Abstract
Objective
To test the commonly held assumption that gastric bypass surgery lowers body weight because it limits the ability to eat large amounts of food.
Design and Methods
Central melanocortin signaling was blocked by ICV infusion of the melanocortin-3/4 receptor antagonist SHU9119 for 14 days in rats who’s high-fat diet-induced obesity had been reversed by Roux-en-Y gastric bypass surgery.
Results
SHU9119 increased daily food intake (+ 100%), body weight (+30%), and fat mass (+50%) in rats with RYGB, surpassing the presurgical body weight and that of saline-treated sham-operated rats. Doubling of food intake was entirely due to increased meal frequency, but not meal size. After termination of SHU9119, body weight promptly returned to near preinfusion levels. In sham-operated rats, SHU9119 produced even larger increases in food intake and body weight.
Conclusions
RYGB rats do not settle at a lower level of body weight because they cannot eat more food as they can easily double food intake by increasing meal frequency. The reversible obesity suggests that RYGB rats actively defend the lower body weight. However, because both RYGB and sham-operated rats responded to SHU9119, central melanocortin signaling is not the critical mechanism in RYGB rats responsible for this defense.
Keywords: Roux-en-Y gastric bypass, high-fat diet, melanocortin, SHU9119, brain
Introduction
Gastric bypass and other types of bariatric surgery are often the only hope for a better life of obese patients with the typical spectrum of comorbid conditions such as type-2 diabetes, cardiovascular disease, sleep apneas, and depression, not to speak of the difficulties in daily life and the social stigma. Although not completely reversing the obese state, Roux-en-y gastric bypass surgery (RYGB) has been shown to reliably and lastingly reduce excess bodyweight by more than 50% and cure or prevent progression of diabetes at a high rate (1–4). Remarkably, RYGB produces sustained suppression of body weight with only minor weight regain for up to 20 years in most patients (5). In contrast, weight loss induced by forced or voluntary calorie restriction (dieting) is often followed by significant weight regain [see (6) for recent review]. This has led to the widely held view that gastric bypass patients and rodents are unable to ingest large amounts of food, with the implication that the surgery imposes a form of perpetual calorie restriction. Recent clinical and preclinical studies have demonstrated a role for reduced motivation to eat (“wanting”) and reduced “liking” of food, particularly high energy dense foods in RYGB-induced anorexia and weight loss (7–14). However, because patients typically undergo strong behavioral counseling therapy and eat widely different foods before and after surgery, they are not ideally suited to dissect the mechanisms of their relative anorexia. It is not clear whether they eat less because of strong counseling expectations, dietary habits, or other biological mechanisms.
Here we use a rat model of RYGB (7, 15–17) allowing strict dietary control and more invasive techniques to answer some of these questions. Specifically, we wanted to know whether formerly diet-induced obese rats that after RYGB had settled at chronically reduced food intake and a low body weight level would be able to substantially increase food intake and regain body weight to the obese level with the proper stimulus – in other words, whether the effect of RYGB could be reversed. Melanocortin-signaling via melanocortin-4 receptors (MC4R) is a crucial effector arm of the homeostatic regulator with strong effects on food intake and energy expenditure (18). Pharmacological agonism at the MC4R powerfully suppresses, while antagonism stimulates food intake, and MC4R null mice are hyperphagic and develop obesity (18, 19). Therefore, we chronically infused the MC3/4R-agonist SHU9119 into the lateral cerebral ventricle of rats with RYGB or sham surgery. Substantial increases of food intake and body weight induced by SHU9119 would strongly argue against restriction as major mechanism for RYGB. At the same time, this pharmacological approach will test the potential role of MC4R signaling in RYGB-induced anorexia and weight loss.
Investigation of the role of MC4R signaling in bariatric surgery-induced weight loss in obese patients carrying MC4R gene variants and in obese MC4R-deficient rodents undergoing various bariatric surgeries has been inconclusive. An impaired weight loss response to gastric banding was found in some patients with some single point mutations (20) and one patient with complete MC4R deficiency (21). Another rare variant was, however, associated with increased weight loss after gastric bypass (22). In contrast, no effect of MC4R integrity on RYGB outcome was found in other human studies (23–26) or with sleeve gastrectomy in rats (27). Similarly, studies in two different MC4R-deficiency mouse models concluded that MC4R signaling is required for weight loss (24) and for weight-independent improvements in glucose homeostasis (22) after RYGB surgery, while a study in MC4R-deficient rats concluded that it is not required for weight loss after vertical sleeve gastrectomy (27). At least some of these disagreements could be due to compensatory changes during the development of these mutant organisms. Therefore, our pharmacological approach with chronic infusion of MC4R antagonist avoids these interpretational problems of germline loss-of-function.
Methods and Procedures
Animals
Male Sprague-Dawley rats initially weighing ~200 g (Harlan Industries, Indianapolis, IN) were housed individually in wire-mesh cages at a constant temperature of 21–23° C with a 12h light-dark cycle (lights on 07:00, off at 19:00). Food and water were provided ad libitum unless otherwise indicated. Animals were made obese by putting them on a two-choice diet for 8 weeks consisting of normal laboratory chow (Kcal%: Carb, 58; Fat, 13.5; Prot, 28.5, # 5001, Purina LabDiet, Richmond IN) and high-sucrose, high-fat diet (sweet HF diet; Kcal%: Carb, 35; Fat, 45; Prot, 20, D12451, Research Diets, New Brunswick, NJ), with each of the diets containing sufficient minerals and vitamins. They were then randomly assigned to either RYGB or sham-surgery. Liquid Ensure diet (Kcal%: Carb, 64; Fat, 21.6; Prot, 14.4, Abbott Laboratories, Columbus, OH) was provided for the first 5 days after surgery or longer if needed.
All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institutes of Health.
Roux-en-Y gastric bypass surgery
Under adequate isoflurane anesthesia, the stomach is first freed from all the ligaments that connect it to the liver and the spleen. Then, the focus of the surgeon turns onto the gastric artery that emerges from the celiac artery. The gastric artery is in very close approximation with the gastroesophagel junction and then runs parallel to the lesser curvature of the stomach branching into one anterior and one posterior vessel that in turn give rise to several smaller branches on the anterior and posterior wall. Before transecting the stomach, the gastric artery is gently dissected off the gastric wall using a microhook. Using a bipolar cautery probe, the first anterior branch that crosses the anterior wall of the stomach, just millimeters from the gastroesophageal junction is cauterized, allowing for transection of the stomach wall with fine scissors without bleeding. After repairing the distal stomach using a 12 mm titanium clip, an end-to-end gastrojejunostomy is then constructed with a 8-0 nylon suture. This surgery resulted in a gastric pouch of no more than about 5% of the gastric volume, as well as Roux-, biliopancreatic, and common limbs of about 20, 30, and 50 % of total intestinal length. Sham-surgery consisted of laparotomy and mobilization of stomach and small intestine. For analgesia, Buprenorphine, meloxicam (1–2 mg/kg, s.c.), and/or carprofen (5 mg/kg, s.c.) were administered as necessary.
To overcome potential deficits in iron absorption and development of anemia, rats were administered a macromolecular dextran-iron complex (Iron Dextran injectable, catalog # 93963, 5 mg, sc; Town and Country, Ashland, OH) once a week for the first two weeks after RYGB surgery. Additional doses were administered to individual anemic animals if indicated.
Chronic brain infusion
At 8–12 weeks after surgery, rats were equipped with ICV cannulas (Plastics One, Roanoke, VA), aimed at the left lateral ventricle as described in detail earlier (28). After recovery, cannula placements were verified by monitoring the acute drinking response to ICV injection of angiotensin-2 (10 pg/rat). All animals drank at least 5 ml of water within 10 minutes of ANG-2 administration. After recovery and adaption to the metabolic chambers at an average post-surgery time of 15 weeks, infusions started with the implantation and connection with minipumps (2 ml/14 days, Durect Corporation, Cupertino CA) loaded with either SHU9119 (1.2 μg [1.12 nmol]/rat/day in sterile saline) or sterile saline in separate groups of rats, with an infusion rate of 0.5 μl/h.
Measurement of food and water intake, oxygen consumption, and locomotor activity
For continuous monitoring of food and water intake, oxygen consumption, and locomotor activity before and during brain infusions, rats were housed in special cages of an automated monitoring system (Phenomaster, TSE). High-fat food (60 % of energy from fat) and water was available ad libitum. One week of adaptation to the hanging food baskets with training lids were followed by three days of adaptation in the metabolic cages. Base line measures were taken during 5 days before implantation of minipumps and the start of infusions. Ten days after the end of infusions, rats were put back into normal cages and food intake was measured weekly.
Measurement of body weight and body composition
Body weight was measured daily throughout the experiment and body composition was measured before and after surgery, by using a Minispec LF 90 NMR Analyzer (Bruker Corporation, The Woodlands, TX). This method uses whole body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility (29).
Statistical analysis
Body weight, body weight changes, and food intake data were analyzed with one-way or two-way ANOVAs, with surgery and infusion as between subjects factors and time as repeated measures factor, as appropriate. Bonferroni corrected multiple comparison tests were used for preplanned comparisons of specific data points. All data are reported as means ± SEM.
Results
RYGB surgery-induced weight loss
Consistent with our earlier observations in this rat model, RYGB effectively reduced body weight and adiposity to levels found in age-matched, chow-fed non-surgical rats, while sham-operated rats continued to gain weight on the high-fat diet (Fig. 1). Food intake recovered from the initial strong suppression to about 10 % lower food intake in RYGB rats compared to sham-operated rats at the time of brain infusion.
Fig. 1.
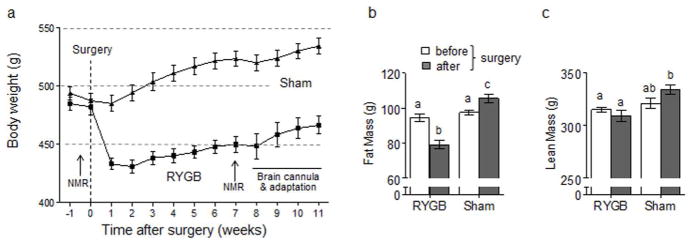
Effect of RYGB on body weight and composition. a: Body weight of rats made obese by feeding high-fat diet for 8 weeks and subjected to RYGB (solid circles, n =20) or sham-surgery (open circles, n = 20). B, c: Fat mass (b) and lean mass (c) before and 4 weeks after RYGB or sham-surgery as measured by NMR.
Effects of SHU9119 infusion on body weight and composition
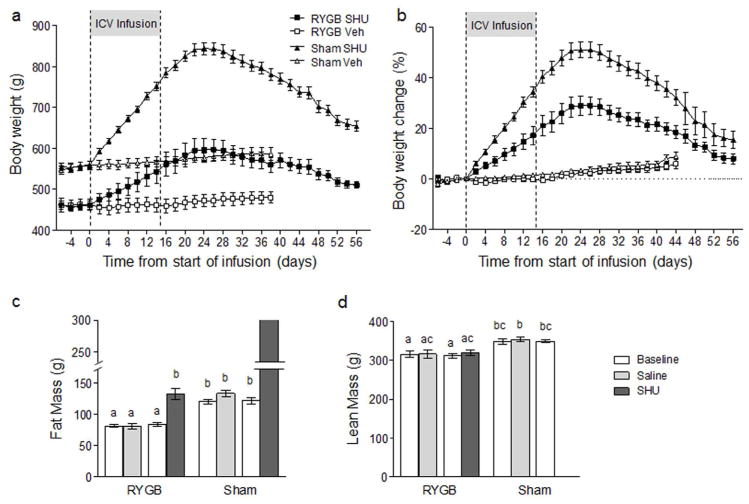
In RYGB rats, body weight steadily increased by ~6.6 g/day to reach a peak of 640 g (+ 31%) about 30 days after the start of infusion (Fig. 2). In sham animals, body weight increased by 13.0 g/day to peak at 850 g (+54%) around 30 days after the start of infusion. Fat mass of RYGB rats increased from 80 g to 170 g (+ 113%), while lean mass increased from 320 g to ~400 g (+25%). Fat mass of Sham rats increased from 126 g to ~300 g (most of the rats were too obese to be placed into the NMR machine). After reaching a peak around 2 weeks after the end of infusion, body weight declined in both RYGB and sham rats to near pre-infusion levels. In RYGB rats, body weight of SHU9119 infused rats was no longer significantly higher than saline infused rats by day 65 after the start of infusion.
Fig. 2.
Brain infusion of SU9119 increases body weight of RYGB and sham-operated rats. a: Absolute body weight. b: Percent change of body weight from start of SHU9119 or vehicle infusion. Eight to twelve weeks after RYGB or sham surgery (Fig. 1), animals were implanted with lateral ventricular cannulas and after recovery adapted to metabolic chambers. The MC3/4 receptor antagonist SHU9119 (100 μg/day) or saline as vehicle was then infused via minipump for 14 days. Note that body weight of both RYGB and sham-operated rats steeply and significantly increased by infusion of SHU9119 but not saline vehicle and returned to near pre-infusion levels after 50–60 days. (n = 10 for each of the 4 treatment groups).
Effects of SHU9119 infusion on food intake and meal structure
Before the start of chronic brain infusion, RYGB rats weighed about 90 g (~16%) less and consumed about 6 Kcal/day (~10%) less than sham-operated rats. Upon Infusion of SHU9119 food intake promptly increased in both RYGB and sham-operated animals (Fig. 3). In RYGB rats, food intake leveled off at about 100 % above baseline after 4 days of infusion. In sham-operated rats, food intake steadily increased to reach a maximal increase of nearly 200 % above baseline by day ten. Control infusion of saline did not significantly change food intake.
Fig. 3.
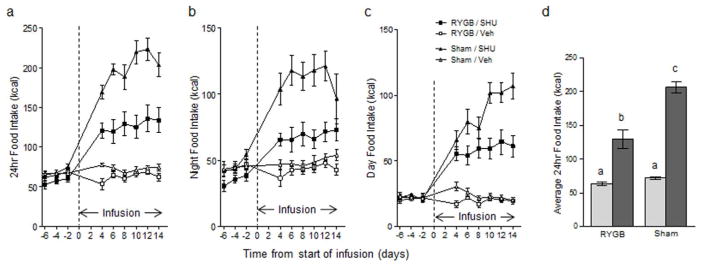
Brain infusion of SHU9119 increases food intake in RYGB and sham-operated rats. 24 h (a), 12 hour dark period (b), and 12 h light period (c) food intake of RYGB (squares) and sham-operated rats (triangles) infused with either SHU9119 (filled symbols) or saline vehicle (open symbols) for 14 days. * p < 0.05, SHU9119 vs. vehicle, for both RYGB and sham groups, n = 10/group.
Meal structure analysis carried out during days 4–14 of infusion revealed the significantly different eating patterns of RYGB compared with sham-operated rats under basal conditions with saline infusion and under stimulated conditions with SHU9119 infusion (Fig. 4). Under basal conditions with saline control infusions, RYGB rats ate significantly smaller meals but more frequently, with relatively small differences in intermeal intervals but a significantly higher satiety ratio. SHU9119 stimulation significantly accentuated these differences, but while sham-operated rats increased total intake primarily by increasing meal size, RYGB rats did it by primarily increasing meal frequency. Overall, SH9119 stimulation led to drastic decreases of the satiety ratio in both surgical groups.
Fig. 4.
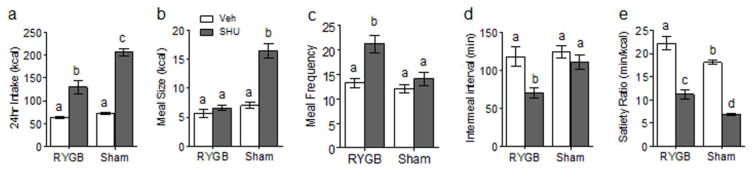
Brain infusion of SHU9119 increases food intake by effects on meal size in sham-operated rats but on meal frequency in RYGB rats. Average total 24 h food intake (a), meal size (b), meal frequency (c), intermeal interval (d), and satiety ratio (e) of RYGB and sham-operated rats with ICV infusion of either SHU9119 (dark gray bars) or saline vehicle (white bars) for 14 days. Bars that do not share the same letters are significantly different from each other (p < 0.05, based on ANOVA followed by Bonferroni-corrected multiple comparison tests, n =10/group).
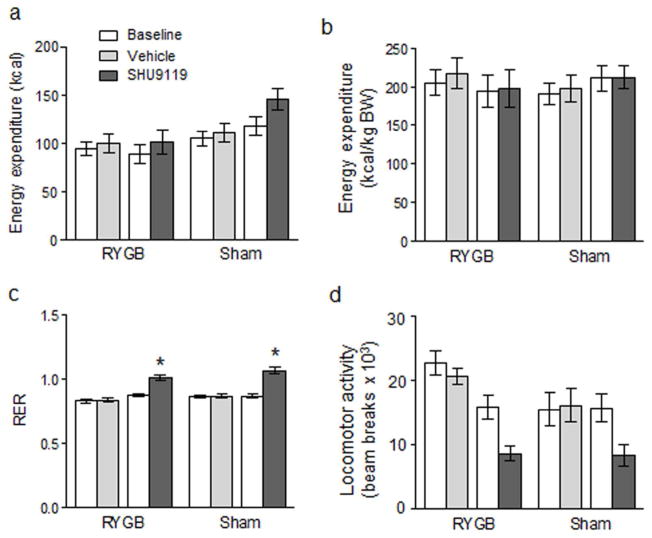
Effects of SHU9119 infusion on energy expenditure
SHU9119 tended to increase uncorrected energy expenditure although the increase did not reach statistical significance (Fig. 5a). If corrected for body weight, energy expenditure was not different in RYGB and sham-operated rats and with saline ar SHU(119 infusion (Fig. 5b). SHU9119 infusions significantly increased RER in both RYGB and sham-operated rats (Fig. 5c). Locomotor activity was significantly decreased by SHU9119 if compared to saline infusion (Fig. 5d).
Fig. 5.
Effect of brain infusion of SHU9119 in RYGB and sham-operated rats on energy expenditure (a, b), respiratory exchange ratio (c), and locomotor activity (d) as measured in metabolic chambers. Note that energy expenditure expressed per animal (a) was slightly, but not significantly lower in RYGB compared with sham-operated rats, but that these differences disappeared when expressed per kg body weight (b). While there were no significant differences in RER and locomotor activity between RYGB and sham-operated rats, SHU9119 infusion significantly increased RER and decreased locomotor activity compared with saline vehicle infusion. Bars that do not share the same letters are significantly different from each other (p < 0.05, based on ANOVA followed by Bonferroni-corrected multiple comparison tests, n =10/group).
Discussion
Bariatric surgeries have been classified as either restrictive, malabsorptive, or both (1), but the meaning of these attributes needs some clarification. While it is clear that the restrictive character of RYGB, limits meal size and rate of ingestion, more frequent meals can entirely make up for these restrictions and even lead to much larger total intake, as clearly demonstrated in the present study. When stimulated with the MC3/4R antagonist SHU9119, RYGB rats were able to increase their total food intake by about 100% and their daytime food intake by almost 200% above their customary level. RYGB rats on SHU9119 gained more than 30% body weight, at a rate of ~7 g/day, from their reduced level 5 months after surgery. They gained almost 100% of fat mass, surpassing pre-surgical (obese) body weight and fat mass and even sham-operated obese rats. SHU9119 completely reversed the effects of RYGB; it restored obesity in a short period of time. Therefore, while RYGB reduces meal size, it is clear that RYGB animals retain the capacity to consume substantially larger amounts of food and gain significantly more body weight. As such, the RYGB-induced decrease in food intake is not due to ‘mechanical’ reduction of maximal food capacity, but instead reflects a physiological decrease in voluntary food consumption. RYGB rats could eat more if they wanted to, but they choose not to.
The role of melanocortin-4 receptor signaling in the beneficial effects of bariatric surgeries has been addressed in clinical studies including subjects with MC4R gene variants and in MC4R-deficient rats and mice and has yielded somewhat inconsistent findings. Most human studies found no, or only negligibly, reduced efficiency of RYGB or sleeve gastrectomy to produce excess weight loss (23–25, 36), and sleeve gastrectomy was just as effective in MC4R-deficient vs. wildtype rats (27). In contrast, weight loss in MC4R-deficient mice was significantly reduced in another study (24), and targeted re-expression of MC4R in brainstem autonomic neurons was demonstrated to mediate some beneficial effects of RYGB on glycemic control (22). The present study clearly demonstrates the power of MC4R-blockade in orchestrating a strong anabolic response with doubling of food intake and considerable fat mass gain in rats that had stabilized at a low body weight level after RYGB. However, an even stronger response in sham-operated rats complicates interpretation of this effect in RYGB animals. Support for a critical role of central melanocortin signaling in RYGB-induced stabilization at a lower body weight level would have required a larger SHU9119-induced body weight gain in RYGB vs. sham-operated rats. If central melanocortin signaling were the crucial mechanism, blockade of this mechanism in RYGB rats should eventually result in catching up with the body weight of sham-operated rats.
There are several potential reasons that this outcome was not observed. First, the SHU9119 infusion lasted only 2 weeks and longer infusion may have changed the outcome. Second, a lower dose of SHU9119 may have stimulated an anabolic response specifically in RYGB but not sham-operated rats. Third, the stronger effect of SHU9119 in sham rats may suggest increased sensitivity of the melanocortin-signaling pathway compared to the RYGB rats which perhaps have reached near maximal signaling capacity already and SHU9119 has little additional effects. Only a full dose-response study would answer these questions. Also, MC4R-blockade at the time of surgery would perhaps provide a clearer answer than blockade at 8–12 weeks after surgery. However, this is unlikely, because in the MC4R-deficient mouse model, the initial weight loss after RYGB was similar for KO and wildtype mice (24). Thus, the present results cannot rule out the possibility that increased central melanocortin signaling is critically involved in the body weight lowering effects of RYGB.
After termination of the MC3/4 receptor blockade, now “obese RYGB rats” reduced food intake and lost all excess body weight and fat mass – they became “true RYGB rats” again. Why did they not stay obese if they have no problem to eat large amounts? Do they come back to pre-SHU9119 levels because they regulate at a lower body weight/adiposity setpoint and if yes, what constitutes this setpoint? These questions have intrigued the field of energy balance regulation since its inception and we still do not have convincing answers. The concept of a lower level of defended body weight is supported by a similar observation in a sleeve gastrectomy model. After a 21-day food restriction regimen leading to additional weight loss, rats with sleeve gastrectomy increased daily food intake by about 30% above their customary level and got back to their pre-restriction body weight level (30). Thus, there must be a mechanism defending the surgery-induced lower level of body weight/adiposity. What could that mechanism be?
The hypothalamus has received most attention as homeostatic regulator of energy balance and body weight, particularly its twin populations of leptin-sensitive neurons in the arcuate nucleus with their powerful effector pathways. The expression level of POMC and AGRP in these crucial neurons is often used to determine whether a rodent is basically “hungry” or not. Increased AGRP and decreased POMC expression is observed after severe food deprivation and, therefore, indicated a “hungry” state; the reverse indicates a “non-hungry” state. These expression levels were measured in a few studies with various rodent bariatric surgery models. The most comprehensive study in rats with sleeve gastrectomy did not find any significant differences, compared to sham-operated rats, in AGRP, POMC, NPY, and MC4R expression either 10 days after surgery, when food intake was suppressed, or 35 days after surgery, when food intake had normalized (30), suggesting that the homeostatic regulator is “fine” with the new lower body weight level and ready to defend it if challenged. In contrast, two studies in rats with duodenal-jejunal (not gastric) bypass, NPY and AGRP expression was increased 10–50 days after surgery, suggesting that the rats were in a hungry state and no stet point shift had occurred (31, 32).
Leptin is an important driver of hypothalamic POMC and AGRP neurons, but the role of leptin signaling in the basomedial hypothalamus and elsewhere after RYGB is not clear. Circulating leptin levels decrease commensurate with the large loss of body weight and fat mass after RYGB (17, 33). A similar decrease of leptin levels is achieved after weight loss induced by calorie restriction. However, while low leptin levels after calorie restriction generate increased hunger, low leptin after RYGB does not stimulate hunger. One explanation for this conundrum is that RYGB increases leptin sensitivity to make up for lower leptin levels, so that even much lower circulating leptin levels are capable of activating hypothalamic POMC neurons and suppress food intake. Leptin-sensitivity can be enhanced by increased leptin receptor expression and/or by increased coupling of the leptin receptor to intracellular signaling cascades. Hypothalamic leptin receptor expression was unchanged, and the food intake suppressing effect of exogenous leptin was not different after sleeve gastrectomy in rats, suggesting that leptin sensitivity is not changed in this model (30). Whether exogenous leptin is able to further decrease body weight after RYGB was recently tested in patients that had lost 31% of body weight at 18 months after surgery (34). The absence of any difference in body weight, percent fat mass, and resting energy expenditure after 16 weeks of leptin or placebo, suggests that there was no increase in leptin sensitivity. Also, because RYGB compared with sham surgery is highly effective in reducing body weight in leptin receptor-deficient Zucker obese rats (35), it appears that leptin signaling is not required for the hypophagia-inducing and body weight lowering effects in this RYGB model. To date, no experiments have been done in leptin-deficient (ob/ob) or leptin receptor-deficient (db/db mouse models, or with molecular indicators of leptin sensitivity such as phosphor-STAT3.
In conclusion, the present study clearly demonstrates that rats eating less and settling at a lower body weight after RYGB can be stimulated to eat large amounts of food and regain the obese body weight level in a reversible fashion, strongly supporting the idea of a defended lower level of body weight.
What is already known about this subject
Gastric bypass surgery leads to a sustained reduction of meal size, food intake, and body weight, but the mechanisms are unknown.
Blockade of brain melanocortin receptor-4 signaling strongly stimulates food intake.
Requirement of melanocortin signaling for weight loss after bariatric surgeries is controversial.
What does this study add
Semi-chronic blockade of brain melanocortin receptor-4 signaling increases food intake and body weight in a reversible fashion.
Semi-chronic blockade of brain melanocortin receptor-4 signaling increases food intake by increasing meal frequency but not meal size.
Body weight level after gastric bypass surgery is defended.
Acknowledgments
Funding agencies: This work was supported by National Institutes of Health grant DK047348
We thank Dr. Neelima Gonugunta for editorial assistance, Jennifer Terrebone for help with the metabolic cages, and Dr. Barry Roberts and his staff for help with care of animals. The study was supported by National Institute of Health Grant DK047348 and the NORC Metabolism and Physiology Core.
Footnotes
Disclosure: The authors declare no conflict of interest
References
- 1.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 2.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 3.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 6.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R581–600. doi: 10.1152/ajpregu.00755.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajnal A, Kovacs P, Ahmed TA, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–G979. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.le Roux CW, Bueter M, Theis N, et al. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1057–1066. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr. 2010;92:277–283. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- 10.Shin AC, Berthoud HR. Food reward functions as affected by obesity and bariatric surgery. Int J Obes (Lond) 2011;35 (Suppl 3):S40–44. doi: 10.1038/ijo.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholtz S, Miras AD, Chhina N, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2013 doi: 10.1136/gutjnl-2013-305008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miras AD, Jackson RN, Jackson SN, et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr. 2012;96:467–473. doi: 10.3945/ajcn.112.036921. [DOI] [PubMed] [Google Scholar]
- 14.Ochner CN, Kwok Y, Conceicao E, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueter M, Lowenstein C, Olbers T, et al. Gastric bypass increases energy expenditure in rats. Gastroenterology. 2010;138:1845–1853. doi: 10.1053/j.gastro.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Zheng H, Shin AC, Lenard NR, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Butler AA, Cone RD. Knockout models resulting in the development of obesity. Trends Genet. 2001;17:S50–54. doi: 10.1016/s0168-9525(01)02481-7. [DOI] [PubMed] [Google Scholar]
- 20.Potoczna N, Branson R, Kral JG, et al. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J Gastrointest Surg. 2004;8:971–981. doi: 10.1016/j.gassur.2004.09.032. discussion 981–972. [DOI] [PubMed] [Google Scholar]
- 21.Aslan IR, Ranadive SA, Ersoy BA, Rogers SJ, Lustig RH, Vaisse C. Bariatric surgery in a patient with complete MC4R deficiency. Int J Obes (Lond) 2011;35:457–461. doi: 10.1038/ijo.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zechner JF, Mirshahi UL, Satapati S, et al. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144:580–590. e587. doi: 10.1053/j.gastro.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aslan IR, Campos GM, Calton MA, Evans DS, Merriman RB, Vaisse C. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg. 2011;21:930–934. doi: 10.1007/s11695-010-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatoum IJ, Stylopoulos N, Vanhoose AM, et al. Melanocortin-4 Receptor Signaling Is Required for Weight Loss after Gastric Bypass Surgery. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goergen M, Manzoni D, De Blasi V, et al. Influence of obesity-susceptibility loci (MC4R and INSIG2) on the outcome of weight loss and amelioration of co-morbidity in obese patients treated by a gastric-bypass. Bull Soc Sci Med Grand Duche Luxemb. 2011:7–24. [PubMed] [Google Scholar]
- 26.Valette M, Poitou C, Le Beyec J, Bouillot JL, Clement K, Czernichow S. Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLoS ONE. 2012;7:e48221. doi: 10.1371/journal.pone.0048221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mul JD, Begg DP, Alsters SI, et al. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. Am J Physiol Endocrinol Metab. 2012;303:E103–110. doi: 10.1152/ajpendo.00159.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR. High-fat intake induced by mu-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R- stimulation. Brain Res. 2010;1350:131–138. doi: 10.1016/j.brainres.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunnecke B, Verry P, Benardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res. 2004;12:1604–1615. doi: 10.1038/oby.2004.200. [DOI] [PubMed] [Google Scholar]
- 30.Stefater MA, Perez-Tilve D, Chambers AP, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–2436. 2436 e2421–2423. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadreau E, Baraboi ED, Samson P, et al. Effects of the biliopancreatic diversion on energy balance in the rat. Int J Obes (Lond) 2006;30:419–429. doi: 10.1038/sj.ijo.0803166. [DOI] [PubMed] [Google Scholar]
- 32.Warne JP, Padilla BE, Horneman HF, et al. Metabolic and neuroendocrine consequences of a duodenal-jejunal bypass in rats on a choice diet. Ann Surg. 2009;249:269–276. doi: 10.1097/SLA.0b013e3181961d5d. [DOI] [PubMed] [Google Scholar]
- 33.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, et al. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia. 2008;51:1901–1911. doi: 10.1007/s00125-008-1118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korner J, Conroy R, Febres G, et al. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity (Silver Spring) 2013;21:951–956. doi: 10.1002/oby.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277–285. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Still CD, Wood GC, Chu X, et al. High allelic burden of four obesity SNPs is associated with poorer weight loss outcomes following gastric bypass surgery. Obesity (Silver Spring) 2011;19:1676–1683. doi: 10.1038/oby.2011.3. [DOI] [PubMed] [Google Scholar]