Abstract
Background
Cardiolipin, a unique phospholipid in the inner mitochondrial membrane, is critical for optimal mitochondrial function. CL abnormalities have been demonstrated in the failing rodent and adult human heart. The aim of this study was to determine whether abnormalities in CL content and the CL biosynthesis and remodeling pathways are present in pediatric idiopathic dilated cardiomyopathy (IDC).
Methods and Results
A cross-sectional analysis of myocardial tissue from 119 IDC and non-failing (NF) control samples was performed. Electrospray ionizing mass spectrometry was used to measure total CL and CL species content in LV tissue. RT-PCR was employed to measure gene expression of the enzymes in the CL biosynthesis and remodeling pathways in both the adult and pediatric heart. Significantly lower total and (18:2)4CL (the beneficial species) content was demonstrated in myocardium from pediatric patients with IDC compared to NF controls. Analysis of mitochondrial gene transcripts was used to demonstrate that there is no decrease in mitochondrial content. Expression of two biosynthesis enzymes and one remodeling enzyme was significantly lower in pediatric IDC compared to NF controls. Expression of two phospholipases involved in CL degradation were also altered, one up and one down-regulated. Except for one remodeling enzyme, these changes are unique from those in the failing adult heart.
Conclusion
Similar to what has been seen in adults and in a rat model of IDC, total and (18:2)4CL are lower in pediatric IDC. Unique CL species profiles are seen in heart tissue from children with IDC compared to adults. Differences in CL biosynthesis and remodeling enzyme expression likely explain the differences in CL profiles observed in IDC and implicate unique age-related mechanisms of disease.
Keywords: Cardiolipin, Pediatric, Heart Failure, Dilated Cardiomyopathy, Mitochondria
1. Introduction
Idiopathic dilated cardiomyopathy (IDC) is a common form of cardiomyopathy and indication for cardiac transplantation in children and adults. The incidence of dilated cardiomyopathy in adults is about 5.5 per 100,000 people per year and between 0.34 and 1.09 per 100,000 people per year in children [1-4]. While the disease is less common in children than adults the clinical consequences are similarly devastating with 1- and 5-year rates of death or transplantation of approximately 30% and 40%, respectively [2,5]. Although the prevalence of heart failure in children is less than in adults, pediatric IDC represents a significant burden of disease and health care cost in the pediatric population [6,7]. IDC is the leading indication for transplantation in children over 1 year of age; additionally these patients are among the highest risk among children for cardiopulmonary resuscitation [2,8]. Evidence-based medical therapy for adults with heart failure (HF) is well established and has improved clinical outcomes [9]. In contrast, modern medical regimens have not significantly improved outcomes in children with HF over those from the 1970s digoxin and diuretic era therapy [5,10,11]. While primary disorders of mitochondrial respiration are a known cause of myocardial dysfunction in children and adults, secondary mitochondrial dysfunction may be a down-stream effect contributing to heart failure [12-15]. Whether mitochondrial dysfunction is a primary cause or consequence of heart failure, improving myocardial energy utilization is an attractive target for new therapies to improve outcomes in the pediatric population [16].
Cardiolipin (CL) is a major cardiac phospholipid found almost exclusively in the mitochondrial inner membrane where it is essential for the optimal function of key energy producing enzymes in the electron transport chain (ETC) [17-20]. A known genetic defect in the CL pathway leads to decreased expression of the CL remodeling enzyme, tafazzin, in X-linked Barth syndrome that is characterized by cardiomyopathy, skeletal myopathy and developmental delay in boys [21]. Evidence from Barth syndrome and other models suggests that CL must be in the tetralinoleoyl form [i.e. 4 linoleic acid side chains, or (18:2)4CL)] in order to allow optimal function of numerous mitochondrial systems related to energy production [11-13]. Nascent CL is synthesized by conjugation of two di-acylglycerols with non-specific fatty acid side chain incorporation, then remodeled into (18:2)4CL via a process where linoleoyl moieties are incorporated onto the di-phosphatidylglycerol backbone via tafazzin and other remodeling transacylases. Calcium-independent phospholipases iPLA2-G6 and iPLA2-γ have both been implicated in removal of specific CL fatty acid side-chains in the remodeling process [22,23]. Proper synthesis, remodeling, and degradation of CL species are essential to maintain mitochondrial electron flux and metabolic integrity of the ETC, preserving the ATP content, and permitting normal myocardial performance [17,24-27].
Previous work has shown that decreases in the linoleoyl content of CL are dramatic in adult idiopathic dilated cardiomyopathy (IDC) and in a rat model of heart failure [28]. Additionally, in the Spontaneously Hypertensive HF rat model (SHHF), a well-established congenital model of IDC, a high linoleic acid diet can restore cardiac (18:2)4CL levels and markedly increase survival [29,30]. This dietary intervention also improves mitochondrial function (measured by cytochrome C oxidase activity), arrests the usual decline in systolic cardiac function, and improves survival in the SHHF rat. Changes in expression of enzymes in the CL biosynthesis pathway have been shown in cardiac tissue from SHHF rats and adults with IDC corresponding with these changes in CL composition [31]. Specifically, we have previously shown that in adult IDC, the biosynthetic enzyme cytidinediphosphatediacylglycerol synthetase (CDS-2), and the remodeling enzyme tafazzin (TAZ) are significantly down-regulated when compared to nonfailing controls, which parallels findings in the rat model. Cardiolipin synthase (CLS), the last step in the biosynthetic pathway, was down-regulated in the failing rat heart as well, although a significant difference was not observed in CLS expression between normal and IDC in human tissue. This work demonstrated that derangements in CL content and composition are observed in human heart failure, likely related to changes in both biosynthesis and remodeling of this mitochondrial phospholipid. The aim of the current study was to directly assess whether CL compositional abnormalities are present in ventricular tissue from children with IDC, compare these to CL composition changes in adults with IDC, and identify changes in expression of biosynthetic and remodeling enzymes associated with changes in CL content and composition.
2. Methods
2.1 Subjects
Subjects were males and females of all races and ethnic backgrounds that donated their heart to the COMIRB approved pediatric and adult transplant tissue banks at the University of Colorado. All non-failing (NF) hearts were donor hearts with normal left ventricular ejection fraction (LVEF) not transplanted for technical reasons and all in HF were idiopathic dilated cardiomyopathic (IDC) hearts (i.e. not ischemic cardiomyopathic hearts or cardiomyopathies from congenital heart disease) with LVEF <30. Pediatric contents were between the ages of 0 and 18 years, 54 IDC (39% male), and 23 NF controls (54% male). Post-hoc analysis was performed to compare pre-pubertal children with adolescent subjects. Adult samples included tissue from individuals aged 20-66 years in the IDC group (27 samples, 56% male) and 44-69 years in the NF group (15 samples, 47% male).
2.2 Left ventricle tissue from pediatric patients with IDC and non-failing controls
At the time of cardiac transplantation, the explanted hearts were immediately cooled in ice cold oxygenated Tyrodes in the operating room. Left ventricle tissue was rapidly dissected flash frozen and stored at −80°C.
2.3 CL molecular species quantification
Lipid was extracted from pediatric LV tissue homogenates (pediatric group n: NF 20, IDC 44, adult group n: NF 10, IDC 15) for quantification by normal phase high pressure liquid chromatography coupled to electrospray ionization mass spectrometry (LC-ESI-MS) as described by Sparagna et al [32]. Using 1,1′,2,2′-tetramyristoyl CL as an internal standard ESI-MS was employed for quantification of total CL from the 7 most prevalent molecular species present in human heart tissue (mass/charge or m/z 1186, 1422, 1424, 1448, 1450, 1472, 1474) measured individually (Table I). These species comprise >95% of CL present in human myocardium. CLs were extracted from LV tissue from IDC and non-failing control samples, and quantitated by ESI-MS. Total (absolute) amounts of detectable CL species were quantitated in nmol per milligram of protein extracted. The fractional content of each CL species was calculated based on the total CL content for each sample as a percentage. Mass/charge species are defined using abbreviations for monolyso (M), palmitic (P), Palmitoleic acid (Po), linoleic (L), oleic (O), and arachidonic (Ar) acid side chains.
Table I.
Major cardiolipin species detected in human heart
| m/z | Fatty acid side chain composition | Abbreviation |
|---|---|---|
| 1186 | (18:2)3 CL | L3 |
| 1422 | (18:2)3(16:1)1 CL | L3Po1 |
| 1424 | (18:2)3(16:0)1 CL | L3P1 |
| 1448 | (18:2)4 CL | L4 |
| 1450 | (18:2)3(18:1)1 CL | L3O1 |
| 1472 | (18:2)3(20:4)1 CL | L3Ar1 |
| 1474 | (18:2)2(18:1)1(20:4)1 CL | L2O1Ar1 |
L- Linoleic acid
O- Oleic acid
Po- Palmitoleic acid
Ar- Arachidonic acid
P- Palmitic acid
2.4 Real-time quantitative PCR (RT-qPCR)
RNA extracted from adult (n: NF 15, IDC 27) and pediatric (n: NF 23, IDC 54) LV tissue (Ambion mirVana isolation kit, manufacturers protocol) was reverse-transcribed to cDNA using the Qiagen miScript II RT kit (per manufacturers protocol). RNA quality was verified using NanoDrop® ND-1000 UV-Vis Spectrophotometer analysis prior to RT-qPCR, performed at the University of Colorado Denver Genomics and Microarray Core. The SYBR Green method was used to quantify enzyme expression using 10 ng cDNA per reaction using the AB StepOne Rapid RT-PCR protocol. All reactions were performed in duplicate with melting curves to ensure specificity of PCR product, and normalized to 18S expression. No difference in expression of 18S between groups was appreciated. RT expression was measured using the delta delta CT method as previously described (values compared to non-failing controls) [33]. Primer sets for target genes are listed in Table II (supplement).
2.5 Statistical analysis
Data is expressed as means +/− SEM. The difference between two groups was evaluated by Students t-test. Comparisons were considered to be significant for p values < 0.05 unless otherwise noted.
3. Results
3.1 Total CL and (18:2)4CL content is depleted in left ventricular myocardium from pediatric patients with IDC
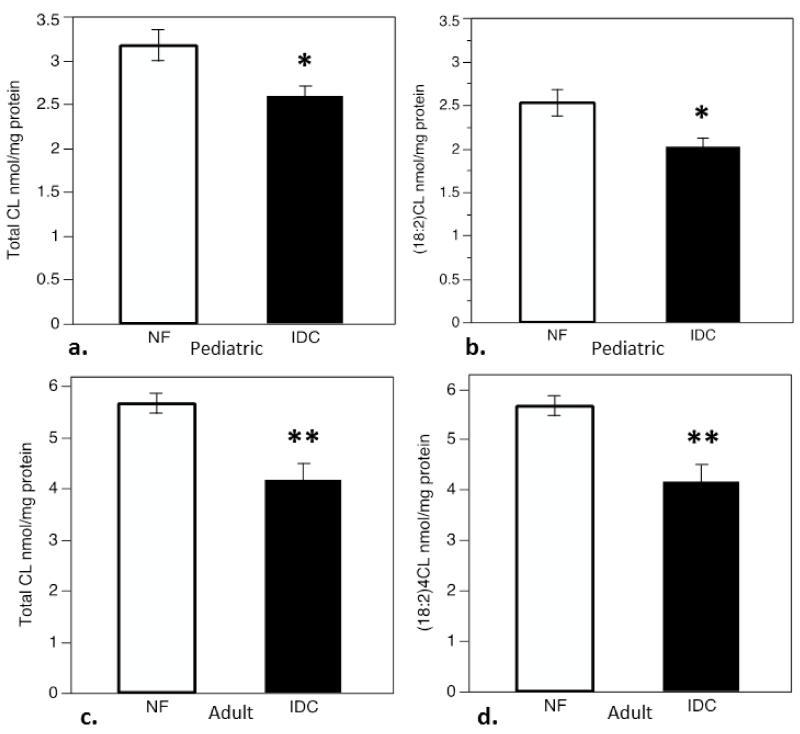
To determine if total and (18:2)4CL content is altered in pediatric IDC, similar to what has been demonstrated in adults, CL species were quantified by LC-ESI-MS. Compared to non-failing controls, total CL content was depleted in pediatric IDC (Figure 1a, p<0.01). The total quantity of tetralinoleoyl (18:2)4CL, m/z species1448 (refer to Table 1), was also significantly lower in pediatric IDC (Figure 1b, p<0.01). This is similar to data previously reported in adults[28]. To verify this comparison, CL quantification by LC-ESI-MS was also performed in the adult cohort (Figure 1c, 1d). Both total and (18:2)4CL, m/z species 1448, content were significantly lower in the adult IDC group, (p<0.005 for both).
Figure 1.
ESI-MS quantitation of total CL and (18:2)4CL, m/z 1448, content in myocardium from patients with IDC compared to nonfailing (NF) controls. a) Total CL content in pediatric NF and IDC groups expressed in nmol/mg total protein, b) (18:2)4CL content in pediatric NF and IDC groups in nmol/mg protein, c) Total CL content in adult NF and IDC groups expressed in nmol/mg total protein, d) (18:2)4CL content in in adult NF and IDC in nmol/mg protein. Asterisks indicate significant difference from NF group (*p<0.01, **p<0.005).
3.2 CL profiles in pediatric IDC are unique from that seen in adults
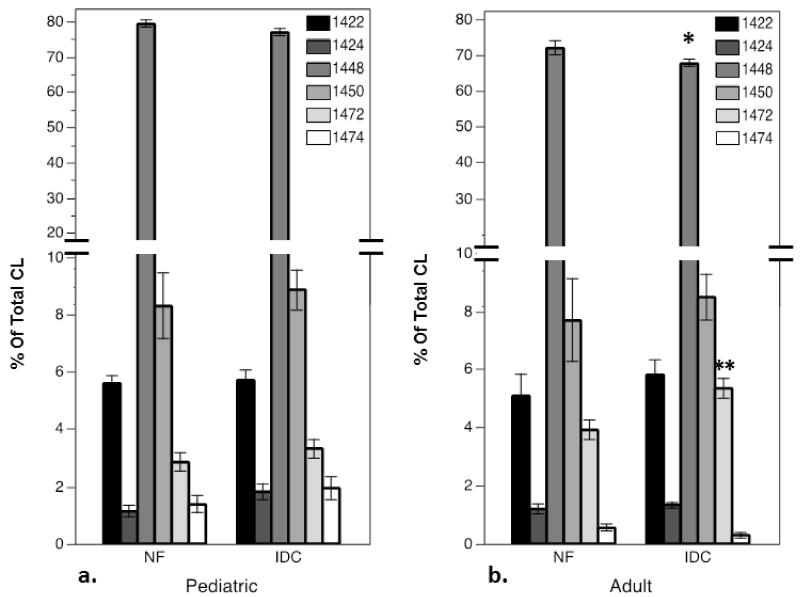
A total of 6 CL lipid species were present in pediatric and adult myocardium in percentages that exceeded 1% of total CL content. The CL species detectable at this level in human heart are listed in Table I with side chain identification and m/z ratio. Content of each CL molecular species was measured as a percentage of total CL content in the pediatric and adult cohorts (Figure 2). When (18:2)4CL is quantitated as a percentage of total CL in the pediatric cohort, there was no significant difference between the IDC and NF groups (Figure 2a). In the adult cohort, however, (18:2)4CL as a percentage of total CL was significantly lower in IDC than the NF group (Figure 2b, p<0.05). Other CL species with arachidonic acid side chains, including CL with 2 linoleic, 1 arachidonic acid and one oleic acid side chain (L2O1Ar1) and CL with three linoleic and one arachidonic acid side chain (L3Ar1) have both been shown to be significantly increased in adult IDC[28]. L2O1Ar1 was detected at a very low level in the pediatric myocardium (1-2% of total CL); with no difference in fractional content in the IDC group compared to controls. L3Ar1CL comprised approximately 2 to 4% of total CL in pediatric myocardium, and was also not significantly different in the IDC group compared to controls. In this adult cohort, as was shown previously, L3Ar1CL is significantly higher in the IDC group compared with controls, but no significant difference between groups was seen in the L2O1Ar1 species as a percentage of total CL. L3P1CL containing one palmitoleic acid side chain (m/z 1422) in total was significantly lower in the IDC group compared to controls (data not shown), with no difference between groups as a percentage of total CL. Monolyso-CL (MLCL, (18:3)3CL), commonly measured as a ratio over (18:2)4CL (1186/1448) has been shown to be elevated in Barth syndrome[34]. We found no significant difference in ratio of MLCL to 1448 in the pediatric or adult IDC groups compared to the control non-failing groups. There was also no difference in total content of MLCL between NF and IDC in pediatric or adult groups. Therefore, loss of total CL in pediatric IDC is accounted for by losses of (18:2)4CL primarily, with levels of L3P1CL also being lower in heart failure. There is no difference in the proportion of (18:2)4CL to total CL in pediatric IDC. In contrast, the proportional content of (18:2)4CL is significantly lower in adult IDC, with a significantly higher percentage of L3Ar1CL Thus, loss of total CL in adult IDC can be attributed to loss of (18:2)4CL alone, with a fractional increase in a species containing an arachidonic acid moiety.
Figure 2.
LC-ESI-MS quantification of CL species in myocardium from patients with IDC compared to nonfailing (NF) controls as percentages of total CL content in the a) pediatric cohort, and b) adult cohort. CL species represented as m/z (See Table I). (18:2)4CL, m/z 1448, is the most prevalent species (~80%). (asterisks indicate significant differences from age-matched NF, *p<0.05, **p<0.01)
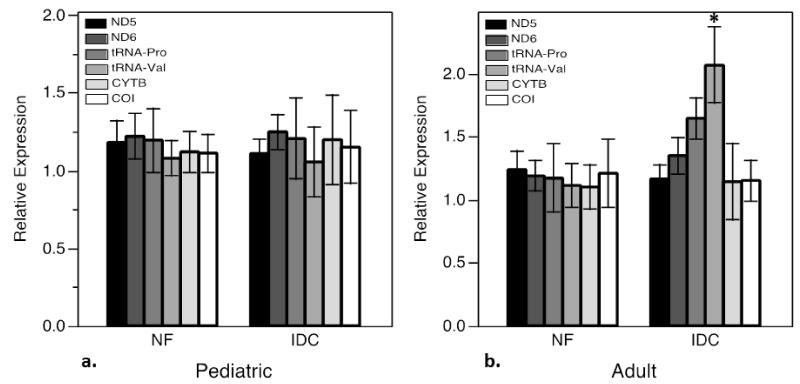
3.3 Loss of total and (18:2)4CL is not associated with changes in mitochondrial content
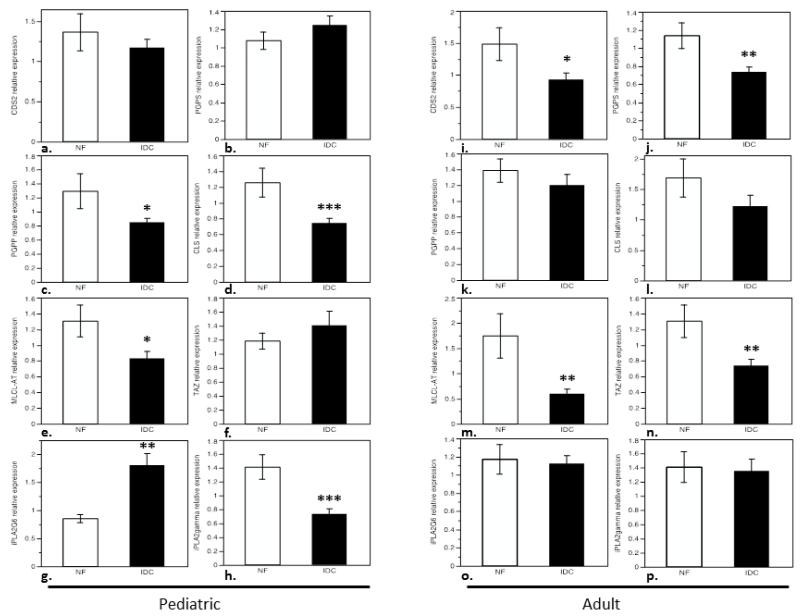
In order to demonstrate whether changes in total CL and (18:2)4CL content in IDC could be attributed to mitochondrial content in diseased myocardium, RT-qPCR analysis of six mitochondrial DNA-encoded proteins was performed as a surrogate for mitochondrial DNA content. ND5 and ND6 (NADH dehydrogenase 5 and 6, both part of Complex I), tRNAs Proline and Valine, cytochrome B of complex III (CYTB), and cytochrome C oxidase subunit I of complex IV (COI) were quantified. Transcripts in pediatric IDC were quantitated relative to NF controls (Figure 3a). There was no significant difference in ND5, ND6, tRNA Pro, tRNA Val, CYTB or COI expression in pediatric IDC. The experiment was replicated in the cohort of adult patients with IDC compared to NF controls (Figure 3b). Similar to what is observed in the pediatric group, there is no difference in ND5, ND6, tRNA Pro, CYTB or COI expression in adult IDC indicating stable mitochondrial content in the diseased heart. Interestingly, there was up-regulation of tRNA Val in adult IDC relative to NF controls (p<0.05). 3.4 Depletion of Total and (18:2)4CL in pediatric IDC is associated with changes in expression of enzymes involved in cardiolipin biosynthesis and remodeling. To investigate the mechanism by which CL changes occur in pediatric IDC, expression of the enzymes involved in CL biosynthesis and remodeling was measured by RT-qPCR. 18S was used to normalize enzyme expression levels of cellular transcripts not subject to regulation in disease. Of note, GAPDH expression was analyzed and found to be significantly down-regulated in IDC samples (children and adults), and therefore not used as a reference transcript. In multiple data sets, and in previously published work, we have verified that no difference in 18S expression is seen between groups[33]. The sequential steps in CL biosynthesis are carried out by the enzymes cytidine diphosphate diacylglycerol synthase (CDS, isoforms 1 and 2), phosphatidylglycerophosphate synthase (PGPS), PGP phosphatase (PGPP), and cardiolipin synthase (CLS), which were all assessed for expression level (Figure 6). While no significant difference in expression of CDS1, CDS2, or PGPS were noted between pediatric groups (Figure 4a, 4b), levels of PGPP and CLS were significantly lower in the IDC group (Figure 4c, 4d). PGPP levels were 37% lower, and CLS levels 43% lower than in non-failing controls (p<0.01, p<0.001 respectively).
Figure 3.
Expression of MT-DNA transcripts, ND5, ND6, tRNA-Pro, tRNA-Val, CYTB, and COI, in pediatric and adult myocardium. a) Expression of mitochondrial transcripts in pediatric tissue, NF controls compared with IDC. B) Expression of mitochondrial transcripts in adult tissue, NF controls compared to IDC.
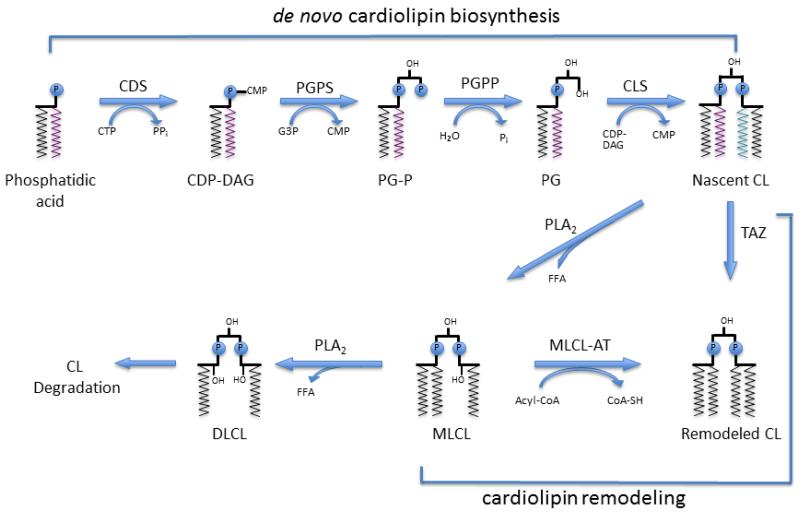
Figure 6.
Model of CL biosynthesis and remodeling in pediatric heart failure: Acyl-CoA-acyl-coenzyme A, CL-Cardiolipin, CDP-DAG-cytidinediphosphate-diacylglycerol, CDS-CDP-DAG synthase, CLS-CL synthase, CMP-cytidinemonophosphate, CoA-SH-coenzyme A (unconjugated), CTP-cytidinetriphosphate, DLCL-Dilyso-CL, FFA-free fatty acid, G3P-glycerol-3-phosphate, MLCL-monolyso-CL, MLCL-AT-MLCL acyltransferase, PG-phosphatidylglycerol, PGP-PG phosphate, PGPS-PG-P synthase, PGPP-PG-P phosphatase, Pi-inorganic phosphate, PLA2-phospholipase A2, TAZ-Tafazzin. Figure adapted from Chicco and Sparagna, 2007 [24].
Figure 4.
Expression of enzymes in the CL biosynthetic and remodeling pathways in pediatric IDC and adult IDC: a) pediatric and i) adult cytidinediphosphate-diacylglycerol synthase-2 (CDS-2), b) pediatric and j) adult phosphatidylglycerophosphate synthase (PGPS), c) pediatric and k) adult phosphatidylglycerophosphate phosphatase (PGPP), d) pediatric and l) adult cardiolipin synthase (CLS), e) pediatric and m) adult monolysocardiolipin acyltransferase (MLCL-AT), f) pediatric and n) adult Tafazzin (TAZ), g) pediatric and o) adult phospholipase A2 group VI (iPLA2G6), and h) pediatric and p) adult phospholipase A2γ (iPLA2γ). (*p<0.05, **p<0.005, ***p<0.001).
Remodeling enzymes tafazzin and monolysocardiolipin acyltransferase (MLCL-AT) remodel nascent, or de-esterified forms of CL to produce a mature phospholipid ((18:2)4CL). The gene that encodes the MLCL-AT enzyme was recently reported to be the trifunctional protein subunit A (HADHA)[35]. MLCL-AT was down-regulated by 36% in the IDC group compared to non-failing controls (Figure 4e, p<0.05). No alteration in expression of tafazzin was observed in pediatric IDC when compared to non-failing controls (Figure 4f).
Additional experiments were conducted to analyze expression of mitochondrial calcium-independent phospholipase A2 (mt-iPLA2) species implicated in the remodeling and possibly in degradation of CL species. iPLA2-G6 was significantly up-regulated in pediatric IDC (by 53%), while, iPLA2-γ was significantly decreased (by 48%) in pediatric IDC (Figure 4g, 4h).
3.5 Cardiolipin Biosynthesis and Remodeling in Pediatric IDC is Regulated in a Manner Unique from Adults
Sparagna et al have previously demonstrated altered expression (down-regulation) of CDS-2 and tafazzin in adult IDC, with no appreciable change in CLS expression. The other enzymatic steps in the biosynthetic and remodeling pathways have not previously been studied. To directly compare the results we obtained in pediatric IDC with that in adults, expression of all cardiolipin biosynthesis and remodeling enzymes was analyzed concurrently in a larger cohort of adults than had previously been studied.
Consistent with previous data[31], CDS-2 and tafazzin expression were significantly lower (37% and 43%, respectively) in adult IDC compared to controls (Figure 4i and 4n). The second step in the biosynthetic pathway, PGPS was significantly downregulated in adult IDC (Figure 4j, 35% lower expression), while no difference was observed in the pediatric group (Figure 4b). PGPP, which was downregulated in pediatric IDC, was not significantly altered in the adult IDC cohort compared to controls (Figure 4k). As was seen previously, no statistically significant difference in CLS expression was seen between the adult control and IDC group (Figure 4l). MLCL-AT expression was significantly lower (by 66%) in the failing heart compared to controls, similar to what was observed in the pediatric cohort (Figure 4m). Unlike what was shown in the pediatric group (Figure 4g, 4h) there were no significant differences in the expression of calcium-independent phospholipases between IDC and non-failing controls (Figure 4o, 4p).
3.6 CL enzyme expression patterns begin to shift during adolescence
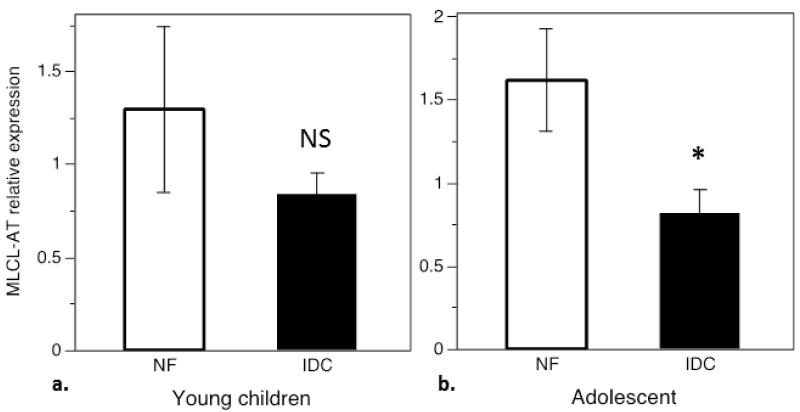
The pediatric cohort was divided into a pre-pubertal group (<12 years, 10 NF, 40 IDC) and an adolescent group (ages 12-18, 13 NF, 14 IDC) for a post-hoc analysis of enzyme expression by age group. In the group of younger children, there was down-regulation of PGPP and CLS similar to what was shown in Figure 4a and 4b (data not shown, p values <0.05 and <0.01 respectively). In this group there was no difference in MLCL-AT expression between ICD and NF samples (Figure 5a), and iPLA2-G6 was up-regulated and iPLA2-γ downregulated as shown in Figure 4e, 4f (data not shown, p values <0.05 and <0.001 respectively). Analysis of the adolescent group also showed that PGPP and CLS appear to be downregulated in IDC, but these did not reach statistical significance (data not shown, p=0.16, p= 0.07). In this group MLCL-AT was downregulated 46% in IDC (Figure 5b, p<0.05), and differences in iPLA2 expression between IDC and NF controls did not reach statistical significance (data not shown).
Figure 5.
Expression of MLCL-AT in young children (a, <12 years) compared with adolescents (b, 12-18 years). a) There is no significant difference in MLCL-AT expression between NF and IDC in young children, b) MLCL-AL is down-regulated in the adolescent group with IDC compared to adolescent controls (p< 0.05).
4. Discussion
Previous work has demonstrated that CL content and composition are altered in myocardium from adults with idiopathic dilated cardiomyopathy [28]. In an animal model of heart failure, the SHHF rat, similar changes in CL content are noted at and before the onset of heart failure [32]. These changes in rat mitochondrial lipid content can be reversed with a diet high in linoleic acid, which raises the possibility of dietary intervention in humans with heart failure [29,30]. Altered expression of the enzymes responsible for biosynthesis and remodeling of CLs during heart failure, both in the rat model and in adult human tissue, has been proposed as a mechanism for changes in CL composition [31].
The present study demonstrates for the first time that changes in CL content are also significantly altered in children with IDC. We have demonstrated that both total CL content and tetralinoleoyl-CL content are significantly lower in children with IDC compared to non-failing controls, similar to what has been shown in a cohort of adults with IDC previously. This work additionally demonstrates that there is loss of the fractional content of (18:2)4CL in response to heart failure in adult IDC that is significant. Conversely, (18:2)4CL as a percentage of total CL in pediatric IDC was not significantly different from controls. This could indicate that in end-stage pediatric heart failure adaptive mechanisms to preserve (18:2)4CL content are intact to a greater degree than is seen in adult heart failure. Reduced quantities of total CL, and CL enriched with linoleic acid as a function of age has been observed in rodent models, providing further support for the hypothesis that remodeling of CL is impaired in older animals compared to the young [28,30,36,37]. Myocardium in the aged rat also demonstrates CL oxidative alterations that are not observed in young rats in response to ischemia-reperfusion, with associated mitochondrial dysfunction, demonstrating impaired myocardial adaptation to injury with age may be related to impaired CL remodeling [38,39].
We confirm previous findings of lower total and tetralinoleoyl CL in adult IDC for comparison. Moreover, we were able to demonstrate that lower amounts of total CL and (18:2)4CL are not related to a change in total mitochondrial content as assessed by expression of mitochondrial-encoded tRNAs and subunits of Complexes I, III, and IV. Therefore, the CL content must be lower as a percentage of total phospholipid in myocardial mitochondria. In further support of normal mitochondrial content, electron microscopy had been performed in biopsy specimens from 19 of the IDC cohort, with all but one specimen showing that mitochondria were normal in appearance, distribution and abundance. While there is conflicting data in the literature related to whether mitochondrial transcripts are up or down-regulated in heart failure, there is other evidence to support increased mitochondrial biogenesis and higher expression of electron transport chain subunits in IDC, that is not seen in ischemic cardiomyopathy or in a mixed population of cardiomyopathies [40-42]. This increase in expression of electron transport chain components does not however correlate with increased mitochondrial metabolic activity. Increased expression of mitochondrial transcripts in human heart failure is not recapitulated in many animal models, indicating that species-specific differences in gene expression may exist, or that heterogeneity and time-course of disease differ in human heart failure when compared to animal models [41].
Monolyso-CL (MLCL) is the intermediary CL species that condenses with acyl-CoA through the enzymatic activity of either tafazzin or MLCL-AT to produce a CL with 4 acyl groups. MLCL has been shown to be dramatically higher in tissues from patients with Barth syndrome [34]. Although MLCL is detected in tissues from pediatric patients, no significant differences in the total amount or proportion of MLCL could be detected between IDC and non-failing controls in the pediatric or adult cohorts. Thus, decreases in MLCL-AT in pediatric IDC do not result in appreciable increases in this CL intermediary, possibly because tafazzin expression is preserved. In adult IDC both MLCL-AT and tafazzin are down-regulated, with no increase observed in total or fractional MLCL. Low activity of CL-specific phospholipases in combination with this down-regulation of transacylases could be a mechanism for a low rate of CL degradation and turnover in IDC. Other CL species in pediatric IDC are unaltered, unlike what has previously been observed in adults. No significant increase in CL species that contain arachidonic acid side-chains was observed in pediatric IDC, whereas in adults a CL species containing an arachidonic acid side-chain (L3Ar1) was present at higher levels in adult IDC as a fraction of total CL. This finding is consistent with previous studies which showed higher levels of L3Ar1 and L2O1Ar1species in adult IDC [28]. Increased content of CL species containing arachidonic acid (L3Ar1CL and L2O1Ar1CL) has also been observed as a function of age alone, reinforcing the concept that CL remodeling is age-dependent [28].
The changes in CL content have been associated with changes in expression of the CL biosynthetic and remodeling enzymes in both a rat model and in tissue from adult humans with IDC. In mammalian heart, de novo biosynthesis of CL occurs in the inner mitochondrial membrane beginning with the conversion of phosphatidic acid to cytidine-5′-diphosphate-1,2-diacylglycerol (CDP-DAG), catalyzed by CDP-DAG synthase (CDS) (Figure 6) [24]. There are 2 CDS isoforms, which are differentially regulated in HF in a species dependent fashion. CDS-2 is downregulated in adult human and rat heart failure, and CDS-1 is up-regulated in the rat with heart failure. We confirmed previous work showing that CDS-2 is down-regulated in adult human IDC, with no change in CDS-1 expression (data not shown). This observation may further implicate CDS-2 as the primary isoform important in human in mitochondria [43]. Unlike what was shown in adults, no change in CDS-1 or CDS-2 expression was appreciated in pediatric IDC.
The first committed step in CL biosynthesis is the condensation of CDP-DAG with sn-glycerol-3-phosphate to form phosphoglycerol-phosphate by PGP synthase (PGPS). In the SHHF rat, PGPS enzyme activity is significantly increased in heart failure, with up-regulation of mRNA early in HF that was attenuated with time [31]. A difference in the pattern of mRNA expression and enzymatic activity raises the possibility of post-translational modification in HF. We show here that in adult IDC, PGPS message is down-regulated, while no significant change is seen in pediatric disease.
The final two steps of mammalian de novo CL biosynthesis involve dephosphorylation of PGP, which is then conjugated with another CDP-DAG to form nascent CL, catalyzed by CLS. Previous studies in the SHHF rat and adult human tissue showed that while CLS enzymatic activity and mRNA expression are down-regulated in the rat, a significant change in expression was not appreciated in adult IDC. PGPP expression was not previously evaluated. Our results show that while PGPP expression is not altered in adult IDC, it is significantly down-regulated as is CLS in pediatric disease. We confirmed that no significant difference in CLS is observed in adult IDC tissue compared to controls.
Mature CL requires transacylases, including tafazzin and MLCL-AT, which exchange non-linoleoyl moieties with linoleic acid to create a mature tetralinoleoyl-CL. In the SHHF rat and in adult IDC, tafazzin was shown to be significantly down-regulated, while MLCL-AT enzymatic activity was elevated in the failing rat heart. mRNA expression was not evaluated, as the gene encoding the transacylase was more recently identified[35]. Here we confirm that tafazzin is down-regulated in adult IDC, with no significant change observed in the pediatric cohort. MLCL-AT expression, in contrast is down-regulated in both adult and pediatric IDC. However, in the pediatric group, lower MLCL-AT expression is accounted for primarily by the adolescent patient samples. MLCL-AT enzyme expression is preserved in the younger pediatric group with IDC. This difference in enzyme expression may indicate a transition from pediatric to adult expression patterns in adolescent patients in response to heart failure. Additionally, downregulation of PGPP, CLS, and iPLA2γ, and upregulation of iPLA2-G6 are not statistically significant in the adolescent group, which may also reflect a transition to the adult expression pattern in response to disease, but may be related to smaller numbers of subjects in the adolescent subset of patients.
The activity of phospholipases is likely to play an additional role in remodeling of CLs, although the critical phospholipases have yet to be fully characterized in humans. Previous work has implicated calcium-independent phospholipases, iPLA2-γ and iPLA2-G6 in particular, as having a critical role in regulating CL composition, with CL remodeling being at least partially dependent on phospholipase activity in a rat myocyte cell culture model [22]. In a iPLA2-γ knock-out mouse, increased mortality occurs with aortic banding due to heart failure with reduced mitochondrial respiration, and depleted total CL and (18:2)4CL [44]. A myocardial iPLA2-γ gain-of-function mouse generated by the same group demonstrates a very interesting phenotype with significantly reduced myocardial phospholipid mass, exacerbated by fasting which results in accumulation of triglycerides and myocardial dysfunction [45]. These mice also have disorganized mitochondrial ultrastructure and evidence of disrupted metabolism at baseline. A group studying a Drosophila model of Barth syndrome was able to reverse the fly sterility phenotype related to abnormal CL remodeling by knocking down iPLA2VIA, the fly homolog to iPLA2-G6 [23]. Thus in flies and mice there is evidence that one or both of these phospholipases is important in CL composition and mitochondrial homeostasis. This body of work suggests that iPLA2-γ and iPLA2-G6 may both play a role in remodeling of CLs to create a mitochondrial environment rich in (18:2)4CL, and also play a role in degradation to generate bioactive lipids for signaling and inflammation. A balanced level of activity is likely necessary to maintain the CL pool. Interestingly, in our study of expression of phospholipases in children with IDC, we show that iPLA2-γ is significantly downregulated, while upregulation of iPLA2-G6 is observed. A counter-balance of activities may be necessary to maintain CL pools by iPLA2-γ, while upregulation of iPLA2-G6 is involved in mobilizing phospholipids for eicosanoid production or other lipid signaling cascades.
5. Conclusions
In summary, Cardiolipin biosynthesis and remodeling is deranged in pediatric heart failure presenting as IDC which results in total myocyte CL depletion and lower levels of (18:2)4CL, necessary for normal mitochondrial function. In adults we have shown that (18:2)4CL as a fraction of total CL is notably lower in IDC, and this trend is observed in a cohort of children with IDC as well. In both children and adults, mitochondrial content is preserved in IDC. The effect of heart failure on total CL levels is similar to that seen in adults, but there may be differences in individual CL isoform content between children and adults with HF. Moreover, there are distinct differences in expression of CL biosynthetic, remodeling and degrading enzymes between adults and children. These differences are likely secondary to unique age and disease-related mechanisms. While a number of other variables, including medical treatment, time-course of heart failure, and genetic etiology of IDC may play a role in mitochondrial phospholipid alterations; this cross-sectional data suggests that age-related mechanisms play a role in progression of heart failure. Pediatric and adult IDC may have similar clinical phenotypes, however, these data support the concept that the mechanisms of disease are different, helping to explain the failure of adult therapies to effectively manage pediatric heart failure. A more tailored therapeutic approach to treating children with IDC may be possible with a better understanding of changes in mitochondrial energy metabolism that occur in heart failure. Moreover, an understanding of how CL biosynthesis and remodeling is regulated and dysregulated in heart failure will also inform use of an appropriate animal model with which a more functional experimental approach can be used to further investigate the role of CL content changes in IDC.
Supplementary Material
Highlights.
Cardiolipin (CL) quantity and composition are altered in adult Idiopathic Dilated Cardiomyopathy (IDC).
We demonstrate that total and (18:2)4CL are also depleted in pediatric IDC.
Total mitochondrial content is preserved in myocardium of children and adults with IDC.
Expression of biosynthesis, remodeling, and degradation enzymes are altered in pediatric and adult IDC.
Expression patterns of CL biosynthesis, remodeling and degradation enzymes are dissimilar in adult and pediatric disease.
Acknowledgments
Funding Sources Supported by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154 and NIH/NHLBI R01HL107715. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures Carmen Sucharov: Equity in miRagen, Inc., Brian Stauffer: Research support from Forest Laboratories, Inc. No other authors have any disclosures.
References
- [1].Arola A, Jokinen E, Ruuskanen O, Saraste M, Pesonen E, Kuusela AL, et al. Epidemiology of idiopathic cardiomyopathies in children and adolescents. A nationwide study in Finland. Am J Epidemiol. 1997;146:385–93. doi: 10.1093/oxfordjournals.aje.a009291. [DOI] [PubMed] [Google Scholar]
- [2].Hollander SA, Bernstein D, Yeh J, Dao D, Sun HY, Rosenthal D. Outcomes of children following a first hospitalization for dilated cardiomyopathy. Circ Heart Fail. 2012;5:437–43. doi: 10.1161/CIRCHEARTFAILURE.111.964510. [DOI] [PubMed] [Google Scholar]
- [3].Nugent AW, Daubeney PEF, Chondros P, Carlin JB, Cheung M, Wilkinson LC, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348:1639–46. doi: 10.1056/NEJMoa021737. [DOI] [PubMed] [Google Scholar]
- [4].Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- [5].Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:1377–84. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- [6].Bublik N, Alvarez JA, Lipshultz SE. Pediatric Cardiomyopathy as a Chronic Disease: A Perspective on Comprehensive Care Programs. Prog Pediatr Cardiol. 2008;25:103–11. doi: 10.1016/j.ppedcard.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rossano JW, Kim J, Decker JA, Price JF, Zafar F, Graves DE, et al. Increasing prevalence and hospital charges in pediatric heart failure related hospitalizations in the United States: a population-based study [abstract] Circulation. n.d.;122. [Google Scholar]
- [8].Lowry AW, Knudson JD, Cabrera AG, Graves DE, Morales DLS, Rossano JW. Cardiopulmonary resuscitation in hospitalized children with cardiovascular disease: estimated prevalence and outcomes from the kids’ inpatient database. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2013;14:248–55. doi: 10.1097/PCC.0b013e3182713329. [DOI] [PubMed] [Google Scholar]
- [9].Cubbon RM, Gale CP, Kearney LC, Schechter CB, Brooksby WP, Nolan J, et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4:396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- [10].Blume ED, Canter CE, Spicer R, Gauvreau K, Colan S, Jenkins KJ. Prospective single-arm protocol of carvedilol in children with ventricular dysfunction. Pediatr Cardiol. 2006;27:336–42. doi: 10.1007/s00246-005-1159-1. [DOI] [PubMed] [Google Scholar]
- [11].Harmon WG, Sleeper LA, Cuniberti L, Messere J, Colan SD, Orav EJ, et al. Treating children with idiopathic dilated cardiomyopathy (from the Pediatric Cardiomyopathy Registry) Am J Cardiol. 2009;104:281–6. doi: 10.1016/j.amjcard.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chinnery PF. Mitochondrial Disorders Overview. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. GeneReviews™. University of Washington, Seattle; Seattle (WA): 1993. [PubMed] [Google Scholar]
- [13].Mohammed S, Bahitham W, Chan A, Chiu B, Bamforth F, Sergi C. Mitochondrial DNA related cardiomyopathies. Front Biosci Elite Ed. 2012;4:1706–16. doi: 10.2741/491. [DOI] [PubMed] [Google Scholar]
- [14].Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- [15].Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–9. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–50. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- [17].Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, et al. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–80. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- [18].Schwall CT, Greenwood VL, Alder NN. The stability and activity of respiratory Complex II is cardiolipin-dependent. Biochim Biophys Acta BBA – Bioenerg. 2012;1817:1588–96. doi: 10.1016/j.bbabio.2012.04.015. [DOI] [PubMed] [Google Scholar]
- [19].Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–6. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- [20].Zhang M, Mileykovskaya E, Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J Biol Chem. 2005;280:29403–8. doi: 10.1074/jbc.M504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schlame M, Ren M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006;580:5450–5. doi: 10.1016/j.febslet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- [22].Zachman DK, Chicco AJ, McCune SA, Murphy RC, Moore RL, Sparagna GC. The role of calcium-independent phospholipase A2 in cardiolipin remodeling in the spontaneously hypertensive heart failure rat heart. J Lipid Res. 2010;51:525–34. doi: 10.1194/jlr.M000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Malhotra A, Edelman-Novemsky I, Xu Y, Plesken H, Ma J, Schlame M, et al. Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proc Natl Acad Sci U S A. 2009;106:2337–41. doi: 10.1073/pnas.0811224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- [25].Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- [26].Kiebish MA, Yang K, Sims HF, Jenkins CM, Liu X, Mancuso DJ, et al. Myocardial regulation of lipidomic flux by cardiolipin synthase: setting the beat for bioenergetic efficiency. J Biol Chem. 2012;287:25086–97. doi: 10.1074/jbc.M112.340521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kiebish MA, Yang K, Liu X, Mancuso DJ, Guan S, Zhao Z, et al. Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. J Lipid Res. 2013;54:1312–25. doi: 10.1194/jlr.M034728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–70. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- [29].Chicco AJ, Sparagna GC, McCune SA, Johnson CA, Murphy RC, Bolden DA, et al. Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared with lard and low-fat diets. Hypertension. 2008;52:549–55. doi: 10.1161/HYPERTENSIONAHA.108.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mulligan CM, Sparagna GC, Le CH, De Mooy AB, Routh MA, Holmes MG, et al. Dietary linoleate preserves cardiolipin and attenuates mitochondrial dysfunction in the failing rat heart. Cardiovasc Res. 2012;94:460–8. doi: 10.1093/cvr/cvs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, et al. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res. 2009;50:1600–8. doi: 10.1194/jlr.M800561-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46:1196–204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- [33].Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Houtkooper RH, Rodenburg RJ, Thiels C, van Lenthe H, Stet F, Poll-The BT, et al. Cardiolipin and monolysocardiolipin analysis in fibroblasts, lymphocytes, and tissues using high-performance liquid chromatography-mass spectrometry as a diagnostic test for Barth syndrome. Anal Biochem. 2009;387:230–7. doi: 10.1016/j.ab.2009.01.032. [DOI] [PubMed] [Google Scholar]
- [35].Taylor WA, Mejia EM, Mitchell RW, Choy PC, Sparagna GC, Hatch GM. Human Trifunctional Protein Alpha Links Cardiolipin Remodeling to Beta-Oxidation. PloS One. 2012;7:e48628. doi: 10.1371/journal.pone.0048628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lewin MB, Timiras PS. Lipid changes with aging in cardiac mitochondrial membranes. Mech Ageing Dev. 1984;24:343–51. doi: 10.1016/0047-6374(84)90119-2. [DOI] [PubMed] [Google Scholar]
- [37].Lee H-J, Mayette J, Rapoport SI, Bazinet RP. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids Health Dis. 2006;5:2. doi: 10.1186/1476-511X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lesnefsky EJ, Hoppel CL. Cardiolipin as an oxidative target in cardiac mitochondria in the aged rat. Biochim Biophys Acta. 2008;1777:1020–7. doi: 10.1016/j.bbabio.2008.05.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lesnefsky EJ, Minkler P, Hoppel CL. Enhanced modification of cardiolipin during ischemia in the aged heart. J Mol Cell Cardiol. 2009;46:1008–15. doi: 10.1016/j.yjmcc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [40].Ahuja P, Wanagat J, Wang Z, Wang Y, Liem DA, Ping P, et al. Divergent Mitochondrial Biogenesis Responses in Human Cardiomyopathy. Circulation. 2013;127:1957–67. doi: 10.1161/CIRCULATIONAHA.112.001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Barth AS, Kumordzie A, Frangakis C, Margulies KB, Cappola TP, Tomaselli GF. Reciprocal transcriptional regulation of metabolic and signaling pathways correlates with disease severity in heart failure. Circ Cardiovasc Genet. 2011;4:475–83. doi: 10.1161/CIRCGENETICS.110.957571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sihag S, Li AY, Cresci S, Sucharov CC, Lehman JJ. PGC-1? and ERR? target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–12. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lykidis A, Jackson PD, Rock CO, Jackowski S. The role of CDP-diacylglycerol synthetase and phosphatidylinositol synthase activity levels in the regulation of cellular phosphatidylinositol content. J Biol Chem. 1997;272:33402–9. doi: 10.1074/jbc.272.52.33402. [DOI] [PubMed] [Google Scholar]
- [44].Mancuso DJ, Sims HF, Han X, Jenkins CM, Guan SP, Yang K, et al. Genetic Ablation of Calcium-independent Phospholipase A2γ Leads to Alterations in Mitochondrial Lipid Metabolism and Function Resulting in a Deficient Mitochondrial Bioenergetic Phenotype. J Biol Chem. 2007;282:34611–22. doi: 10.1074/jbc.M707795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mancuso DJ, Han X, Jenkins CM, Lehman JJ, Sambandam N, Sims HF, et al. Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent phospholipase A2gamma. J Biol Chem. 2007;282:9216–27. doi: 10.1074/jbc.M607307200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.