Abstract
In (NZB × NZW)F1 (B/W) mice, moderate caloric intake [10 kcal (41.8 kJ) per day] from the time of weaning was associated with maintenance of lower body weight, greater capacity of spleen cells to be stimulated with T-cell mitogens, and better preserved capacity to generate cytotoxic cells in response to in vitro and in vivo stimulation with allogeneic tumor cells. Plaque-forming cell response to sheep erythrocytes was also well maintained in animals on the restricted diets when sensitization was accomplished either in vitro or in vivo. Spontaneous suppressor cell activity against plaque-forming cells that developed in controls did not appear in the mice on the restricted diet. Significantly less circulating antibody to native DNA was present in the blood of mice 10 months of age when their dietary intake had been restricted. Histological analysis revealed that the development of renal disease and the deposition of gamma globulin in the glomerular capillaries was markedly inhibited in the mice on restricted diets. Dietary restriction from the time of weaning thus appears to prolong significantly the life of autoimmunity-prone (NZB × NZW)F1 male and female mice and to alter lymphoid cell immune function, thereby decreasing the autoimmune processes and immunological assault associated with progressive renal disease in these animals.
Keywords: calories, autoimmunity, longevity, immunity, histopathology
Full text
PDF
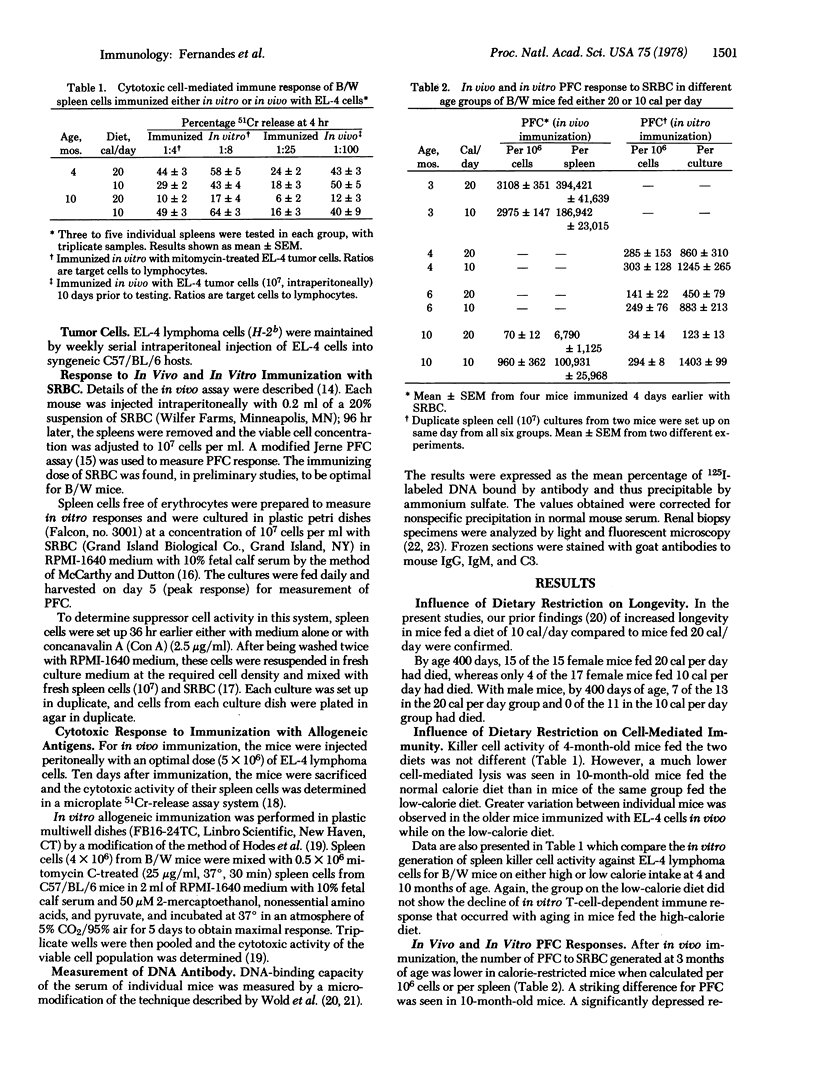
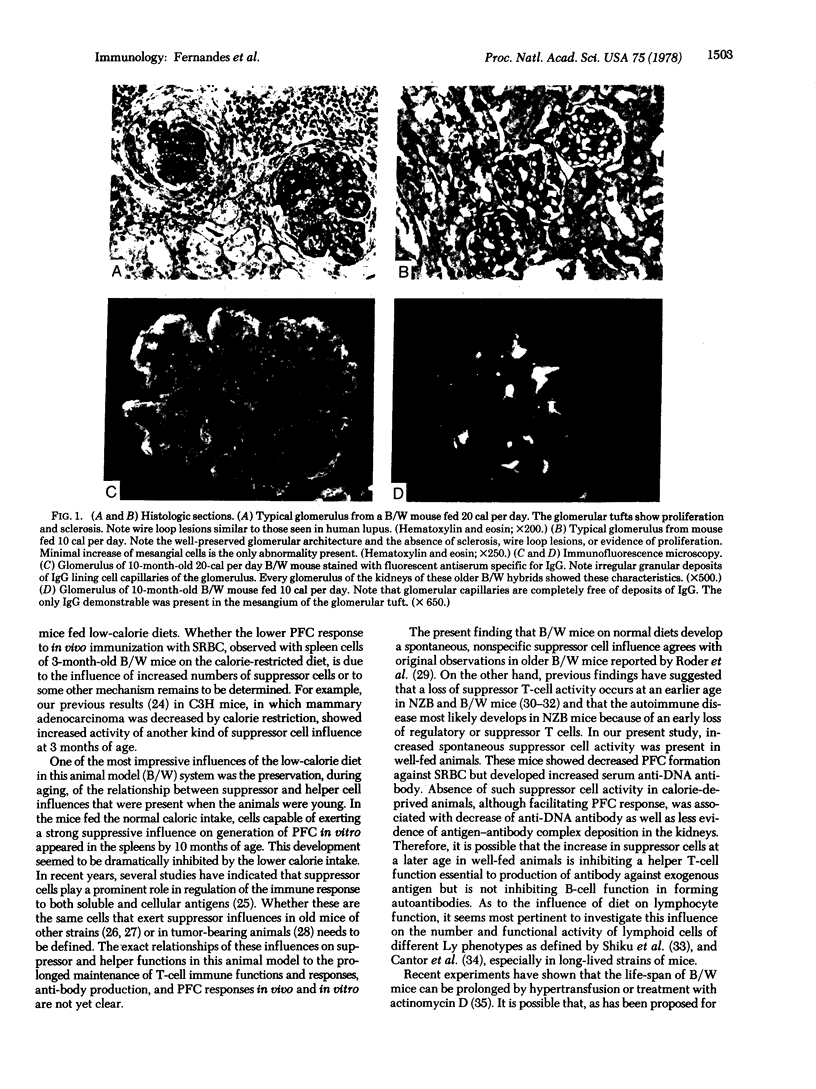

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullock W. W., Möller E. "Spontaneous" B cell activation due to loss of normal mouse serum suppressor. Eur J Immunol. 1972 Dec;2(6):514–517. doi: 10.1002/eji.1830020609. [DOI] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois E. L., Strain L. Effect of diet on survival and nephropathy of NZB-NZW hybrid mice. Biochem Med. 1973 Apr;7(2):336–342. doi: 10.1016/0006-2944(73)90091-4. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Halberg F., Yunis E. J., Good R. A. Circadian rhythmic plaque-forming cell response of spleens from mice immunized with SRBC. J Immunol. 1976 Sep;117(3):962–966. [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Depression of cytotoxic T cell subpopulation in mice by hydrocortisone treatment. Clin Immunol Immunopathol. 1975 Jul;4(2):304–313. doi: 10.1016/0090-1229(75)90066-5. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Influence of protein restriction on immune functions in NZB mice. J Immunol. 1976 Mar;116(3):782–790. [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Good R. A. Suppression of adenocarcinoma by the immunological consequences of calorie restriction. Nature. 1976 Oct 7;263(5577):504–507. doi: 10.1038/263504b0. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Jose D. G., Good R. A. Dietary influence on antinuclear antibodies and cell-mediated immunity in NZB mice. Int Arch Allergy Appl Immunol. 1973;44(6):770–782. doi: 10.1159/000230981. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Yunis E. J., Smith J., Good R. A. Dietary influence on breeding behavior, hemolytic anemia, and longevity in NZB mice. Proc Soc Exp Biol Med. 1972 Apr;139(4):1189–1196. doi: 10.3181/00379727-139-36327. [DOI] [PubMed] [Google Scholar]
- Friend P. S., Kim Y., Michael A. F., Donadio J. V. Pathogenesis of membranous nephropathy in systemic lupus erythematosus: possible role of nonprecipitating DNA antibody. Br Med J. 1977 Jan 1;1(6052):25–25. doi: 10.1136/bmj.1.6052.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen A. E., Lubert A. S., Olsen C. T. Suppression of murine lupus erythematosus by dactinomycin. Nature. 1976 Dec 2;264(5585):439–440. doi: 10.1038/264439a0. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Ihle J. N., Pillarisetty R. J., Talal N., Dubois E. L., Levy J. A. Type C virus expression and host response in diet-cured NZB/W mice. Nature. 1977 Jul 28;268(5618):341–344. doi: 10.1038/268341a0. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Goidl E. A., Innes J. B., Weksler M. E. Immunological studies of aging. II. Loss of IgG and high avidity plaque-forming cells and increased suppressor cell activity in aging mice. J Exp Med. 1976 Oct 1;144(4):1037–1048. doi: 10.1084/jem.144.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good R. A., Fernandes G., Yunis E. J., Cooper W. C., Jose D. C., Kramer T. R., Hansen M. A. Nutritional deficiency, immunologic function, and disease. Am J Pathol. 1976 Sep;84(3):599–614. [PMC free article] [PubMed] [Google Scholar]
- Good R. A., Yunis E. Association of autoimmunity, immunodeficiency and aging in man, rabbits, and mice. Fed Proc. 1974 Sep;33(9):2040–2050. [PubMed] [Google Scholar]
- Hodes R. J., Handwerger B. S., Terry W. D. Synergy between subpopulations of mouse spleen cells in the in vitro generation of cell-mediated cytotoxicity: evidence for the involvement of a non-T cell. J Exp Med. 1974 Dec 1;140(6):1646–1659. doi: 10.1084/jem.140.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose D. G., Good R. A. Absence of enhancing antibody in cell mediated immunity to tumour heterografts in protein deficient rats. Nature. 1971 Jun 4;231(5301):323–325. doi: 10.1038/231323b0. [DOI] [PubMed] [Google Scholar]
- Jose D. G., Good R. A. Quantitative effects of nutritional essential amino acid deficiency upon immune responses to tumors in mice. J Exp Med. 1973 Jan 1;137(1):1–9. doi: 10.1084/jem.137.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose D. G., Good R. A. Quantitative effects of nutritional protein and calorie deficiency upon immune responses to tumors in mice. Cancer Res. 1973 Apr;33(4):807–812. [PubMed] [Google Scholar]
- Kirchner H., Chused T. M., Herberman R. B., Holden H. T., Lavrin D. H. Evidence of suppressor cell activity in spleens of mice bearing primary tumors induced by Moloney sarcoma virus. J Exp Med. 1974 Jun 1;139(6):1473–1487. doi: 10.1084/jem.139.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer R. S., Strober W., Rippeon D. L., Waldmann T. A. Prevention of autoimmunity in experimental lupus erythematosus by soluble immune response suppressor. Science. 1977 Apr 1;196(4285):56–59. doi: 10.1126/science.300174. [DOI] [PubMed] [Google Scholar]
- Makinodan T., Adler W. H. Effects of aging on the differentiation and proliferation potentials of cells of the immune system. Fed Proc. 1975 Feb;34(2):153–158. [PubMed] [Google Scholar]
- McCarthy M. M., Dutton R. W. The humoral response of mouse spleen cells to two types of sheep erythrocytes. I. Genetic control of the response to H and L SRBC. J Immunol. 1975 Nov;115(5):1316–1321. [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiku H., Kisielow P., Bean M. A., Takahashi T., Boyse E. A., Oettgen H. F., Old L. J. Expression of T-cell differentiation antigens on effector cells in cell-mediated cytotoxicity in vitro. Evidence for functional heterogeneity related to the surface phenotype of T cells. J Exp Med. 1975 Jan 1;141(1):227–241. doi: 10.1084/jem.141.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talal N. Disordered immunologic regulation and autoimmunity. Transplant Rev. 1976;31:240–263. doi: 10.1111/j.1600-065x.1976.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Walford R. L., Liu R. K., Gerbase-Delima M., Mathies M., Smith G. S. Longterm dietary restriction and immune function in mice: response to sheep red blood cells and to mitogenic agents. Mech Ageing Dev. 1973 Dec;2(6):447–454. doi: 10.1016/0047-6374(73)90035-3. [DOI] [PubMed] [Google Scholar]
- Westberg N. G., Naff G. B., Boyer J. T., Michael A. F. Glomerular deposition of properdin in acute and chronic glomerulonephritis with hypocomplementemia. J Clin Invest. 1971 Mar;50(3):642–649. doi: 10.1172/JCI106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold R. T., Young F. E., Tan E. M., Farr R. S. Deoxyribonucleic acid antibody: a method to detect its primary interaction with deoxyribonucleic acid. Science. 1968 Aug 23;161(3843):806–807. doi: 10.1126/science.161.3843.806. [DOI] [PubMed] [Google Scholar]