Abstract
Many important components of the cardiovascular system display circadian rhythmicity. In both humans and mice, cardiac damage from ischemia/reperfusion (I/R) is greatest at the transition from sleep to activity. The causes of this window of susceptibility are not fully understood. In the murine heart we have reported high amplitude circadian oscillations in expression of the cardioprotective protein regulator of calcineurin 1 (Rcan1). This study was designed to test whether Rcan1 contributes to the circadian rhythm in cardiac protection from I/R damage. Wild type (WT), Rcan1 KO, and Rcan1-Tg mice, with cardiomyocyte-specific overexpression of Rcan1, were subjected to 45 minutes of myocardial ischemia followed by 24 hours of reperfusion. Surgeries were performed either during the first two hours (AM) or the last two hours (PM) of the animal’s light phase. The area at risk was the same for all genotypes at either time point; however, in WT mice, PM-generated infarcts were 78% larger than AM-generated infarcts. Plasma cardiac troponin I levels were likewise greater in PM-operated animals. In Rcan1 KO mice there was no significant difference between the AM- and PM-operated hearts, which displayed greater indices of damage similar to that of PM-operated WT animals. Mice with cardiomyocyte-specific over expression of human RCAN1, likewise, showed no time-of-day difference, but had smaller infarcts comparable to those of AM-operated WT mice. In vitro, cardiomyocytes depleted of RCAN1 were more sensitive to simulated I/R and the calcineurin inhibitor, FK506, restored protection. FK506 also conferred protection to PM-infarcted WT animals. Importantly, transcription of core circadian clock genes was not altered in Rcan1 KO hearts. These studies identify the calcineurin/Rcan1-signaling cascade as a potential therapeutic target through which to benefit from innate circadian changes in cardiac protection without disrupting core circadian oscillations that are essential to cardiovascular, metabolic, and mental health.
Keywords: circadian rhythms, ischemia-reperfusion, signal transduction, calcineurin, Rcan1, cardioprotection
1. Introduction
Circadian rhythms are self-sustaining, 24-hour cycles in molecular, biochemical, and behavioral parameters that help an organism prepare for anticipated changes in physiological demand. The molecular basis of the circadian clock consists of cell-autonomous interlocking positive and negative transcriptional and posttranscriptional feedback loops [1]. The “master clock”, located in the suprachiasmatic nucleus (SCN) within the hypothalamus, responds to changes in daily light cycles and influences the phase of independent molecular clocks found in peripheral organs, including the heart. Many important cardiovascular factors, including metabolism, heart rate, blood pressure, and hormone release, oscillate over a 24-hour period [2–6]. Clinical and experimental studies have demonstrated repeatedly the importance of circadian rhythms in cardiovascular health and the need to maintain proper coordination of rhythmic processes. For instance, in humans, the incidence of myocardial infarction (MI) [7, 8], sudden cardiac death [9, 10], ventricular tachyarrhythmias [11], and rupture of aortic aneurysms [12–14] peaks in the morning around the time of transition from sleep to waking [15, 16]. The strength and persistance of the intrinsic circadian clock is such that the incidence of sudden cardiac death in long distance travelers peaks at a time corresponding to the early morning in the time zone from which the traveler originated [17].
Although it is well accepted that circadian rhythms influence the timing of MI in humans, whether there is a specific time of day when the heart sustains more damage from a heart attack remains controversial in both the clinical and basic literature. Both cardiac intrinsic and extrinsic oscillations contribute to maintenance of cardiac function and many of these certainly have the potential to impact the susceptibility ot the heart to damage. Retrospective studies in humans argue both for and against the time-of-day of the on-set of ST segment elevation myocardial infarction (STEMI) influencing infarct size and/or long-term outcomes [18–28]. Using an elegant closed-chest model of I/R, Durgan et al demonstrated that the murine heart is more susceptible to damage from I/R at the time of day when mice become active, than when they transition to a period of rest [3]. However, other studies using different techniques and modes of analysis have drawn the opposite conclusion [29].
In addition to cycling of transcriptional clock components, many cells and tissues also display persistent circadian fluctuations in cytoplasmic Ca2+ levels [30, 31]. Dysregulation of Ca2+ handling is a hallmark of heart disease. Several Ca2+-responsive signaling pathways have been causally linked to the progression of heart failure [32]. Prominent among these is the Ca2+-activated protein phosphatase calcineurin. Sustained activation of calcineurin is sufficient to drive pathological hypertrophic remodeling of the myocardium with subsequent heart failure and premature death [33]. The regulators of calcineurin (RCANs) are a family of proteins that can bind directly to the catalytic subunit of calcineurin and inhibit its activity [34]. Expression of the exon 4 isoform of Rcan1 (Rcan1.4) is under the control of calcineurin and thus functions as an endogenous feedback inhibitor, protecting cells from unrestrained calcineurin activity [35]. In mice, cardiac-specific expression of an Rcan1 transgene blunts hypertrophic growth and inhibits pathological remodeling of the heart in response to a variety of stresses [36–38]. Mice with a disruption of the Rcan1 gene are reported to sustain more damage from I/R than in WT mice [39].
Our laboratory recently demonstrated a strong circadian oscillation in protein and transcript levels of Rcan1.4 in the hearts of normal, healthy mice indicating a circadian pattern of activation of the cardioprotective calcineurin/Rcan1.4 feedback loop [40]. The peaks in both transcript and protein levels occurred in the early morning coinciding with the transition to rest and the time of day when the murine heart is reported to be most resistant to damage from I/R [3]. Based on these observations we postulated that circadian changes in RCAN1 abundance or calcineurin activity could contribute to the circadian rhythm in protection of the heart to damage from I/R.
2. Materials and Methods
2.1. Animals
C57BL/6:129 mixed background, male, wild type (WT), Rcan1 KO [41], and Rcan1-Tg [37] mice were raised and maintained in ventilated chambers outfitted with independent lighting systems on a 12:12 light:dark cycle. One chamber was set for lights to come on at 10 AM and off at 10 PM (AM Box). The other chamber was set for lights to come on at midnight and off at noon (PM Box). Cardiac function was assessed by echocardiography in unanaesthetized animals using the VisualSonics Vevo 770 imaging system. All animal procedures were carried out with the oversight and approval of the University’s Institutional Animal Care and Use Committee and conformed to the current Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health.
2.2. Ischemia/reperfusion (I/R)
Surgeries were performed on males between 10 and 14 weeks of age using standard procedures [42]. Briefly, animals were anesthetized with a cocktail of 8 mg/kg xylazine and 60 mg/kg ketamine (IP). Anesthesia was maintained via inhalation of isofluorane. A warming pad was used to maintain constant body heat at 37°C. Ligation of the left anterior descending (LAD) coronary artery was performed at a level resulting in a large ischemic area encompassing 60% of the left ventricle (LV). After 45 min of ischemia the ligation was removed and reperfusion confirmed visually. Animals that did not achieve complete reperfusion were excluded from the study. The chest cavity was closed and sutured. After recovery from anesthesia the animal was returned to it’s respective light cycle box. FK506 or vehicle was administered immediately following reperfusion (cutting of the ligation) at a dose of 0.05 mg/kg in 0.1% cremophore in physiological saline injected directly into the LV lumen through the wall of the apex using a tuberculin syringe. Subsequent coronary perfusion then allowed delivery of the drug directly to the area at risk.
2.3. Assessment of area at risk (AAR) and infarct (INF)
After 24 hrs of reperfusion, the animal was re-anesthetized, intubated, and blood drawn for assessing cardiac troponin I levels. The right carotid artery was cannulated and 100µl of heparin (50 units/ml) perfused through the carotid artery. The LAD was ligated in the same location as the previous day’s surgery. Evan’s blue was injected through the catheter to delineate the ischemic (AAR) from nonischemic zones. The excised heart was sliced coronally at 1mm intervals from the point of LAD ligation to the apex. The slices were placed in 1% 2,3,5-Triphenyltetrazolium chloride (TTC) for 4 minutes to differentiate between live and necrotic (INF) areas of the myocardium. Photographs were taken of each side of each heart slice to calculate AAR, INF, and remote area using Image J. Heart slices were weighed and the percent AAR per left ventricle (%AAR/LV) and percent infarct area per AAR (%INF/AAR) calculated. Troponin levels in the cleared plasma were measured using a High Sensitivity ELISA assay for cardiac troponin-I in mouse plasma (Life Diagnostics). For terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), hearts were excised 24-hours after reperfusion and fixed in 4% paraformaldehyde. Five-micron thick coronal sections were cut every 500 microns from the LAD ligation to the apex. Sections were stained using the Promega DeadEnd Fluorometric TUNEL System kit (G3250).
2.3. Real-time PCR and Immuno-analysis
Total RNA was extracted using Trizol (Invitrogen) and first strand cDNA synthesized from 2 µg of RNA using Superscript III (Invitrogen). Real-time PCR was carried out in a Roche 480 Lightcycler. Primers sequences were as reported previously [40]. Transcript levels were normalized to 18s rRNA. To assess circadian changes in gene expression WT and Rcan1 KO mice were entrained to a 12:12 light:dark cycle for two weeks and then shifted to constant darkness at the beginning of a dark phase. Three hearts from each genotype were harvested for each time point.
Total soluble protein extracts were isolated in M-PER reagent (Pierce) with protease and phosphatase inhibitors using a Dounce homogenizer. Protein extracts (20µg) were fractionated by SDS-PAGE, transferred onto a nitrocellulose, blocked, then probed with primary antibodies for RCAN1 (Sigma D6694), α-tubulin (Sigma T5168), or calcineurin (Chemicon AB1695). Secondary antibodies were conjugated with an infrared label. Blots were scanned using the Odyssey Imaging System (LI-COR Biosciences).
2.4. Simulated ischemia reperfusion (sim-I/R)
Neonatal rat ventricular myocytes (NRVMs) were isolated and cultured as described previously [43]. Cells were grown for 48 hours in DMEM:M199 (4:1), 10% FBS, with BrdU and antibiotics, then transfected with a control siRNA (UGGUUUACAUGUCGACUAA) or ones targeting RCAN1 (UGGAGGAGGUGGAUCUGCAUUU and GAUGAUGUCUUCAGCGAAAUU) (Dharmacon ON-TARGETplus) using Lipofectamine®RNAiMax reagent. 48 hours after transfection, media was changed to ischemia-mimicking solutions containing 5mM HEPES, 10mM 2-deoxy-D-glucose, 139mM, 12mM KCl, 0.5mM MgCl2, 1.3mM CaCl2, 20mM and lactic acid, pH 6.2, then incubated under 100% nitrogen (O2<1%) at 37°C for 6 hours. The cultures were then returned to normal culture conditions for 12 hours, DMEM/M199 (4:1), 5% FBS; 37°C ambient air, 5% CO2. Release of lactate dehydrogenase (LDH) into the media was measured using the Promega CytoTox 96® kit.
2.5. Statistical analysis
Analysis of Variance statistics were performed with a Bonferonni post-test using GraphPad Prism to determine statistical significance for %AAR/LV, %INF/AAR, circulating troponin I, and %FS. Student’s t-test was used for protein and transcript analysis.
3. Results
3.1. Damage is greater in hearts subjected to I/R at the end of the light phase than at the beginning of the light phase
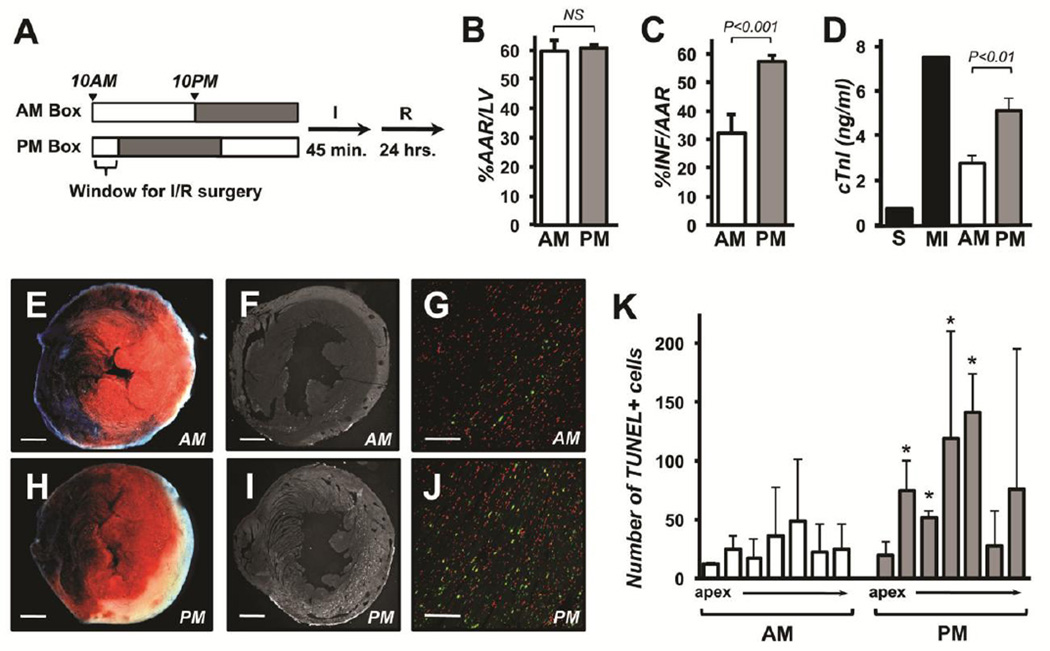
To test our hypothesis, we designed a model in which the surgeon was blinded to the circadian time of the animal being operated upon (Fig 1A). Male, WT, C56BL/6:129 mixed background, mice were raised and maintained in light-tight ventilated chambers outfitted with independent lighting systems. The AM box was set for the lights to go on at 10:00 AM and off at 10:00 PM. The PM box was set for the lights to come on at midnight and off at noon. The two chambers were identical so that the surgeon was unaware as to which was the AM or PM box. All surgeries were performed between 10:00 AM and noon, corresponding to zeitgeber time (ZT) 0–2 for the animals from the AM chamber and ZT:10–12 for the PM animals. Performing surgeries only during the light phase of the circadian day avoided the problem of exposing the animals to a phase-shifting stimulus during the operation. Ligation of the left anterior artery (LAD) was performed as described in methods. Following 45 minutes of ischemia, arterial flow was restored and mice were returned to their respective light chambers. Hearts were analyzed twenty-four hours later to quantify AAR and INF.
Figure 1.
Damage from I/R is greater at the end of the light phase (PM) than at the beginning of the light phase (AM) in wild type mice. The schematic illustrates the timing of light entrainment and I/R surgeries for the AM and PM boxes (A). Grey shadowing indicates periods of “lights-off”. Area at risk (%AAR/LV) (B), infarct (%INF/AAR) (C), and circulating cardiac troponin I levels (D) in the AM (n=7) or PM (n=10) operated animals 24 hours after I/R. Troponin levels in sham operated animals (S) and animals with permanent LAD (MI) are provided for comparison. Representative images of TTC and Evans blue staining (E and H) and TUNEL-positive signal (F, G, I and H) are provided for AM (E, F, and G) PM (H, I, and J) surgeries. Green indicates TUNEL-positive nuclei and red total nuclei in the image overlays in G and J. The inset bar indicates either 1 mm (in E, F, H, and I) or 0.1 mm (G and H). Quantification of TUNEL-positive signals in individual 1mm slices arrayed from apex to the site of ligation from AM and PM hearts 24 hours after I/R. (n=3 per slice). Error bars indicate standard error.
The %AAR/LV was the same between the two groups (Fig 1B). However, %INF/AAR was 78% larger in hearts subjected to I/R at the end of the light phase than in those challenged at the beginning of the light phase (Fig 1C, E, and H). Consistent with this, circulating cardiac troponin I levels were higher in the PM group than in the AM group indicative of greater muscle damage (Fig 1D). There were fewer TUNEL-positive cells in LV cross-sections from the AM hearts (Fig 1F, G, and K) than in sections from the PM hearts (Fig 1I, J, and K). These data demonstrate that the time of day has a large influence on the extent of damage in the open-chest model of I/R, an animal model used widely by investigators. The peak in cardioprotection occurred at the dark to light transition similar to that reported earlier in a closed-chest model of I/R using WT C57BL/6 mice [3]. Thus, the circadian changes in sensitivity to I/R are not specific to the technique used, genetic background, or operator bias, as the surgeon in our study was blinded as to the circadian time of the mouse.
3.2. Rcan1.4 expression increases in response to I/R
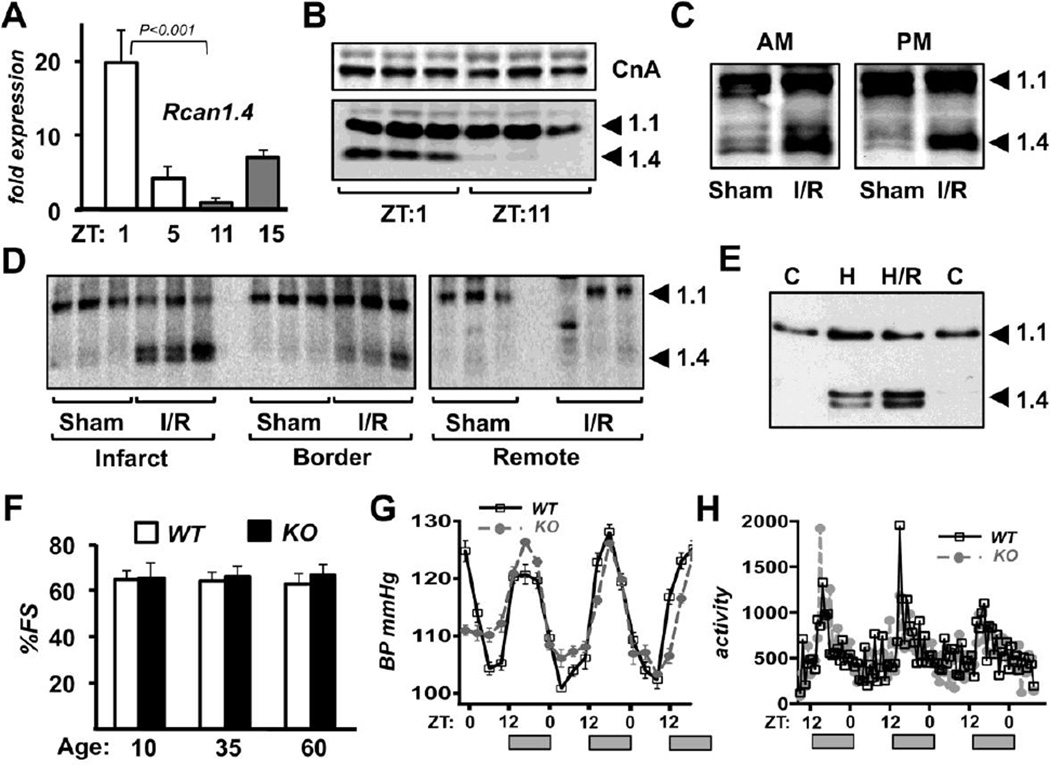
There was a circadian pattern of Rcan1.4 expression in the hearts of the C57BL/6:129 mixed background mice similar to that reported for inbred C57BL/6, C3H, and 129 lines [40]. Rcan1.4 expression was highest at ZT:1, around the time of the AM surgeries, and lowest at ZT:11, around the time of the PM surgeries (Fig 2A). RCAN1.4 protein levels were also higher at ZT:1 than at ZT:11 (Fig 2B and S1A). Protein levels of RCAN1.1 and the catalytic subunit of calcineurin (CnA) were not different between the two time points (Fig 2B, S1B-C). Three hours after I/R RCAN1.4 protein levels were elevated in the infarct zone regardless of whether I/R occurred in the AM or PM (Fig 2C and S1D) indicating that the calcineurin/Rcan1.4 feedback loop is activated in response to I/R regardless of the time of day the challenge occurs. Three hours after I/R corresponds approximately to ZT:5 for the AM animals and ZT:15 for the PM animals when Rcan1.4 transcript levels are similar but either declining or increasing respectively (Fig 2A). Thus, at least at these two time points, it appears that stress activation of Rcan1.4 expression over-rides underlying circadian control. Twenty-four hours after I/R, RCAN1.4 protein levels were still elevated in the infarct and border zones but not in the remote tissue (Fig 2D and S1E), indicating sustained activation of the calcineurin/Rcan1.4 feedback loop primarily in the regions of the heart with greatest damage. Extended hypoxia and hypoxia/reperfusion increased RCAN1.4 protein levels in cultured NRVMs (Fig 2E and S1F) suggesting that oxidative stress can activate the Rcan1.4 feedback loop in isolated cardiomyocytes. Taken as a whole, these data demonstrate that prior to I/R the circadian pattern of Rcan1.4 expression coincides with resistance of the myocardium to I/R damage. Subsequent stress-dependent activation of Rcan1.4 is largely independent of time of day. Therefore, we hypothesize that differential levels of Rcan1.4 at the time of I/R, rather than its subsequent activation in response to stress contributes to the window of cardiac resistance at ZT:0–2.
Figure 2.
Rcan1.4 expression is highest in the AM and activated in response to I/R. Real-time PCR was used to compare Rcan1.4 transcript levels in the hearts of wild type mice at the Zeitgeber times (ZT) indicated in unoperated control animals (A). Signal was normalized to 18s rRNA. RCAN1 protein levels were assessed by western blot in control hearts (B) and hearts 3 hours (C) or 24 hours (D) after I/R. The surgeries in D were performed and analyzed at 4 PM (ZT:10) when RCAN1.4 levels are normally low. Western blot analysis was used to compare RCAN1 protein in NRVMs cultured at ambient O2 (C), or exposed to 1% O2 (99% N2) for 24 hours (H), then reperfused for 3 hours (H/R) (E). Cardiac function in wild type and Rcan1 littermates was assessed at 10, 35 and 60 weeks of age (F) (n=5 for each genotype). Blood pressure was monitored over a 60 hour time period starting at 6AM (G). Physical activity was monitored over a 60 hour time period starting at 6PM (H) (n=3 each genotype). Grey bars along x-axis indicate timing of light cycle relative to each graph.
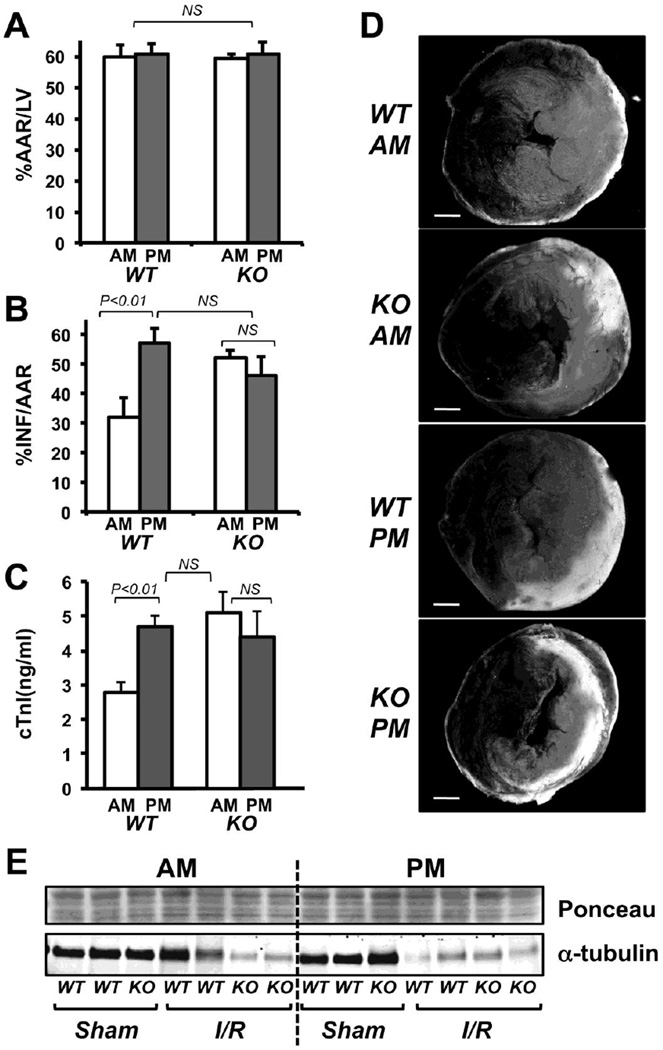
3.3. Hearts from Rcan1 KO mice lack time-of-day differences in susceptibility to I/R damage
Cardiac function in Rcan1 KO mice was compared to that of WT littermates by echocardiography. There was no difference in %FS at 10, 35 and 60 weeks of age (Fig 2F). Thus, there was no evidence of an age-dependent decline in cardiac function in Rcan1 KO mice. Circadian patterns in systolic blood pressure (Fig 2G) and physical activity (Fig 2H) were also comparable in Rcan1 KO and WT siblings. Rcan1 KO C57BL/6:129 mixed background mice were raised and entrained in the AM and PM light boxes then subjected to I/R at ZT:0–2 or ZT:10–12 as described for WT. There was no significant difference in %AAR/LV (Fig 3A), %INF/AAR (Fig 3B), or circulating cardiac troponin I levels (Fig 3C) in the AM operated Rcan1 KO hearts compared to the PM operated KO. Furthermore, damage was similar in the Rcan1 KO from either time point to that found in WT hearts subjected to I/R at the PM time point, when WT is most susceptible to damage (Fig 3B-D). Disruption of the microtubule network is an early sign of irreversible ischemic injury [44] and maintenance of microtubule integrity has been implicated in preconditioning resistance to I/R [45, 46]. We therefore examined changes in tubulin levels as an indication of disruption of the microtubular network. Western blot analysis of protein extracts from the infarct zone three hours after I/R showed a decrease in α-tubulin abundance compared to controls that mirrored the circadian and genotype pattern of damage (Fig 3E and S1G). In other words, the decrease in α-tubulin was greatest in the Rcan1 KO AM, Rcan1 KO PM and WT PM I/R samples but minimal in the WT AM I/R samples indicative of disruption of the microtubular network at 3 hours in a pattern mirroring the extent of myocardial damage at 24 hours post I/R. Taken together these results suggest that the Rcan1 gene is necessary for the window of protection from I/R in WT mice during the active to resting transition.
Figure 3.
Damage from I/R is greater in Rcan1 KO hearts than in wild type hearts and shows no AM window of protection as assessed by area at risk (A), infarct (B), and circulating cardiac troponin I levels (C). Representative images of TTC and Evans blue staining are provided (D). AM (n=5); PM (n=7). Alpha-tubulin levels in heart extracts three hours after surgery were compared for the various genotypes and treatments using western blot analysis (E). Ponceau staining is provided as a loading control. Error bars indicate standard error. The white bar in D indicates 1 mm.
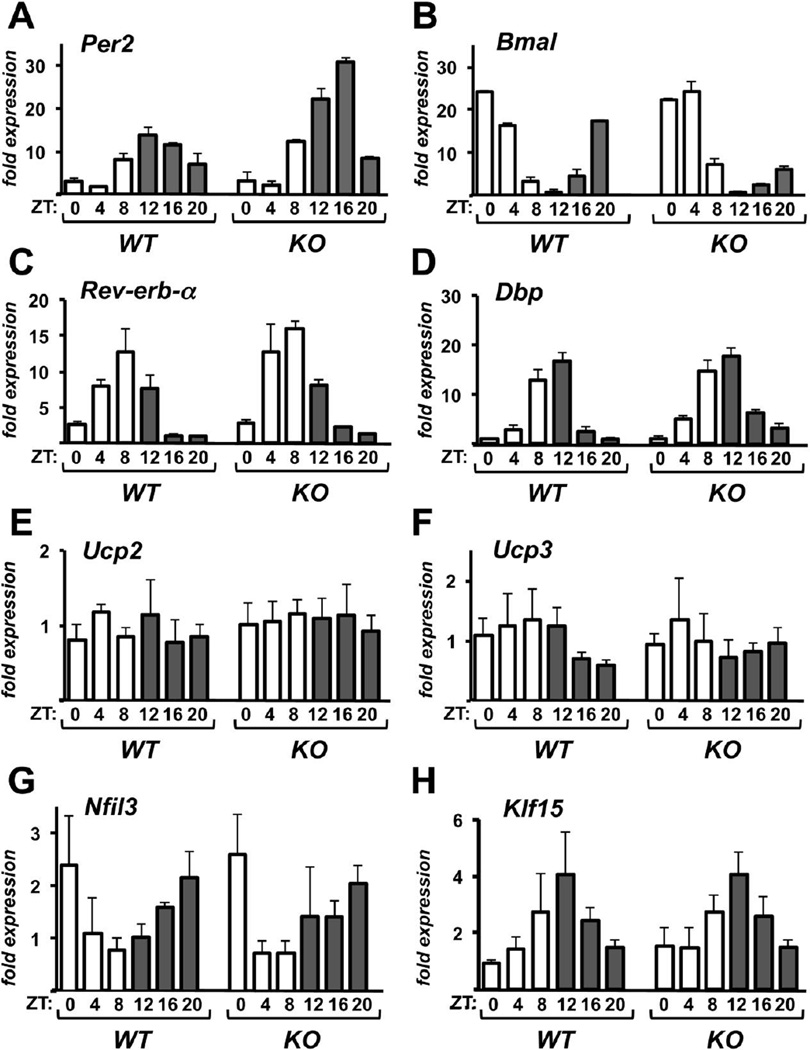
3.4. The hearts of Rcan1 KO mice have an intact transcriptional circadian clock
Our findings suggest that Rcan1 KO mice have lost a circadian oscillation in susceptibility of the heart to damage from I/R. We therefore asked whether the transcriptional circadian clock is either lost or substantially altered in Rcan1 KO hearts. The negative core clock gene Per2 (Fig 4A) and the positive core clock gene Bmal (Fig 4B) continued to oscillate under free-running conditions with opposing phases as in WT hearts. Expression of additional components of the transcriptional clock, including Rev-erb-α (Fig 4C) and Dbp (Fig 4D), showed circadian patterns of expression indistinguishable from that in WT hearts. Mitochondrial uncoupling is protective in I/R and expression of uncoupling proteins is circadian in many tissues including the heart [2]. Expression of the uncoupling proteins Ucp2 and Ucp3 was not significantly altered in the Rcan1 KO hearts (Fig 4E-F). Likewise, we did not see significant changes in expression of the apoptosis related proteins Bax and Bcl-2 (Fig S3A-B). Expression of the cardiac survival factor Nfil3 is circadian in the heart and in phase with Rcan1.4 expression [3]. However, the circadian expression of Nfil3 was also preserved in the Rcan1 KO (Fig 4G). The transcription factor Klf15 has been identified recently as an important regulator of circadian processes in the heart [47, 48]. Circadian expression of Klf15 was preserved in the Rcan1 KO (Fig 4G). These results demonstrate that the transcriptional circadian clock is intact in the hearts of Rcan1 KO mice. Therefore, the loss of time-of-day changes in cardiac susceptibility to I/R is not simply a secondary consequence of lost rhythmicity but more directly related to some downstream component of Rcan1 activity.
Figure 4.
Circadian expression of core clock genes is not disrupted in hearts from Rcan1 KO mice. Transcript levels for Per2 (A), Bmal (B), Rev-erb-α (C), Dbp (D), Ucp2 (E), Ucp3 (F), Klf15 (G), and Bax (H) were quantified by real-time PCR in the hearts of wild type and Rcan1 KO mice at the Zeitgeber times (ZT) indicated. Signal was normalized to 18s rRNA. RNA from three hearts was pooled for each time point. Error bars indicate standard deviation. (n=3 per time point)
3.5. Cardiomyocytes deficient for RCAN1 are more susceptible to I/R in vitro. Inhibiting calcineurin restores protection
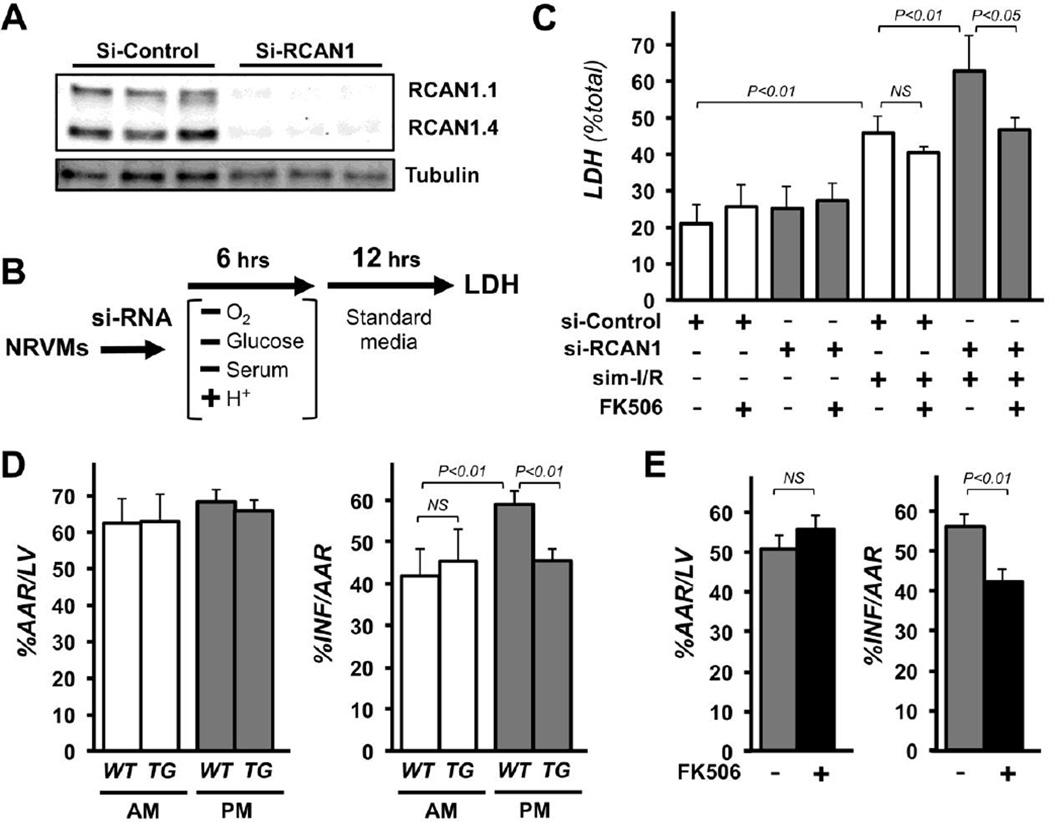
To assess whether RCAN1-mediated protection is myocyte autonomous or mediated through functions external to the heart, NRVMs were depleted of both RCAN1 isoforms using RCAN1-targeted siRNAs (Fig 5A). Two days after siRNA transfection cultures were subjected to simulated I/R (sim-I/R) as outlined in Fig 5B. Knock down of RCAN1 had no effect on cell viability at base line, but reduced cell survival following sim-I/R (Fig 5C). Furthermore, pharmacological inhibition of calcineurin using FK506 had no significant effect on viability of control cells but restored survival of RCAN1-depleted NRVMs following sim-I/R to that of control siRNA transfected NRVMs subjected to sim-I/R (Fig 5C). Taken together these studies suggest that RCAN1-dependent protection from I/R is cardiomyocyte autonomous, and that protection is conferred via its ability to inhibit calcineurin activity.
Figure 5.
Loss of RCAN1 sensitizes cardiac myocytes to I/R damage. Calcineurin inhibition restores protection. Depletion of RCAN1 proteins from NRVMs was verified by western blot 48 hours after transfection with control (si-control) or RCAN1-targeted si-RNA (si-RCAN1) (A). Tubulin was used as a loading control. Schematic depicts the parameters for simulated I/R (B). LDH release into the media was used to quantify cell death following sim-I/R. Cultures were treated with vehicle or FK506 prior to sim-I/R as indicated (C). A cardiomyocyte-specific RCAN1 transgene (TG) conferred protection from I/R damage in PM operated animals, but provides no increase in protection to AM-operated animals (D) (n=5 each genotype and time point). Injection of FK506 just prior to reperfusion confers protection to the PM-operated animals (E) (n=5 each treatment).
3.6. Calcineurin inhibition confers protection from I/R to PM-operated hearts
Genetic and pharmacological approaches were used to test whether time-of-day differences in calcineurin activity contribute to the time-of-day-dependent differences we observed in susceptibility of the heart to I/R damage. Transgenic mice expressing a human RCAN1 transgene under the control of the cardiomyocyte-specific αMHC promoter (αMHC-RCAN1) and WT littermates were subjected to I/R during either the AM or PM time point as previously described. The αMHC-RCAN1 transgene encodes aa 81–197 of hRCAN1 [37] which is common to both RCAN1 isoforms and provides potent calcineurin inhibition. In WT animals infarct size was significantly larger in PM-operated WT hearts than in AM-operated hearts as previously shown. The αMHC-RCAN1 transgene conferred protection from I/R to PM-operated animals, whereas, it provided no additional protection at the AM time point (Fig 5D). Pharmacological inhibition of calcineurin by administering FK506 immediately upon reperfusion verified that calcineurin inhibition was capable of providing protection from reperfusion damage at the PM time point (Fig 5E). These studies indicate that activation of calcineurin contributes to cardiac damage when I/R occurs near the transition to waking, but is less of a contributing factor when I/R occurs near the transition to rest.
4. Discussion
Taken as a whole, the current findings suggest that Rcan1 underlies circadian changes in cardiac tolerance to I/R and that it functions, at least in part, by controlling the activation of cardiomyocyte-localized calcineurin in response to I/R. Furthermore, Rcan1 accomplishes this, not by altering global circadian rhythmicity, but likely acts downstream as a mediator between the transcriptional clock mechanism and circadian changes in cardiac susceptibility to I/R damage. This is important because it positions calcineurin activity and Rcan1 as potential therapeutic targets through which to benefit from innate circadian changes in cardiac protection without disrupting core circadian oscillations that are essential to cardiovascular, metabolic, and mental health.
We show that in WT mice, peak expression of Rcan1.4 coincides with the time of greater resistance of the mouse heart to damage from I/R, at the transition from the active to resting phase. Rcan1 KO mice no longer have this window of protection and are equally susceptible to cardiac I/R damage at both the beginning and end of the light phase. Rcan1 KO mice lack both the Rcan1.4 and the Rcan1.1 isoforms; so either one may contribute protective functions. Repeated studies demonstrate that either isoform can act as a potent inhibitor of calcineurin. There is one study suggesting that, in the context of angiogenesis, RCAN1.4 inhibits calcineurin, whereas, RCAN1.1 may act to potentiate calcineurin-dependent responses [49], however, the molecular mechanism of any such isoform-specific functions remains undefined. Transcription of Rcan1.1 and Rcan1.4 is under the control of independent promoters and only Rcan1.4 acts a feedback inhibitor of calcineurin. Given the pronounced circadian oscillations in Rcan1.4 expression, it is reasonable to postulate that Rcan1.4 confers a key activity relevant to our studies. The cardiomyocyte-specific αMHC-RCAN1 transgene confers protection at the end of the light period as does systemic administration of FK506, whereas, αMHC-RCAN1 confers no additional protection at the beginning of the light phase. This suggests that differential calcineurin activation is a key element of the circadian response, thus RCAN1’s primary function in this context likely relates to its ability to inhibit calcineurin.
Many studies have implicated activation of calcineurin in I/R damage in neurons [50, 51], lung [52], kidney [53], and heart [54–57]. Pharmacological studies using cyclosporine A (CsA) to inhibit calcineurin need to be interpreted with caution because CsA also inhibits the mitochondrial permeability transition pore (MPTP) in a calcineurin-independent fashion and MPTP is an important component of the cascade through which I/R damage occurs [58]. However, FK506, does not inhibit MPTP and has also been shown to be cardioprotective in rat models of I/R [54–57, 59]. Our studies would suggest there are time of day differences in the activation of calcineurin in response to I/R, however, we were not able to detect significant AM/PM differences in in vitro biochemical assays of total calcineurin activity in heart tissue extracts (data not shown). These assays only assess total potential calcineurin activity, not the actual activity of calcineurin in the tissue. Likewise, they provide no information regarding subcellular pools of calcineurin or microdomains, which can be important for specificity of calcineurin signaling [60, 61].
Calcineurin has diverse functions in many tissues and processes, including synaptic signaling [62], survival of neurons [63, 64], immune responses [51], cytokine production [52], and angiogenesis [53], many of which are known to contribute to survival of the myocardium after I/R. It is therefore possible that multiple systems in the Rcan1 KO mice contribute to increased susceptibility to I/R and loss of the early morning window of protection from I/R damage, however, the αMHC-RCAN1 transgenic mice, along with our in vitro studies, demonstrate that an important component of this protective process is cardiomyocyte-intrinsic rather than the result of Rcan1-dependent functions in other tissue systems.
Our results, showing an increase in cardiac susceptibility to I/R at the time of transition to waking is consistent with the report of Durgan et al., who also measured infarct size and troponin levels 24 hours after I/R [3]. Interestingly, another recent study, analyzing infarct size only 3 hours after I/R, reported the opposite phase for peak sensitivity [29]. We speculate that these contrasting findings may reflect fundamental differences in the mechanism of cell damage during ischemia versus those set into play at reperfusion.
Retrospective studies in humans argue both for and against the time-of-day of STEMI onset influencing infarct size and/or long-term outcomes [18–28]. It has been suggested that in these epidemiological studies the type of medical intervention received is a key factor. In general, when the majority of patients underwent revascularization, a circadian component was observed, whereas, when most of the patients did not receive reperfusion intervention, no circadian-dependent differences were noted. Consistent with this, we find no evidence of circadian differences in the size of infarct following permanent LAD in WT mice, suggesting that circadian timing has an impact on events triggered by reperfusion, whereas death of myocytes during sustained ischemia is independent of the time of onset.
Our findings highlight the danger of ignoring the influence of the circadian clock in studies of cardiac I/R. For instance, it was previously reported that the Rcan1 KO is more susceptible to damage from I/R [39]. Our studies show that this is indeed true for animals subjected to I/R early in the light phase, however, the same experiment carried out at the end of the light phase would have drawn the conclusion that I/R damage in the Rcan1 KO was similar to that seen in wild type mice. Certainly there are circadian oscillations in many factors that can influence cardiovascular health. It is therefore essential to carefully control for the contribution of the circadian clock in experimental design. This includes not only controlling the window of time in which a procedure such as I/R is performed, but also considering the impact that a specific treatment might have on the entrainment of the animal prior to experimentation, such as forced treadmill exercise during an animal’s normal sleep period.
5. Conclusions
The present study demonstrates that circadian rhythms have a profound influence on the susceptibility of the heart to damage in a widely used mouse model of I/R and identifies Rcan1 control of calcineurin activation as necessary for this daily oscillation in cardiac resistance to damage. Rcan1 accomplishes this, not by altering global circadian rhythmicity, but likely acts downstream as a mediator between the transcriptional clock and circadian changes in protective mechanisms. Numerous studies have demonstrated links between cardiovascular disease and disruption of circadian homeostasis in humans. Man is diurnal (active during the day) whereas mice are nocturnal. Therefore, whether there is a reverse oscillation of Rcan1.4 in human heart remains a critical question, as does identifying the downstream nodal points through which Rcan1 confers protection.
Supplementary Material
Highlights.
Infarct size in the standard mouse model of cardiac I/R is highly dependent on the time of day.
The heart is protected from I/R at the transition to rest when RCAN1.4 levels peak.
Rcan1 KO mice lack this window of protection yet maintain normal circadian clock function.
Rcan1 protects via calcineurin inhibition and acts cardiomyocyte-autonomously.
Exogenous calcineurin inhibition protects when the heart is most vulnerable to I/R damage.
Acknowledgments
The authors would like to thank Dr. Steven McKnight for the generous use of his circadian entrainment equipment, Ms. Sandi Estill for technical assistance and advice, and Drs. Thomas Gillette and Anwarul Ferdous for their lively discussions and careful critiques of this manuscript.
Funding
This work was supported by the National Institutes of Health [HL072016 and HL097768] and the American Heart Association [0655202Y].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none declared.
Contributor Information
David Rotter, Email: david.rotter@utsouthwestern.edu.
D. Bennett Grinsfelder, Email: david.grinsfelder@utsouthwestern.edu.
Valentina Parra, Email: valentine.parra@utsouthwestern.edu.
Zully Pedrozo, Email: zpedrozo@gmail.com.
Sarvjeet Singh, Email: sarvjeet.singh@utsouthwestern.edu.
Nita Sachan, Email: nsachan@gmail.com.
Beverly A. Rothermel, Email: Beverly.rothermel@utsouthwestern.edu.
References
- 1.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 2.Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, et al. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530–H1541. doi: 10.1152/ajpheart.00406.2005. [DOI] [PubMed] [Google Scholar]
- 3.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, et al. Short communication: ischemia/reperfusion tolerance is time-of-daydependent: mediation by the cardiomyocyte circadian clock. Circ Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portman MA. Molecular clock mechanisms and circadian rhythms intrinsic to the heart. Circ Res. 2001;89:1084–1086. [PubMed] [Google Scholar]
- 5.Richards AM, Nicholls MG, Espiner EA, Ikram H, Cullens M, Hinton D. Diurnal patterns of blood pressure, heart rate and vasoactive hormones in normal man. Clin Exp Hypertens A. 1986;8:153–166. doi: 10.3109/10641968609074769. [DOI] [PubMed] [Google Scholar]
- 6.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290:H1–H16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 7.Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 9.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 10.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- 11.Eksik A, Akyol A, Norgaz T, Aksu H, Erdinler I, Cakmak N, et al. Circadian pattern of spontaneous ventricular tachyarrhythmias in patients with implantable cardioverter defibrillators. Med Sci Monit. 2007;13:CR412–CR416. [PubMed] [Google Scholar]
- 12.Manfredini R, Fabbian F, Manfredini F, Salmi R, Gallerani M, Bossone E. Chronobiology in aortic diseases - "is this really a random phenomenon?". Prog Cardiovasc Dis. 2013;56:116–124. doi: 10.1016/j.pcad.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Manfredini R, Boari B, Gallerani M, Salmi R, Bossone E, Distante A, et al. Chronobiology of rupture and dissection of aortic aneurysms. J Vasc Surg. 2004;40:382–388. doi: 10.1016/j.jvs.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Sumiyoshi M, Kojima S, Arima M, Suwa S, Nakazato Y, Sakurai H, et al. Circadian, weekly, and seasonal variation at the onset of acute aortic dissection. Am J Cardiol. 2002;89:619–623. doi: 10.1016/s0002-9149(01)02311-6. [DOI] [PubMed] [Google Scholar]
- 15.Martino TA, Sole MJ. Molecular time: an often overlooked dimension to cardiovascular disease. Circ Res. 2009;105:1047–1061. doi: 10.1161/CIRCRESAHA.109.206201. [DOI] [PubMed] [Google Scholar]
- 16.la Sierra de A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–472. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]
- 17.Couch RD. Travel, time zones, and sudden cardiac death. Emporiatric pathology. Am J Forensic Med Pathol. 1990;11:106–111. doi: 10.1097/00000433-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Suárez-Barrientos A, López-Romero P, Vivas D, Castro-Ferreira F, Núñez-Gil I, Franco E, et al. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97:970–976. doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- 19.Ibanez B, Suárez-Barrientos A, López-Romero P. Circadian variations of infarct size in STEM1. Circ Res. 2012;110 doi: 10.1161/CIRCRESAHA.111.262816. e22–authorreplye23. [DOI] [PubMed] [Google Scholar]
- 20.De Luca G, Suryapranata H, Ottervanger JP, van 't Hof AWJ, Hoorntje JCA, Gosselink ATM, et al. Absence of seasonal variation in myocardial perfusion, enzymatic infarct size, and mortality in patients with ST-segment elevation myocardial infarction treated with primary angioplasty. Am J Cardiol. 2005;95:1459–1461. doi: 10.1016/j.amjcard.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 21.De Luca G, Suryapranata H, Ottervanger JP, van 't Hof AWJ, Hoorntje JCA, Gosselink ATM, et al. Circadian variation in myocardial perfusion and mortality in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Am Heart J. 2005;150:1185–1189. doi: 10.1016/j.ahj.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 22.Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ Res. 2012;110:105–110. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arroyo Úcar E, Dominguez-Rodriguez A, Abreu-Gonzalez P. [Influence of diurnal variation in the size of acute myocardial infarction] Med Intensiva. 2012;36:11–14. doi: 10.1016/j.medin.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Fournier S, Eeckhout E, Mangiacapra F, Trana C, Lauriers N, Beggah AT, et al. Circadian variations of ischemic burden among patients with myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2012;163:208–313. doi: 10.1016/j.ahj.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Manfredini R, Boari B, Bressan S, Gallerani M, Salmi R, Portaluppi F, et al. Influence of circadian rhythm on mortality after myocardial infarction: data from a prospective cohort of emergency calls. Am J Emerg Med. 2004;22:555–559. doi: 10.1016/j.ajem.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Ammirati E, Cristell N, Cianflone D, Vermi A-C, Marenzi G, De Metrio M, et al. Questing for circadian dependence in ST-segment-elevation acute myocardial infarction: a multicentric and multiethnic study. Circ Res. 2013;112:e110–e114. doi: 10.1161/CIRCRESAHA.112.300778. [DOI] [PubMed] [Google Scholar]
- 27.Ammirati E, Maseri A, Cannistraci CV. Still need for compelling evidence to support the circadian dependence of infarct size after ST-elevation myocardial infarction. Circ Res. 2013;113:e43–e44. doi: 10.1161/CIRCRESAHA.113.301908. [DOI] [PubMed] [Google Scholar]
- 28.Fournier S, Muller O. Finding the real culprit between circadian rhythm and “out of hours effect” to explain the higher myocardial infarction size among patients with symptom onset occurring at night. Circ Res. 2012;110 doi: 10.1161/CIRCRESAHA.112.269076. e67–authorreplye68. [DOI] [PubMed] [Google Scholar]
- 29.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honma S, Honma KI. The biological clock: Ca2+ links the pendulum to the hands. Trends Neurosci. 2003;26:650–653. doi: 10.1016/j.tins.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda M. Calcium dynamics and circadian rhythms in suprachiasmatic nucleus neurons. Neuroscientist. 2004;10:315–324. doi: 10.1177/10738584031262149. [DOI] [PubMed] [Google Scholar]
- 32.Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Ann N Y Acad Sci. 2005;1047:86–98. doi: 10.1196/annals.1341.008. [DOI] [PubMed] [Google Scholar]
- 33.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothermel BA, Vega RB, Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med. 2003;13:15–21. doi: 10.1016/s1050-1738(02)00188-3. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, et al. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–E68. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 36.Hill JA, Rothermel B, Yoo K-D, Cabuay B, Demetroulis E, Weiss RM, et al. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. Preservation of systolic function. J Biol Chem. 2002;277:10251–10255. doi: 10.1074/jbc.M110722200. [DOI] [PubMed] [Google Scholar]
- 37.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, et al. Myocyteenriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci USa. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Rooij E, Doevendans PA, Crijns HJGM, Heeneman S, Lips DJ, van Bilsen M, et al. MCIP1 overexpression suppresses left ventricular remodeling and sustains cardiac function after myocardial infarction. Circ Res. 2004;94:e18–e26. doi: 10.1161/01.RES.0000118597.54416.00. [DOI] [PubMed] [Google Scholar]
- 39.Sanna B, Brandt EB, Kaiser RA, Pfluger P, Witt SA, Kimball TR, et al. Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo. Proc Natl Acad Sci USa. 2006;103:7327–7332. doi: 10.1073/pnas.0509340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachan N, Dey A, Rotter D, Grinsfelder DB, Battiprolu PK, Sikder D, et al. Sustained hemodynamic stress disrupts normal circadian rhythms in calcineurin-dependent signaling and protein phosphorylation in the heart. Circ Res. 2011;108:437–445. doi: 10.1161/CIRCRESAHA.110.235309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vega RB, Rothermel BA, Weinheimer CJ, Kovacs A, Naseem RH, Bassel-Duby R, et al. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc Natl Acad Sci USa. 2003;100:669–674. doi: 10.1073/pnas.0237225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmeyer MR, Jones SP, Ross CR, Sharp B, Grisham MB, Laroux FS, et al. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res. 2000;87:812–817. doi: 10.1161/01.res.87.9.812. [DOI] [PubMed] [Google Scholar]
- 43.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwai K, Hori M, Kitabatake A, Kurihara H, Uchida K, Inoue M, et al. Disruption of microtubules as an early sign of irreversible ischemic injury. Immunohistochemical study of in situ canine hearts. Circ Res. 1990;67:694–706. doi: 10.1161/01.res.67.3.694. [DOI] [PubMed] [Google Scholar]
- 45.Xiao J, Liang D, Liu Y, Zhang H, Liu Y, Zhao H, et al. Taxol, a microtubule stabilizer, improves cardiac functional recovery during postischemic reperfusion in rat in vitro. Cardiovasc Ther. 2012;30:12–30. doi: 10.1111/j.1755-5922.2010.00163.x. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Miura T, Nakano A, Ichikawa Y, Yano T, Kobayashi H, et al. Role of microtubules in ischemic preconditioning against myocardial infarction. Cardiovasc Res. 2004;64:322–330. doi: 10.1016/j.cardiores.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeyaraj D, Scheer FAJL, Ripperger JA, Haldar SM, Lu Y, Prosdocimo DA, et al. Klf15 orchestrates circadian nitrogen homeostasis. Cell Metab. 2012;15:311–323. doi: 10.1016/j.cmet.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin L, Zhao D, Liu X, Nagy JA, Hoang MV, Brown LF, et al. Down syndrome candidate region 1 isoform 1 mediates angiogenesis through the calcineurin-NFAT pathway. Mol Cancer Res. 2006;4:811–820. doi: 10.1158/1541-7786.MCR-06-0126. [DOI] [PubMed] [Google Scholar]
- 50.Shioda N, Han F, Moriguchi S, Fukunaga K. Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia. J Neurochem. 2007;102:1506–1517. doi: 10.1111/j.1471-4159.2007.04600.x. [DOI] [PubMed] [Google Scholar]
- 51.Kaminska B, Gaweda-Walerych K, Zawadzka M. Molecular mechanisms of neuroprotective action of immunosuppressants--facts and hypotheses. J Cell Mol Med. 2004;8:45–58. doi: 10.1111/j.1582-4934.2004.tb00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCourtie AS, Merry HE, Wolf PS, FitzSullivan E, Keech JC, Farivar AS, et al. Synergistic protection in lung ischemia-reperfusion injury with calcineurin and thrombin inhibition. Ann Thorac Surg. 2010;89:1766–1771. doi: 10.1016/j.athoracsur.2010.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Xie X. Tacrolimus attenuates myocardium damage to the total hepatic ischemia-reperfusion via regulation of the mitochondrial function. J Surg Res. 2012;172:e47–e54. doi: 10.1016/j.jss.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Jin Z, Duan W, Chen M, Yu S, Zhang H, Feng G, et al. The myocardial protective effects of adenosine pretreatment in children undergoing cardiac surgery: a randomized controlled clinical trial. Eur J Cardiothorac Surg. 2011;39:e90–e96. doi: 10.1016/j.ejcts.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 55.Obasanjo-Blackshire K, Mesquita R, Jabr RI, Molkentin JD, Hart SL, Marber MS, et al. Calcineurin regulates NFAT-dependent iNOS expression and protection of cardiomyocytes: co-operation with Src tyrosine kinase. Cardiovasc Res. 2006;71:672–683. doi: 10.1016/j.cardiores.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 56.Hotta H, Miura T, Miki T, Togashi N, Maeda T, Kim SJ, et al. Angiotensin II type 1 receptor-mediated upregulation of calcineurin activity underlies impairment of cardioprotective signaling in diabetic hearts. Circ Res. 2010;106:129–132. doi: 10.1161/CIRCRESAHA.109.205385. [DOI] [PubMed] [Google Scholar]
- 57.Lu X, Moore PG, Liu H, Schaefer S. Phosphorylation of ARC is a critical element in the antiapoptotic effect of anesthetic preconditioning. Anesth Analg. 2011;112:525–531. doi: 10.1213/ANE.0b013e318205689b. [DOI] [PubMed] [Google Scholar]
- 58.Miura T, Tanno M, Sato T. Mitochondrial kinase signalling pathways in myocardial protection from ischaemia/reperfusion-induced necrosis. Cardiovasc Res. 2010;88:7–15. doi: 10.1093/cvr/cvq206. [DOI] [PubMed] [Google Scholar]
- 59.Leshnower BG, Kanemoto S, Matsubara M, Sakamoto H, Hinmon R, Gorman JH, et al. Cyclosporine preserves mitochondrial morphology after myocardial ischemia/reperfusion independent of calcineurin inhibition. Ann Thorac Surg. 2008;86:1286–1292. doi: 10.1016/j.athoracsur.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ Res. 2011;108:265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 61.Heineke J, Ritter O. Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolemma to the nucleus and beyond. J Mol Cell Cardiol. 2012;52:62–73. doi: 10.1016/j.yjmcc.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Hoeffer CA, Dey A, Sachan N, Wong H, Patterson RJ, Shelton JM, et al. The Down syndrome critical region protein RCAN1 regulates long-term potentiation and memory via inhibition of phosphatase signaling. J Neurosci. 2007;27:13161–13172. doi: 10.1523/JNEUROSCI.3974-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee EJ, Lee JY, Seo SR, Chung KC. Overexpression of DSCR1 blocks zinc-induced neuronal cell death through the formation of nuclear aggregates. Mol Cell Neurosci. 2007;35:585–595. doi: 10.1016/j.mcn.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Porta S, Serra SA, Huch M, Valverde MA, Llorens F, Estivill X, et al. RCAN1 (DSCR1) increases neuronal susceptibility to oxidative stress: a potential pathogenic process in neurodegeneration. Hum Mol Genet. 2007;16:1039–1050. doi: 10.1093/hmg/ddm049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.