator and nonpredator odour. Physiol. Behav. 1996;60:1469–1472. doi: 10.1016/s0031-9384(96)00245-4. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Wood S, Shanks N, Perks P, Gonde GL, da Costa APC, Ingram CD, Lightman SL. Endocrine and behavioural responses to noise stress: comparison of virgin and lactating female rats during non-disrupted maternal activity. J. Neuroendocrinol. 1997a;9:407–414. doi: 10.1046/j.1365-2826.1997.00587.x. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997b;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wright SL, Brown RE. The importance of paternal care on pup survival and pup growth in Peromyscus californicus when required to work for food. Behav. Processes. 2002;60:41–52. doi: 10.1016/s0376-6357(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Timonin ME. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm. Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Young BJ, Cook CJ. Stress-induced modification of anxiety in rats is dependent on reproductive status. Physiol. Behav. 2004;80:569–575. doi: 10.1016/j.physbeh.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Zhang J-X, Cao C, Gao H, Yang Z-S, Sun L, Zhang Z-B, Wang Z-W. Effects of weasel odor on behaviour and physiology of two hamster species. Physiol. Behav. 2003;79:549–552. doi: 10.1016/s0031-9384(03)00123-9. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Wegner FH, Snowdon CT. Hormonal responses to parental and nonparental conditions in male cotton-top tamarins, Saguinus oedipus, a New World Primate. Horm. Behav. 1996;30:287–297. doi: 10.1006/hbeh.1996.0035. [DOI] [PubMed] [Google Scholar]
- Zuluaga MJ, Agrati D, Pereira M, Uriarte N, Fernandez-Guasti A, Ferreira A. Experimental anxiety in the black and white model in cycling, pregnant and lactating rats. Physiol. Behav. 2005;84:279–286. doi: 10.1016/j.physbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]