Abstract
Background
Some recent studies in older, largely white populations suggest that vitamin D, measured by 25-hydroxyvitamin D [25(OH)D], is important for cognition, but such results may be affected by reverse-causation. Measuring 25(OH)D in late-middle age before poor cognition affects behavior may provide clearer results.
Methods
Prospective cohort analysis of 1,652 participants (52% white; 48% black) in the Atherosclerosis Risk in Communities (ARIC) Brain MRI Study. 25(OH)D was measured from serum collected in 1993–1995. Cognition was measured by the Delayed Word Recall Test (DWRT), the Digit Symbol Substitution Test (DSST), and the Word Fluency Test (WFT). Dementia hospitalization was defined by ICD-9 codes. Adjusted linear, logistic, and Cox proportional hazards models were used.
Results
Mean age of participants was 62 years and 60% were female. Mean 25(OH)D was higher in whites than blacks (25.5 ng/ml versus 17.3 ng/ml, p<0.001). Lower 25(OH)D was not associated with lower baseline scores or with greater DWRT, DSST, or WFT decline (p>0.05). Over a median of 16.6 years, there were 145 incident hospitalized dementia cases. Though not statistically significant, lower levels of 25(OH)D were suggestive of an association with increased dementia risk (HR lowest versus highest race-specific tertile: whites 1.32 [95% CI: 0.69, 2.55]; blacks 1.53 [95% CI: 0.84, 2.79]).
Conclusions
In contrast to prior studies performed in older white populations, our study did not find significant associations between lower levels of 25(OH)D measured in late-middle age black or white participants with lower cognitive test scores at baseline, change in scores over time, or dementia risk.
Keywords: Vitamin D, Cognition, Dementia, ARIC Study
INTRODUCTION
Vitamin D insufficiency and deficiency, as defined by 25-hydroxyvitamin D [25(OH)D] <30 ng/ml and <20 ng/ml, respectively [1], have been estimated to affect approximately 1 billion people worldwide [2]. Vitamin D insufficiency and deficiency are more prevalent among older individuals [2] and among racial minorities, especially blacks [3]. Low serum concentrations of 25(OH)D have been associated with increased risk of mortality [4], cardiovascular disease [5], and diabetes [6]. These associations are important because vitamin D insufficiency and deficiency are amenable to treatment through supplementation or increased sunlight exposure.
It has also been suggested that low concentrations of 25(OH)D may be associated with poor cognitive functioning and increased risk for dementia, particularly among older individuals [7]. However, results of studies published on this topic are inconsistent, with some studies reporting a significant association of 25(OH)D with cognition and dementia [7–12], and other studies not reporting a significant association [13–16]. A recent systematic review of the existing literature concluded that lower 25(OH)D concentration is associated with cognitive impairment and dementia [7]. However, most of the studies included in this review were cross-sectional in design and were performed among older whites, some of whom already had dementia diagnoses [7, 12].
Among the longitudinal studies published, mean follow-up time was <7 years [8–10, 13, 14]. Given that sun exposure and subsequent skin synthesis is a major source of vitamin D [17], and people with cognitive impairment spend less time outdoors [18], exploring the association between vitamin D and cognitive function cross-sectionally or in studies with short follow-up is potentially problematic, due to the possibility of reverse causation. This highlights the need for prospective studies with long follow-up (>10 years) of middle-aged individuals, who are not likely to be cognitively impaired at baseline. Furthermore, given racial variation in vitamin D levels, it is important to evaluate this association in diverse populations.
The Atherosclerosis Risk in Communities (ARIC) brain MRI ancillary study [19] has advantages for studying the association of 25(OH)D with cognition and dementia risk. This population had 25(OH)D measured in late-middle age, is comprised of approximately 50% black participants, and has a median of 10.6 years follow-up for cognitive testing and a median of 16.6 years follow-up for incident dementia. We hypothesized that lower concentrations of 25(OH)D would be associated cross-sectionally with lower cognitive test scores, prospectively with greater decline in cognitive test scores, and with increased risk of hospitalization with a ICD-9 code for dementia in both black and white participants of the ARIC Brain MRI study.
METHODS
Study Population
The ARIC study is an ongoing, community-based prospective cohort of 15,792 adults aged 45–65 years at baseline (1987–1989) from four U.S. communities [20]. The ARIC Brain MRI ancillary study [19] is comprised of a subset of participants aged ≥55 years from two of the communities, Forsyth County, North Carolina and Jackson, Mississippi, who were invited for a cerebral MRI and cognitive testing during visit 3 (1993–1995).
25(OH)D was measured in serum samples from ARIC Brain MRI Ancillary Study participants who attended visit 3 (which serves as the baseline for the present study). Of the 1,934 participants included in the visit 3 Brain MRI Ancillary Study, we excluded those missing stored serum, insufficient serum for 25(OH)D measurement, or samples that did not pass internal quality control (n=165), those with a history of stroke, transient ischemic attack, or prior hospitalization with an ICD-9 code for dementia (n=44), those of self-reported non-white and non-black race (n=5), those missing cognitive test data (n=53), and those missing covariates included in statistical models (n=15), leaving 1,652 participants included in our main analyses.
The ARIC study has been approved by the Institutional Review Boards of all participating institutions (Johns Hopkins University, University of Minnesota, University of Mississippi, University of North Carolina). All participants gave written informed consent at each study visit.
Measurement of 25(OH)D and associated biomarkers
25(OH)D was measured in 2012 from serum samples that were stored at −70°C since collection during visit 3 (1993–1995) using liquid chromatography-tandem mass spectrometry (Waters Alliance e2795, Milford, Massachusetts). The inter-assay coefficient of variations (CVs) for 25(OH)D2 are 6.2% and 5.3% at concentrations of 7.9 and 12.9 ng/mL, respectively. The inter-assay CVs for 25(OH)D3 are 4.8% for concentrations of 30.1 and 55.8 ng/mL. 25(OH)D2 and 25(OH)D3 were added together for total 25(OH)D concentration.
Using the same stored serum samples, calcium, phosphorus, and parathyroid hormone (PTH) were also measured (calcium and phosphate: Roche Modular P-Chemistry Analyzer [Roche Diagnostics, Indianapolis, Indiana], PTH: Elecsys 2010 [Roche Diagnostics, Indianapolis, Indiana]).
Measures of cognitive function
Cognitive functioning was assessed at visit 3 (first brain MRI visit, 1993–1995), visit 4 (1996–1998) and the second brain MRI visit (2004–2006) using three standard tests: the delayed word recall test (DWRT) [21], the digit symbol substitution test (DSST) [22], and the word fluency test (WFT) [23].
The DWRT is a test of verbal learning and recent memory. Participants were given 10 common nouns that they were asked to learn by using each word in one or two sentences. After a five-minute delay, participants were given 60 seconds to recall the 10 words. The score is the number of words correctly recalled.
The DSST is a test of executive function and processing speed. Participants were asked to translate numbers to symbols using a key. The score (range 0–93) is the total number of numbers correctly translated to symbols within 90-seconds.
The WFT is a test of executive function and language, and tests the ability to spontaneously generate words beginning with a particular letter, excluding proper names or places. Participants were given 60 seconds for each of the letters “F”, “A”, and “S”. The score is the total number of words generated across the three trials.
Incident dementia hospitalization
The ARIC Study ascertains hospitalizations from annual telephone contact with study participants and through active surveillance of all hospitalizations in the study communities. For the present study, follow-up was available through December 31, 2010. We defined time to first hospitalization with an ICD-9 code for dementia using the following ICD-9 codes (listed anywhere in the hospital discharge record): Alzheimer’s disease (331.0), vascular dementia (290.4), or dementia of other etiology (290.0, 290.1, 290.2, 290.3, 290.9, 294.1, 294.2, 294.8, 294.9, 331.1, 331.2, 331.8, 331.9). This definition has been used previously [24, 25].
Covariates
All covariates used in the regression models were assessed at visit 3 (1993–1995), unless otherwise stated. Covariates in our main model included: age, gender, education (<high school; high school or equivalent; college, graduate or professional school; assessed at visit 1), income (<$35,000/year; ≥$35,000/year; not reported), physical activity (score range 1 to 5, based on replies to the Baecke Physical Activity questionnaire [26]), cigarette smoking (current; former; never), alcohol consumption (current; former; never), body mass index, waist circumference, and use of vitamin D supplements.
Covariates in supplemental models included: diabetes (self-reported physician diagnosis, medication use, or fasting blood glucose ≥126 mg/dl), systolic and diastolic blood pressure (mmHg, mean of the second and third measurements), use of hypertension medication, total and HDL cholesterol (mg/dL), estimated glomerular filtration rate (eGFR, calculated using the chronic kidney disease epidemiology collaboration formula [27], assessed at Visit 2 (1990–1992)), calcium, phosphate, and PTH.
Statistical Analysis
25(OH)D concentrations vary by season [28]. Therefore we adjusted 25(OH)D for seasonal change by computing the residuals from a linear regression model (25[OH]D, dependent variable; month of visit, independent variable). The residuals were added back to the mean to determine an estimated annual 25(OH)D value. We performed this adjustment separately for whites and for blacks, as 25(OH)D concentrations vary by race [3]. This estimated annual 25(OH)D value was used in all analyses.
25(OH)D concentrations were divided into race-specific tertiles (whites: ≥28.3 ng/ml, 21.8 to <28.3 ng/ml, <21.8 ng/ml; and blacks: ≥19.3 ng/ml, 14.0 to <19.3 ng/ml, <14.0 ng/ml). Baseline characteristics (1993–1995) of the study population were summarized across these race-specific tertiles of 25(OH)D. We also repeated our analyses using clinical categories of 25(OH)D: [1]: ≥30 ng/ml as sufficient, 20 to <30 ng/ml as insufficient, and <20 ng/ml as deficient.
Linear regression models were used to assess the cross-sectional association between 25(OH)D concentration and cognitive test scores at visit 3 (1993–1995). We analyzed change in cognitive test scores in 2 subpopulations: 1.) those with repeat cognitive testing at visit 4 (1996–1998) (n=1,429), and 2.) those with repeat cognitive testing at the second Brain MRI Ancillary Study visit (2004–2006) (n=915). In these analyses, the annual cognitive change for each person was calculated using visit 3 as the baseline (1993–1995) and was categorized into quintiles. Using logistic regression, we estimated the odds ratio (95% confidence interval) of having severe cognitive decline, defined by being in the top quintile of decline for each test, compared to the other four quintiles. To allow for greater power in our change analyses, we also used linear regression to estimate the β-coefficients (95% confidence intervals) for change in cognitive test score. To assess for possible survival bias in these analyses, we compared baseline characteristics of participants included in and excluded from our longitudinal analyses. We additionally performed a sensitivity analysis in which we placed all living participants who had a hospitalization with an ICD-9 code for dementia between baseline and visit 4 and/or the second Brain MRI Ancillary Study visit and who did not attend these later visits in the “top quintile” of decline.
For the prospective association of 25(OH)D with incident hospitalization with dementia, we estimated hazard ratios (95% confidence intervals) using Cox proportional hazard models. The proportional-hazards assumptions were checked with the use of Schoenfeld residuals and graphic methods. We also modeled the association of 25(OH)D with incident hospitalization with dementia continuously using a restricted cubic spline model.
Our primary model was adjusted for demographic factors (age, gender) and behavioral/socioeconomic variables (education, income, physical activity, smoking, alcohol use, body-mass index, waist circumference, and vitamin D supplementation). We performed two additional analyses: 1.) primary model + potential mediators (diabetes, systolic and diastolic blood pressure, use of hypertension medication, total and HDL cholesterol, and eGFR), and 2.) primary model + potential mediators + biomarkers related to vitamin D metabolism (calcium, phosphate, and PTH). We formally tested for interaction by age in our analyses.
All reported p-values are two-sided and p<0.05 was considered statistically significant. Analyses were performed using Stata Version 13 (StataCorp, College Station, Texas).
Role of the Funding Source
The ARIC Study is supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI). The NHLBI was not involved in the conception or execution of this paper.
RESULTS
Baseline (1993–1995) characteristics of the study population by race-specific 25(OH)D tertiles are shown in Table 1. Overall, the mean age of participants was 62 years, 60% of participants were female, and 48% of participants were black. Mean (SD) 25(OH)D concentration was 25.5 (7.9) ng/ml among whites and 17.3 (6.3) ng/ml among blacks. Vitamin D supplement use was low in both whites and blacks (2.5% and 1%, respectively). Among whites, compared to those with 25(OH)D concentration in the highest tertile, those with 25(OH)D concentration in the lowest tertile were of similar age (63 years, p=0.664), were more likely to be female (67% versus 48%, p<0.001), and had a lower physical activity index score (2.4 versus 3.0, p<0.001). Among blacks, compared to those with 25(OH)D concentration in the highest tertile, those with 25(OH)D concentration in the lowest tertile were younger (61 years versus 62 years, p=0.003), were more likely to be female (78% versus 52%, p<0.001), and had a lower physical activity index score (2.3 versus 2.5, p<0.001). Characteristics of the study population by race and by clinical categories (≥30 ng/ml; 20–<30 ng/ml; <20 ng/ml) of 25(OH)D are shown in Appendix Table 1.
Table 1.
Baseline (ARIC Visit 3, 1993–1995) characteristics by race-specific 25(OH)D tertiles (n=1,652).
| Highest 25(OH)D Tertile | Middle 25(OH)D Tertile | Lowest 25(OH)D Tertile | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Whites (≥28.3 ng/ml) (n=285) | Blacks (≥19.3 ng/ml) (n=267) | Whites (21.8– <28.3 ng/ml) (n=283) | Blacks (14.0– <19.3 ng/ml) (n=272) | Whites (<21.8 ng/ml) (n=284) | Blacks (<14.0 ng/ml) (n=261) | |
| Age (years), mean (SD) | 63.1 (4.3) | 62.2 (4.4) | 63.3 (4.5) | 61.4 (4.6) | 62.9 (4.4) | 61.0 (4.5) |
| Female, % | 48.4 | 52.4 | 53.7 | 63.2 | 66.9 | 78.2 |
| Study Site, % | ||||||
| Jackson, Mississippi | 0.0 | 88.8 | 0.0 | 86.8 | 0.0 | 85.4 |
| Forsyth County, North Carolina | 100.0 | 11.2 | 100.0 | 13.2 | 100.0 | 14.6 |
| Education, % | ||||||
| <High School | 14.4 | 43.8 | 15.2 | 37.1 | 11.6 | 35.3 |
| High School or Vocational School | 41.4 | 22.5 | 44.9 | 26.8 | 43.7 | 26.1 |
| College, Graduate, or Professional School | 44.2 | 33.7 | 39.9 | 36.1 | 44.7 | 38.7 |
| Family Income, % | ||||||
| <$35,000/year | 45.3 | 71.6 | 48.1 | 72.1 | 46.5 | 74.7 |
| ≥$35,000/year | 50.2 | 18.7 | 49.1 | 15.8 | 48.2 | 17.2 |
| Not Reported | 4.5 | 9.7 | 2.8 | 12.1 | 5.3 | 8.1 |
| Smoking Status, % | ||||||
| Never | 36.8 | 48.3 | 43.1 | 48.5 | 40.8 | 52.5 |
| Former | 48.8 | 36.3 | 41.0 | 34.6 | 34.9 | 27.6 |
| Current | 14.4 | 15.4 | 15.9 | 16.9 | 24.3 | 19.9 |
| Alcohol Consumption, % | ||||||
| Never | 30.2 | 40.5 | 34.3 | 46.3 | 38.4 | 46.4 |
| Former | 16.8 | 33.3 | 23.3 | 25.8 | 14.8 | 26.1 |
| Current | 53.0 | 26.2 | 42.4 | 27.9 | 46.8 | 27.6 |
| Physical activity index, mean (SD) | 3.0 (0.9) | 2.5 (0.8) | 2.6 (0.8) | 2.3 (0.7) | 2.4 (0.7) | 2.3 (0.7) |
| Body mass index (kg/m2), mean (SD) | 25.8 (4.0) | 29.2 (4.9) | 26.8 (4.6) | 29.6 (5.5) | 26.9 (5.1) | 30.0 (5.6) |
| Waist circumference (cm), mean (SD) | ||||||
| Men | 99.5 (11.2) | 98.6 (10.3) | 101.3 (10.2) | 97.7 (11.1) | 102.1 (12.4) | 99.9 (12.3) |
| Women | 89.4 (11.5) | 102.9 (15.1) | 95.5 (13.4) | 102.7 (14.8) | 96.0 (14.4) | 103.3 (14.0) |
| Diabetes, % | 6.3 | 24.0 | 12.4 | 27.4 | 13.4 | 24.5 |
| Hypertension, % | 30.6 | 60.9 | 36.5 | 60.9 | 31.0 | 67.2 |
| Total Cholesterol (mg/dl), mean (SD) | 209.0 (34.6) | 209.3 (40.9) | 210.1 (33.3) | 210.0 (39.9) | 208.5 (38.4) | 206.1 (39.9) |
| HDL (mg/dl), mean (SD) | 55.7 (21.3) | 56.6 (19.8) | 51.7 (18.6) | 56.1 (16.8) | 53.1 (19.8) | 56.0 (22.4) |
| LDL (mg/dl), mean (SD) | 125.0 (31.5) | 125.7 (36.5) | 126.7 (30.9) | 131.6 (37.6) | 124.5 (34.1) | 129.9 (38.7) |
| Triglycerides (mg/dl), mean (SD) | 142.6 (85.5) | 114.5 (60.6) | 162.8 (119.0) | 112.2 (59.2) | 151.5 (85.5) | 121.6 (70.7) |
| * Creatinine (mg/dl), mean (SD) | 1.16 (0.18) | 1.22 (0.23) | 1.12 (0.20) | 1.16 (0.26) | 1.09 (0.19) | 1.15 (0.36) |
| * Estimated GFR (ml/min), mean (SD) | 84.2 (12.6) | 91.4 (17.0) | 86.7 (13.2) | 96.2 (16.5) | 86.8 (13.0) | 95.1 (18.7) |
| PTH (pg/ml), mean (SD) | 37.0 (13.3) | 44.0 (18.2) | 40.6 (14.1) | 49.1 (23.3) | 47.3 (40.9) | 58.0 (37.0) |
| Calcium (mg/dl), mean (SD) | 9.4 (0.4) | 9.6 (0.4) | 9.4 (0.3) | 9.5 (0.4) | 9.4 (0.4) | 9.5 (0.5) |
| Phosphate (mg/dl), mean (SD) | 3.35 (0.52) | 3.45 (01) | 3.39 (0.48) | 3.51 (0.46) | 3.46 (0.57) | 3.51 (0.50) |
| Vitamin D Supplementation Use, % | 4.2 | 2.3 | 1.4 | 0.0 | 1.8 | 0.4 |
| C-reactive protein (mg/L), median (IQR) | 2.0 (0.9, 4.9) | 3.4 (1.4, 6.6) | 2.0 (1.2, 6.1) | 3.2 (1.3, 7.0) | 2.3 (1.0, 5.3) | 3.5 (1.4, 7.0) |
| Visit 3 Cognitive Test Scores | ||||||
| DWRT (words), mean (SD) | 6.79 (1.43) | 5.89 (1.72) | 6.77 (1.46) | 6.08 (1.76) | 6.81 (1.49) | 6.22 (1.78) |
| DSST (points), mean (SD) | 46.5 (11.2) | 27.6 (12.0) | 45.9 (10.6) | 29.2 (13.1) | 47.6 (11.6) | 31.2 (13.6) |
| WFT (words), mean (SD) | 34.4 (12.1) | 27.4 (13.3) | 32.7 (10.9) | 28.4 (14.2) | 35.3 (11.3) | 28.7 (13.6) |
Measured at ARIC Visit 2 (1990–1992).
Note: The following variables had missing data: diabetes (n=1), hypertension (n=9), total cholesterol (n=1), HDL cholesterol (n=1), LDL cholesterol (n=22), triglycerides (n=1), 10-year Framingham risk (n=137), 10-year risk of stroke (n=96), creatinine (n=18), estimated GFR (n=18), PTH (n=8), calcium (n=2), and C-reactive protein (n=209).
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; DWRT, Delayed Word Recall Test; DSST, Digit Symbol Substitution Test; GFR, Glomerular Filtration Rate; HDL, high-density lipoprotein; IQR, inter-quartile range; LDL, low-density lipoprotein; PTH, parathyroid hormone; SD, standard deviation; WFT, Word Fluency Test.
Table 2 shows the β-coefficients (95% confidence intervals) for the cross-sectional association of 25(OH)D with cognitive tests scores by race-specific 25(OH)D tertile. Lower concentrations of 25(OH)D were not significantly associated with lower cognitive test scores on any of the three cognitive tests in whites or in blacks (all p>0.05). Similar results were found when the analyses were repeated using clinical categories of 25(OH)D (Appendix Table 2).
Table 2.
Cross-sectional (ARIC Visit 3, 1993–1995) association of race-specific 25(OH)D tertiles with cognitive test scores.
| DWRT β (95% CI) |
DSST β (95% CI) |
WFT β (95% CI) |
|
|---|---|---|---|
|
| |||
| Whites (n=852) | |||
| 25(OH)D ≥28.3 ng/ml | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| 25(OH)D 21.8–<28.3 ng/ml | −0.04 (−0.27, 0.20) | −0.20 (−1.78, 1.37) | −0.86 (−2.64, 0.93) |
| 25(OH)D <21.8 ng/ml | −0.11 (−0.36, 0.13) | 0.46 (−1.16, 2.09) | 1.35 (−0.49, 3.20) |
| Blacks (n=800) | |||
| 25(OH)D ≥19.3 ng/ml | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| 25(OH)D 14.0–<19.3 ng/ml | −0.02 (−0.31, 0.26) | −0.06 (−1.85, 1.73) | 0.12 (−1.86, 2.10) |
| 25(OH)D <14.0 ng/ml | −0.01 (−0.31, 0.28) | 0.66 (−1.19, 2.51) | −0.18 (−2.23, 1.86) |
Model is adjusted for demographics variables (age, sex) and behavioral/socioeconomic variables (education, income, physical activity, smoking, alcohol use, body mass index, waist circumference, and vitamin D supplementation).
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; DWRT, Delayed Word Recall Test; DSST, Digit Symbol Substitution Test; WFT, Word Fluency Test.
The odds ratios (95% confidence intervals) for the quintile of greatest cognitive test score decline versus the other 4 cognitive decline categories (over a median of 3.0 years for change between Visit 3 [1993–1995] and Visit 4 [1996–1998] and over a median of 10.6 years for change between Visit 3 [1993–1995] and the second Brain MRI ancillary visit [2004–2006]) for each of the three tests (DWRT, DSST, WFT) are shown by race-specific 25(OH)D categories in Table 3 and by clinical categories of 25(OH)D in Appendix Table 3. 25(OH)D concentrations were largely not significantly associated with the top quintile of decline on any of the three cognitive tests in whites or blacks at either median follow-up of 3 years or 10.6 years (p>0.05). The association of 25(OH)D concentrations with linear change in cognitive test scores was also non-significant for all three cognitive tests (all p>0.05) (Appendix Table 4).
Table 3.
Odds ratios (95% confidence intervals) for the association of race-specific 25(OH)D tertiles with the top quintile of cognitive test score decline on the DWRT, DSST, and WFT versus the 4 quintiles of lesser change in cognitive test scores over a median follow-up period of 3.0 years and 10.6 years, respectively.
| DWRT OR (95% CI) |
DSST OR (95% CI) |
WFT OR (95% CI) |
|
|---|---|---|---|
|
| |||
|
Visit 3 (1993–1995) – Visit 4 (1996–1998)
|
|||
| Whites (n=766) | |||
| 25(OH)D ≥28.3 ng/ml | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25(OH)D 21.8–<28.3 ng/ml | 1.28 (0.80, 2.05) | 1.44 (0.91, 2.27) | 0.76 (0.48, 1.21) |
| 25(OH)D <21.8 ng/ml | 1.09 (0.66, 1.81) | 1.13 (0.70, 1.84) | 1.04 (0.65, 1.66) |
| Blacks (n=663) | |||
| 25(OH)D ≥19.3 ng/ml | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25(OH)D 14.0–<19.3 ng/ml | 1.28 (0.80, 2.05) | 1.07 (0.67, 1.72) | 0.86 (0.53, 1.40) |
| 25(OH)D <14.0 ng/ml | 1.38 (0.86, 2.23) | 0.82 (0.50, 1.36) | 1.11 (0.68, 1.82) |
|
| |||
|
Visit 3 (1993–1995) – Second Brain MRI Ancillary Visit (2004–2006)
|
|||
| Whites (n=467) | |||
| 25(OH)D ≥28.3 ng/ml | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25(OH)D 21.8–<28.3 ng/ml | 0.94 (0.52, 1.69) | 1.37 (0.78, 2.42) | 0.79 (0.42, 1.48) |
| 25(OH)D <21.8 ng/ml | 1.24 (0.68, 2.26) | 1.71 (0.95, 3.09) | 1.14 (0.61, 2.15) |
| Blacks (n=448) | |||
| 25(OH)D ≥19.3 ng/ml | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25(OH)D 14.0–<19.3 ng/ml | 1.03 (0.58, 1.84) | 0.49 (0.25, 0.97) | 0.65 (0.37, 1.15) |
| 25(OH)D <14.0 ng/ml | 0.72 (0.38, 1.34) | 0.72 (0.37, 1.40) | 0.83 (0.47, 1.48) |
Model is adjusted for demographics variables (age, sex) and behavioral/socioeconomic variables (education, income, physical activity, smoking, alcohol use, body mass index, waist circumference, and vitamin D supplementation).
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval; DWRT, Delayed Word Recall Test; DSST, Digit Symbol Substitution Test; WFT, Word Fluency Test.
In these longitudinal analyses, it is important to consider the possibility of survival bias. Indeed, as shown in Appendix Table 5, compared to participants excluded from the 1993–1995 to 2004–2006 change analysis (n=737), participants included in this analysis (n=915) were significantly younger (62 years versus 63 years, p<0.001), performed better on all cognitive tests at baseline (DWRT: 6.6 words versus 6.2 words, p<0.001; DSST: 41 points versus 35 points, p<0.001; WFT: 33 words versus 28 words, p<0.001), and were less likely to have an incident hospitalization with dementia during follow-up (4.7% versus 13.8%, p<0.001). In our sensitivity analyses in which all living participants who had a hospitalization with an ICD-9 code for dementia between baseline and visit 4 and/or the second Brain MRI Ancillary Study visit and who did not attend these later visits were placed in the “top quintile” of decline, lower concentrations of 25(OH)D remained not significantly associated with the “top quintile” of decline (data not shown).
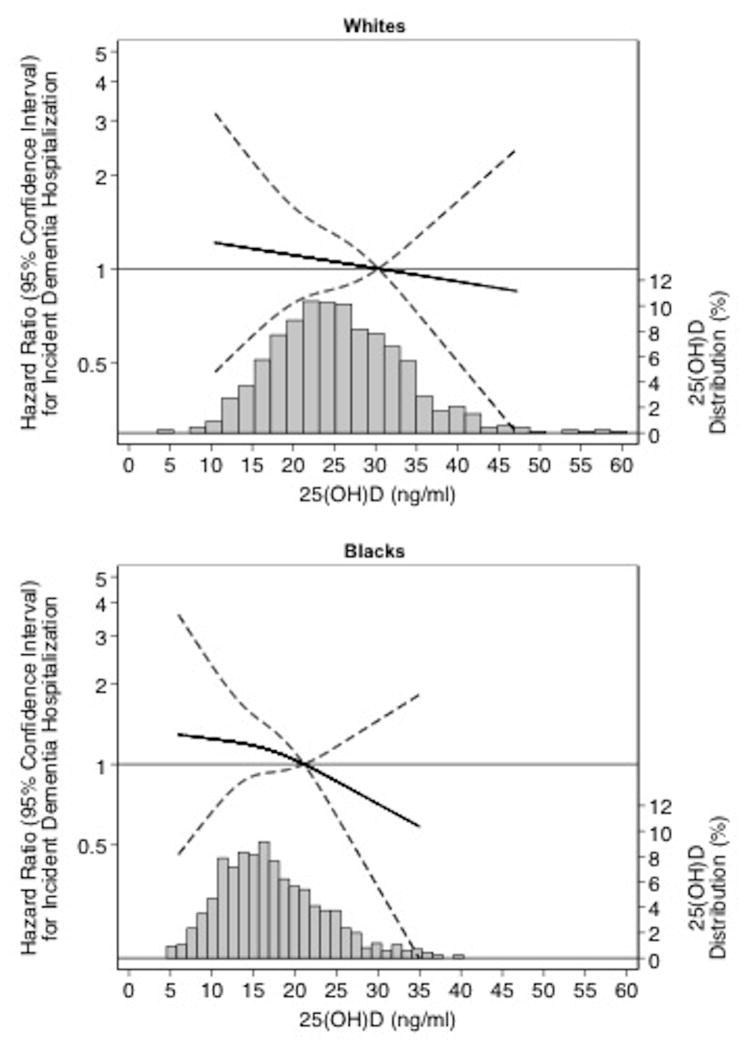
Among our baseline population of 1,652 persons, there were 145 incident hospitalizations with an ICD-9 code for dementia hospitalization that occurred over a median of 16.6 years of follow-up. Table 4 shows the hazard ratios (95% confidence intervals) for the association of 25(OH)D at baseline with risk for incident hospitalization with dementia. There was a non-significant increase in dementia risk for lower levels of 25(OH)D in both whites and blacks (lowest versus highest tertile: whites, HR: 1.32 [95% CI: 0.69, 2.55]; blacks, HR: 1.53 [95% CI: 0.84, 2.79]) Similar results were found when the analyses were repeated using clinical categories (Appendix Table 6). Figure 1 shows the continuous association of 25(OH)D with incident dementia-related hospitalization using a restricted cubic spline model, separately in whites and in blacks.
Table 4.
Hazard ratios (95% confidence intervals) for the association of race-specific 25(OH)D tertiles with incident dementia hospitalization risk (n=1,652).
| n dementia cases/n total | HR (95% CI) | |
|---|---|---|
|
| ||
| Whites (n=852) | ||
| 25(OH)D ≥28.3 ng/ml | 18/285 | 1.00 (reference) |
| 25(OH)D 21.8–<28.3 ng/ml | 31/283 | 1.74 (0.95, 3.18) |
| 25(OH)D <21.8 ng/ml | 24/284 | 1.32 (0.69, 2.55) |
| Blacks (n=800) | ||
| 25(OH)D ≥19.3 ng/ml | 23/267 | 1.00 (reference) |
| 25(OH)D 14.0–<19.3 ng/ml | 24/272 | 1.22 (0.68, 2.19) |
| 25(OH)D <14.0 ng/ml | 25/261 | 1.53 (0.84, 2.79) |
Model is adjusted for demographics variables (age, sex) and behavioral/socioeconomic variables (education, income, physical activity, smoking, alcohol use, body mass index, waist circumference, and vitamin D supplementation).
Figure 1.
Legend. Adjusted* restricted cubic spline model showing the hazard ratios (95% confidence intervals) for the association of 25(OH)D with incident dementia in whites and blacks. The solid line represents the hazard ratios and the dashed lines represents the 95% confidence intervals. Knots at 10th, 50th, and 90th percentiles. Spline centered at the 75th percentile. Histogram shows the distribution of concentrations of 25(OH)D. Restricted cubic spline truncated at 1st and 99th percentile of 25(OH)D.
*Model is adjusted for demographics variables (age, sex) and behavioral/socioeconomic variables (education, income, physical activity, smoking, alcohol use, body mass index, waist circumference, and vitamin D supplementation).
In all analyses, additional adjustment for potential mediators and biomarkers related to vitamin D metabolism did not appreciably alter the results. There was no evidence of interaction by age in our analyses (all p-for-interaction >0.05).
CONCLUSIONS
In this community-based population comprised of approximately 50% white and 50% black participants, lower levels of 25(OH)D measured in late-middle age were not significantly associated with lower cognitive test scores at baseline or with greater decline in cognitive test score over time. Lower levels of 25(OH)D measured in late-middle age were also not significantly associated with increased dementia risk during a median of 16.6 years of follow-up.
Our study is consistent with several prior studies that reported no association between 25(OH)D levels and cognition and dementia [13–16]. Like our study, many of these previously published null studies were performed in younger participants [15, 16]. In contrast, many of the previous studies reporting significant associations between 25(OH)D levels and cognition and dementia were performed in older populations [7–12], some of which included persons with a diagnosis of dementia [12]. Indeed, most studies [7–12] that report a significant association required participants to be at least 60 years of age to be included in the study, which was approximately the mean age of our population.
The age when 25(OH)D concentrations are measured is important when studying the association of 25(OH)D levels with cognition and dementia due to the possibility of reverse causation. Vitamin D insufficiency and deficiency are more prevalent among older individuals [2], as are cognitive impairment and dementia [29]. Therefore if 25(OH)D and cognition are both assessed in older individuals, it is possible that an association seen between 25(OH)D levels and cognition and dementia means that low 25(OH)D is a marker of poor health rather than a causative factor in dementia pathogenesis. This is supported by studies showing that persons who are institutionalized due to poor physical health or dementia have less sun exposure, and therefore these persons are more likely to have lower concentrations of 25(OH)D than their healthier counterparts [18]. Our study is less susceptible to reverse causation as we measured 25(OH)D in late-middle age among community dwelling participants, often years prior to a hospitalization for dementia. Alternatively, it is possible that 25(OH)D is a causal factor for the risk of adverse cognitive outcomes, and that 25(OH)D in late-life is more important than 25(OH)D in mid-life in this association. However, two prior randomized controlled trials, one in younger and one in older participants [30, 31] and a post-hoc analysis of the Women’s Health Initiative (WHI) Calcium and Vitamin D Trial [32] did not find any beneficial effect of vitamin D supplementation (400–5000 IU/day) on cognitive function.
Certain limitations should be taken into consideration when interpreting the results of this study. First, we used ICD-9 hospitalization codes to define incident dementia and we did not have information to determine if dementia was the primary reason for hospitalization. However, dementia hospitalization is a relatively specific marker of dementia, but likely disproportionately identifies the most severe cases of dementia (those who were primarily hospitalized for their dementia) and dementia in the presence of other diseases. Indeed, in a prior analysis of ARIC data [24], it was shown that age-specific incidence rates of dementia hospitalization were lower than age-specific incidence rates of dementia in other studies [33, 34]. Second, we had only a single measurement of 25(OH)D measured in late-middle age, which has been shown to vary over time within individuals [28, 35, 36]. However, we adjusted 25(OH)D concentrations for seasonal change. Lastly, although we had a large sample size (n=1,652), we cannot exclude the possibility that our non-significant results are due to a lack of power to detect a true association.
Our study also has a number of important strengths, including a large sample size comprised of approximately 50% white and 50% black participants. Additionally, our study also had comprehensive measurement of confounders and data on three cognitive tests, which allowed us to explore the association of 25(OH)D with different cognitive domains. The same three cognitive tests were repeated across 3 time-points, allowing us to explore the prospective relation between vitamin D and cognitive change over a median of 3.0 and 10.6 years of follow-up, respectively. For our incident dementia analyses, we excluded participants who had a prior hospitalization with an ICD-9 code for dementia, and had a median of 16.6 years of follow-up. Measurements of 25(OH)D concentrations took place in serum collected in late-middle age, before the onset of clinical dementia, which helps to avoid the possibility of reverse causation in the association between 25(OH)D and cognitive decline and dementia.
In conclusion, in this community-based population of whites and blacks, we did not find significant associations between lower levels of 25(OH)D measured in late-middle age with lower cognitive test scores at baseline, change in score over time, or increased dementia risk. Future studies are needed to determine if 25(OH)D measured in late-life is a marker of poor health (reverse causation) rather than a causal factor in the risk of adverse cognitive outcomes, or alternatively, if 25(OH)D is a causal factor that is possibly more important for cognitive function in late-life than in mid-life.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
FUNDING SOURCES
This work was supported by NIH/NINDS grant R01 NS072243. A.L.C.S. was supported by NIH/NHLBI training grant T32HL007024. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The ARIC Brain MRI study was supported by grant R01-HL70825.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- ARIC
Atherosclerosis Risk in Communities
- CV
Coefficient of Variation
- DSST
Digit Symbol Substitution Test
- DWRT
Delayed Word Recall Test
- WFT
Word Fluency Test
Footnotes
CONFLICT OF INTEREST/DISCLOSURES
None.
References
- 1.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. The Journal of clinical endocrinology and metabolism. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. The New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 4.Rush L, McCartney G, Walsh D, Mackay D. Vitamin D and subsequent all-age and premature mortality: a systematic review. BMC Public Health. 2013;13:679. doi: 10.1186/1471-2458-13-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Current Opinion in Clinical Nutrition and Metabolic Care. 2008;11:7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–1428. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Schaft J, Koek HL, Dijkstra E, Verhaar HJ, van der Schouw YT, Emmelot-Vonk MH. The association between vitamin D and cognition: A systematic review. Ageing research reviews. 2013 doi: 10.1016/j.arr.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Slinin Y, Paudel M, Taylor BC, et al. Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:1092–1098. doi: 10.1093/gerona/gls075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Beauchet O. Serum vitamin D deficiency as a predictor of incident non-Alzheimer dementias: a 7-year longitudinal study. Dementia and Geriatric Cognitive Disorders. 2011;32:273–278. doi: 10.1159/000334944. [DOI] [PubMed] [Google Scholar]
- 10.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Archives of Internal Medicine. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80:722–729. doi: 10.1136/jnnp.2008.165720. [DOI] [PubMed] [Google Scholar]
- 12.Oudshoorn C, Mattace-Raso FU, van der Velde N, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2008;25:539–543. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- 13.Breitling LP, Perna L, Muller H, Raum E, Kliegel M, Brenner H. Vitamin D and cognitive functioning in the elderly population in Germany. Experimental gerontology. 2012;47:122–127. doi: 10.1016/j.exger.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Slinin Y, Paudel ML, Taylor BC, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74:33–41. doi: 10.1212/WNL.0b013e3181c7197b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolppanen AM, Williams DM, Lawlor DA. The association of serum ionized calcium and vitamin D with adult cognitive performance. Epidemiology. 2011;22:113–117. doi: 10.1097/EDE.0b013e3181f74683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29:49–54. doi: 10.1159/000108918. [DOI] [PubMed] [Google Scholar]
- 17.Ashwell M, Stone EM, Stolte H, et al. UK Food Standards Agency Workshop Report: an investigation of the relative contributions of diet and sunlight to vitamin D status. The British journal of nutrition. 2010;104:603–611. doi: 10.1017/S0007114510002138. [DOI] [PubMed] [Google Scholar]
- 18.Pilz S, Dobnig H, Tomaschitz A, et al. Low 25-hydroxyvitamin D is associated with increased mortality in female nursing home residents. The Journal of clinical endocrinology and metabolism. 2012;97:E653–657. doi: 10.1210/jc.2011-3043. [DOI] [PubMed] [Google Scholar]
- 19.Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 20.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 21.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–145. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Adult Intelligence Scale Revised Manual. 1981. [Google Scholar]
- 23.Benton AL, Hamsher K. Multilingual Aphasia Examination. 2. 1989. [Google Scholar]
- 24.Alonso A, Mosley TH, Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80:1194–1201. doi: 10.1136/jnnp.2009.176818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider AL, Gottesman RF, Mosley T, et al. Cognition and incident dementia hospitalization: results from the atherosclerosis risk in communities study. Neuroepidemiology. 2013;40:117–124. doi: 10.1159/000342308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 27.Pugliese G, Solini A, Bonora E, et al. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) Study formula in subjects with type 2 diabetes. Atherosclerosis. 2011;218:194–199. doi: 10.1016/j.atherosclerosis.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) Serum Seasonality in the United States. PLoS ONE. 2013;8:e65785. doi: 10.1371/journal.pone.0065785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2011;26:477–484. doi: 10.3233/JAD-2011-110149. [DOI] [PubMed] [Google Scholar]
- 31.Dean AJ, Bellgrove MA, Hall T, et al. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults--a randomised controlled trial. PLoS ONE. 2011;6:e25966. doi: 10.1371/journal.pone.0025966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossom RC, Espeland MA, Manson JE, et al. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J Am Geriatr Soc. 2012;60:2197–2205. doi: 10.1111/jgs.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 34.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 35.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. American Journal of Epidemiology. 2010;171:903–908. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 36.Berger C, Greene-Finestone LS, Langsetmo L, et al. Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. Journal of bone and mineral research. 2012;27:1381–1389. doi: 10.1002/jbmr.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.