Abstract
Objective
Two psychological interventions for rheumatoid arthritis (RA) are cognitive-behavioral coping skills training (CST) and written emotional disclosure (WED). These approaches have developed independently, and their combination may be more effective than either one alone. Furthermore, most studies of each intervention have methodological limitations, and each needs further testing.
Method
We randomized 264 adults with RA in a 2 × 2 factorial design to one of two writing conditions (WED vs. control writing) followed by one of two training conditions (CST vs. arthritis education control training). Patient-reported pain and functioning, blinded evaluations of disease activity and walking speed, and an inflammatory marker (C-reactive protein) were assessed at baseline and 1-, 4-, and 12-month follow-ups.
Results
Completion of each intervention was high (> 90% of patients), and attrition was low (10.2% at 12-month follow-up). Hierarchical linear modeling of treatment effects over the follow-up period, and ANCOVAs at each assessment point, found no interactions between writing and training; however, both interventions had main effects on outcomes, with small effect sizes. Compared to control training, CST decreased pain and psychological symptoms through 12 months. The effects of WED were mixed: compared with control writing, WED reduced disease activity and physical disability at 1 month only, but WED had more pain than control writing on one of two measures at 4 and 12 months.
Conclusions
The combination of WED and CST does not improve outcomes, perhaps because each intervention has unique effects at different time points. CST improves health status in RA and is recommended for patients, whereas WED has limited benefits and needs strengthening or better targeting to appropriate patients.
Keywords: Rheumatoid arthritis, emotional disclosure, expressive writing, cognitive behavioral therapy, coping skills training, pain
Rheumatoid arthritis (RA) is an autoimmune disease that affects 1 to 2% of adults, more often women, and exacts substantial economic, social, and psychological costs (Helmick et al., 2008; Wolfe, Allaire, & Michaud, 2007). Research supports a bidirectional relationship between psychological stress and RA onset, progression, and remission. On the one hand, RA is a serious and complex disease, requiring patients to cope not only with pain, disability, and possible joint disfigurement, but also with stressors such as disrupted work, family, and marital functioning (Meenan, Yelin, Nevitt, & Epstein, 1988). On the other hand, major life stressors, interpersonal conflicts, and subsequent difficulty regulating negative emotions can trigger RA onset or increase its severity (Potter & Zautra, 1997; Thomason Brantley, Jones, Dyer, & Morris, 1992; Zautra, Burleson, Matt, Roth, & Burrows, 1994; Zautra et al., 1989).
Interventions for RA
Pharmacological advances hold promise for many patients with RA, but residual pain and disability is common, and some patients avoid newer medications, such as biological response modifiers, due to their high cost or side effects (Muller, Kallikorm, Polluste, & Lember, 2012). There is, therefore, interest in psychosocial interventions for RA. Both cognitive-behavioral and emotion processing approaches have some support as effective interventions for RA.
A substantial literature indicates that active coping strategies such as problem-solving, relaxation, and cognitive restructuring predict better adaptation, whereas catastrophizing and passive coping strategies predict worse adjustment (e.g., Affleck, Urrows, Tennen, & Higgins, 1992; Jensen, Turner, Romano, & Karoly, 1991; Keefe, Brown, Wallston, & Caldwell, 1989). Based on these findings, researchers have developed and tested various cognitive-behavioral therapy protocols. This general approach has two major components. First, it helps patients reconceptualize their pain as a biopsychosocial experience rather than solely a biomedical problem by teaching them how pain is influenced by thoughts, feelings, and behavior. Second, patients are trained in various cognitive and behavioral techniques or skills to enhance their ability to cope with pain and improve their behavioral and psychological functioning. Such coping skills training (CST) is a very common component of cognitive behavioral protocols.
We know of 15 published, randomized controlled trials of cognitive behavioral, skills-based protocols for RA (Applebaum, Blanchard, Hickling, & Alfonson, 1988; Barsky et al., 2010; Bradley et al., 1987; Evers, Kraaimaat, van Riel, & de Jong., 2002; Freeman, Hammond, & Lindoln, 2002; Germond, Schomer, Meyers, & Weight, 1993; Kraaimaat, Brons, Geenen, & Bijlsma, 1995; Leibing, Pfingsten, Bartmann, Rueger, & Schuessler, 1999; O’Leary, Shoor, Lorig, & Holman, 1988; Parker et al., 1988; Radojevic, Nicassio, & Weisman, 1992; Sharpe et al., 2001; Sharpe, Sensky, Timberlake, Ryan, & Allard, 2003; Shearn & Fireman, 1985; Taal, 1993; Zautra et al., 2008). Two meta-analyses, (Astin, Beckner, Soeken, Hochberg, & Berman, 2002; Dixon, Keefe, Scipio, Perri, & Abernethy, 2007), which included many of these 15 studies, concluded that cognitive-behavioral protocols have small to medium effect sizes (ranging from 0.18 to 0.35 SD) on arthritis pain, disability, joint status, and psychological symptoms (depression and anxiety). These 15 clinical trials, however, are limited in various ways. Only six studies used active controls, and only three of those used a control condition with equivalent duration and therapist contact; thus, non-specific effects cannot be ruled out for most of these studies. Also, samples have been quite small, averaging only about 27 patients per condition, and the largest study averaged only 56 patients per condition. Results from small clinical trials such as these often are not reliable (Ioanniddis, 2005). Finally, the median time to follow-up in these studies was only 6 months. Thus, there is a need for studies of CBT-based interventions for RA that have larger samples, active controls, and longer follow-ups.
A second, more recent but distinct literature indicates that awareness, expression, and processing of emotions can be adaptive, whereas emotional avoidance or suppression is often maladaptive (Gross, 2002; Kennedy-Moore & Watson, 1999; Taylor, Bagby, & Parker, 1997). Thus, a second general approach to reducing stress and improving health is to express and process emotions. The technique of written emotional disclosure (WED), or expressive writing (Pennebaker & Beall, 1986), asks participants to write (or in some studies, speak) privately for 15 to 30 minutes daily for 3 or 4 sessions about stressful experiences and their deepest thoughts and feelings. It is believed that such expression, processing, and resolution will reduce stress and thereby improve health. A meta-analysis of early studies conducted on healthy people showed a moderate sized benefit (0.47 SD) of WED versus control writing across a range of outcomes (Smyth, 1998). In the last 15 years, research on emotional disclosure has exploded, and many studies have been done with clinical populations. The largest meta-analysis of this literature reported a small, yet positive effect of disclosure (effects size = 0.15 SD; Frattaroli, 2006).
Researchers have tested emotional disclosure in people with RA, due in part to the increased life stress and reactivity found in this population. Eight studies of either written or verbal (spoken) emotional disclosure in RA have been published. An early highly-controlled study demonstrated that WED led to reduced disease activity (Smyth, Stone, Hurewitz, & Kaell, 1999), although this same team subsequently found little or no benefit of WED when done as part of routine clinical practice (Broderick, Stone, Smyth, & Kaell, 2004). Although one other study found no benefits of spoken disclosure (Keefe et al., 2008), the other studies of written or spoken disclosure in RA have found some benefits (Danoff-Burg, Agee, Romanoff, Kremer, & Strosberg, 2006; Kelley, Lumley, & Leisen, 1997; Lumley et al., 2011; van Middendorp, Geenen, Sorbi, van Doornen, & Bijlsma, 2009; Wetherell et al., 2005), although the benefits are often limited to one or a few outcomes (pain, joint status, inflammatory markers, behavioral functioning, psychological status), which often vary by study. Samples sizes have been rather small (from 21 to 40 participants per condition, including the “protocol adherent” patients in Broderick et al., 2004), and only one study had a follow-up beyond 6 months (Keefe et al., 2008). Furthermore, half of these studies have examined speaking, in part due to the assumption that arthritic hand joints would prohibit writing; however, writing has been by far the most validated approach to emotional disclosure. The control topic used in most of these studies (writing about time management) may not be ideal; a more face-valid, health-relevant control condition might provide a stronger test of the hypothesis that disclosing and processing one’s stressors and emotions is the central process involved. Finally, there is evidence that enhancing the instructions for writing might be worthwhile, such as instructing writers to remain on topic, reflect on the meaning of the stressor, and to write about coping possibilities (Broderick et al., 2004; Cameron & Nicholls, 1998). Thus, research is needed in which emotional disclosure through writing is tested on large samples of people with RA, using longer follow-ups, instructions that encourage more powerful disclosure, and a better control condition.
Independent and Combined Effects of CST and WED
These two approaches have developed independently and differ fundamentally in their assumptions regarding stress and negative emotions. Cognitive-behavioral approaches such as CST encourage new thoughts and behaviors to manage pain and improve functioning, but do not target unresolved stress or confront avoided emotional experiences. Indeed, negative emotions are generally viewed as needing down-regulation by techniques such as relaxation, reappraisal of catastrophic thoughts, engaging in pleasant activities, and developing new ways to solve problems. Emotional disclosure, in contrast, views the experience of negative emotions as potentially adaptive, reversing unhelpful patterns of avoidance or inhibition, and motivating and guiding behavior toward adaptive ends. Thus, emotional disclosure seeks to temporarily augment negative emotions by encouraging people to experience, express, process, and learn from their negative emotional experiences.
These two interventions may also affect different outcomes. There is some evidence that emotional disclosure is most likely to improve physiological indices of health (Frisina et al., 2004; Harris, 2006; Smyth, 1998), and in RA, emotional disclosure has improved immune markers in one study (van Middendorp et al., 2009), and disease status in others (Smyth et al., 1999; Wetherall et al., 2005). In contrast, disclosure’s effects on subjective health might be weaker, in part, because disclosure usually first increases negative emotions. Coping skills training, in contrast, targets specifically pain and behavioral functioning, but one might not expect this intervention to improve disease activity or physiological indices.
The somewhat modest effects of both CST and WED suggest a need to strengthen them. Given that these two interventions appear to have different targets, processes, and possibly outcomes, combining these approaches may achieve better outcomes than either one alone. For example, patients may need to both resolve their emotional stressors or conflicts and learn skills to manage their condition, so that a combination of WED and CST should provide a more powerful intervention for those patients. Alternatively, there may be subsets of patients—some who need primarily disclosure to address unresolved emotional issues, and others who need to learn cognitive-behavioral skills; if so then a combined intervention should cover a larger number of patients than either intervention alone. More generally, pain and adjustment are broad constructs that encompasses behavioral, cognitive, and affective dimensions, and it is possible that CST more effectively influences the behavior and cognition, whereas WED targets emotion. It is possible, of course, that these two approaches are largely redundant or even opposing in their processes or outcomes, such that a combined intervention would add nothing to the effects of each approach alone.
How should WED and CST be combined? Emotional disclosure targets stress but provides no guidance or training on how to change cognitive, behavioral, or interpersonal functioning. Researchers of WED assume that reduced stress following emotional disclosure is sufficient to improve health, or that disclosure will somehow guide and motivate patients to make subsequent changes on their own. Yet, behavioral change after disclosure does not appear to occur spontaneously (Stone, Smyth, Kaell, & Herewitz, 2000). Furthermore, emotional disclosure can be unsettling, and at least one team has recommended that subsequent training in coping skills might be needed to improve outcomes (Gidron, Peri, Connolly, & Shalev, 1996). In addition, the transtheoretical / stages of change model (Norcross, Krebs, & Prochaska, 2011) suggests that techniques that encourage insight and increase motivation to change, particularly emotion-oriented approaches, should precede action techniques, such as cognitive and behavior change approaches. Thus, we reasoned that WED should occur prior to CST so that patients might first become aware of their sources of stress, increase their motivation for change, and increase the value that they put on learning coping skills.
Design of the Study, Goals, and Hypotheses
We conducted a large-scale, multi-site, active-control randomized clinical trial of the independent and combined effects of WED and CST in people with RA (the RAISED trial: Rheumatoid Arthritis Instruction in Skills and Emotional Disclosure). We used a 2 × 2 factorial design, in which patients first engaged in a one of two writing conditions (WED or control writing) followed by one of two training conditions (CST or control training). This design allowed us to compare not only the unique combination of WED + CST—that is, their interaction—against each approach alone (and against neither approach), but also allowed us to examine the main effects of WED (vs. control writing) and CST (vs. control training) in larger samples. Patients were assessed at baseline and three follow-up assessments over 12 months on a range of outcomes, including self-report measures, blinded physical examination and walking speed test, and an inflammatory marker. We hypothesized that there would be main effects; each intervention would improve health compared to its control, and specifically the primary outcomes of disease activity for WED and pain for CST. We also hypothesized that the combined condition would be more effective than either intervention alone.
Methods
Participants
Participants were recruited in nearly equal numbers from two sites: metropolitan Detroit, Michigan and the catchment area of Duke University Medical Center in North Carolina. To be included, participants needed to be adults who met the 1987 American College of Rheumatology criteria for non-juvenile RA and who reported pain or morning stiffness due to RA during week prior to screening. Patients were excluded if they: a) had another autoimmune disorder; b) had a current, life-threatening disease (e.g., cancer); c) were illiterate or cognitively impaired; d) were in a formal behavioral pain management program; e) had experienced a major stressful life change in the prior 6 months; or f) were unable to write or walk.
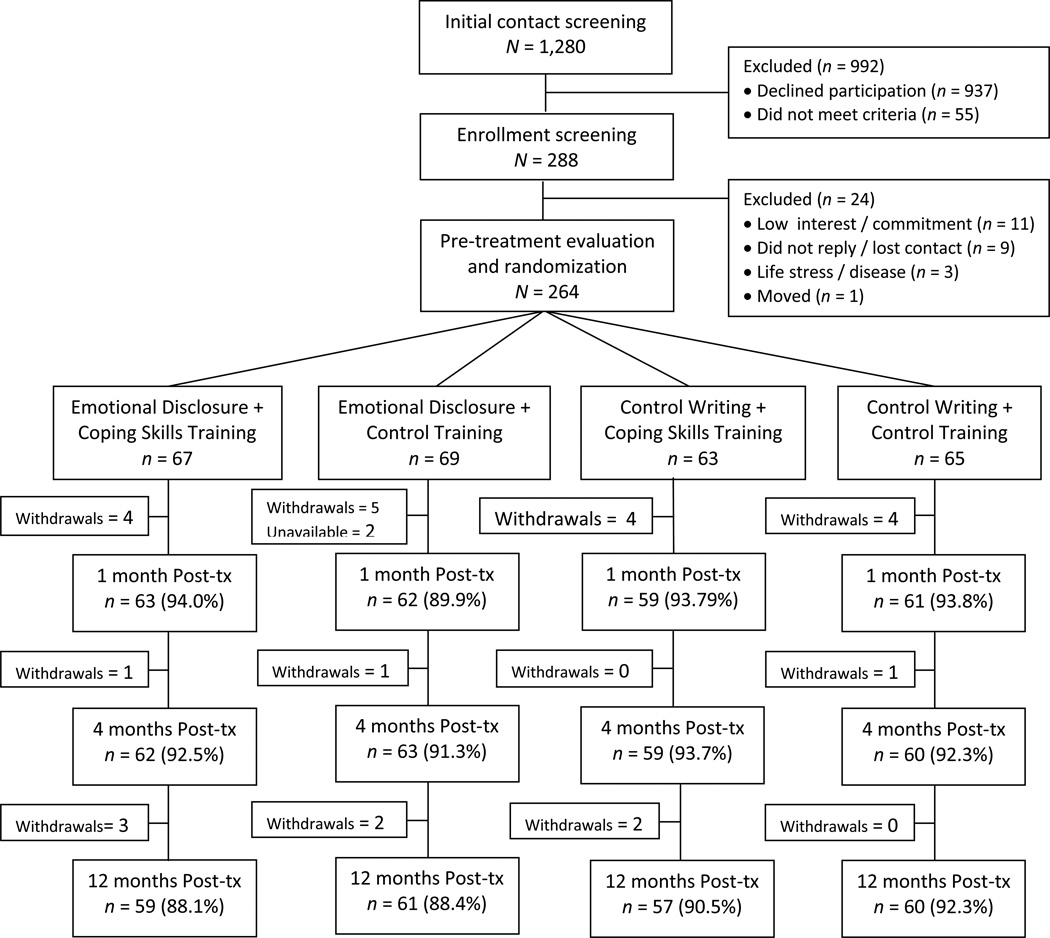
Figure 1 depicts patient flow through the study. There were 1,280 potential participants who had an initial contact screening about the study (i.e., they contacted the study team or were informed about it at a rheumatology clinic). Although most people were not interested in participating (n = 937; 73.2%), those who were interested were screened by a study rheumatologist; 55 (4.3%) were excluded because they did not meet the above study criteria. Of the remaining 288 patients, 24 were excluded prior to randomization, leaving a final, intent-to-treat sample of 264 patients who were randomized. This final sample included 214 women (81.1%) and 50 men (18.9%), averaged 55.1 years of age (SD = 12.1; range 22 – 82 years), and was 68.2% European American, 28% African American, 1.5% Asian, 1.5% mixed race, and 0.8% Asian. The sample was 65.6% married or living together, 14% divorced, 11.4% never married, and 8% widowed; had a mean education of 14.7 years (i.e., 2.7 years of college; SD = 2.5); and averaged 13.1 (SD = 11.4) years since RA diagnosis.
Figure 1.
RAISED study flow of participants.
Procedure
The study was approved by the institutional review boards of both sites and registered on Clinicaltrials.gov (#NCT00088764) before recruitment, which ran from March 2005 to November 2007; follow-up assessments were completed by April 2009. The Michigan site recruited largely through community advertisements, flyers in rheumatologists’ offices, and letters sent to patients from the local Arthritis Foundation. The initial contact screening in Michigan was conducted primarily by phone and included a full study description and screening by the team rheumatologist, who examined the patient or corresponded with patient’s physician, as needed, to confirm diagnosis and study criteria. The North Carolina site recruited directly through its rheumatology clinics, and the initial contact screening, including confirmation of criteria, was conducted by referring rheumatologists and staff at those clinics.
At both sites there was a subsequent in-person enrollment screening visit, at which a post-bachelor’s level research assistant, following a standard protocol, obtained written consent, interviewed the patient for background and medical history, had the patient complete background questionnaires, and instructed the patient in the completion of daily diaries at home for the next month. (Diary data will be presented elsewhere.) Patients who passed this screening and remained interested and committed (operationalized as submitting at least 21 consecutive baseline daily diaries), came to a subsequent pre-treatment (baseline) evaluation, where they completed health-related questionnaires, had a physical examination and walking speed test by a rheumatologist, and had a blood draw.
Prior to recruitment, a person independent of the study staff used randomization software to develop the condition assignments. To balance the conditions by important variables, randomization was stratified by the two study sites as well as three current medication classes; patients were classified as taking biological response modifiers, disease modifying anti-rheumatic drugs (but not biologicals), or neither of these two. Randomization was done in blocks of 8 patients to one of two writing conditions (WED or control writing) and one of two training conditions (CST or education control), and assignments were sealed in envelopes.
At the end of the pre-treatment evaluation, the research assistant and each patient jointly opened the next envelope in the sequence, which contained the patient’s randomly assigned writing and training conditions. The assistant read aloud a script describing the two assigned conditions while the patient read silently. Patients started their assigned writing condition, which continued for the next week, after which they immediately began their assigned training condition, with eight sessions held at weekly intervals. The three post-treatment health evaluations were identical to the pre-treatment evaluation and occurred approximately 1 month, 4 months, and 12 months after the training ended. (In actuality, consistent with ideal clinical trials methodology, we scheduled evaluations exactly 4 months, 7 months, and 15 months after randomization rather than the end of treatment.) Patients were paid for $15 for the screening visit and $30 for each evaluation. Interventions were provided at no charge.
Writing Conditions
After the pre-treatment evaluation and randomization, the research assistant gave patients a blank journal with instructions for each session of writing. The assistant read the condition-specific daily writing instructions (while patients read along), and patients then conducted their first writing session for their assigned condition, in the laboratory or clinic, for 20 minutes. Following this writing, the assistant discussed a writing plan with the patient, including day, time, and location for the next three, 20-minute writing sessions, which occurred at home, for both writing conditions, within the next week. The assistant telephoned patients during this interval to verify that the writing was conducted (or to remind patients to do so), and all writings were returned to the research team, to verify adherence. There were two writing conditions:
Written emotional disclosure (WED)
The rationale given to patients for this condition was that stressful experiences, including experiences from childhood, difficult problems in life currently, and even health problem such as RA, are common. Stressful experiences that are kept private or not talked about increase pain and disability, and writing about one’s thoughts and feelings about stressful experiences can decrease stress and improve health. We used enhanced or guided daily instructions for writing, which were as follows: In session 1, patients were instructed to identify stressful or traumatic events that continued to cause them stress and to write about their most vivid memories and innermost thoughts and feelings about those events. In session 2, patients were asked to continue writing about their thoughts and feelings about their stressful events. In session 3, patients were instructed to try to find meaning from their stressful events—how the events affected their beliefs or their health, and to write about anything they learned from their experiences. In session 4, patients were asked to write about how they coped with their stressful experiences, whether those coping strategies were useful, and how they would cope with their stressors or their feelings in the future.
Control writing
This condition was adapted from the commonly used “time management” control writing condition, but with a different health behavior focus each session. This condition was designed to be face valid as relevant to RA, to create equivalent expectation for improvement as WED, but to focus solely on behaviors and avoid emotional disclosure and processing of stressful experiences. The introductory rationale paralleled that of WED by noting that practicing healthy behaviors helps to maintain good health, and that writing about one’s health behaviors in detail can lead to better health. In session 1, patients wrote about how they spent and managed their time during the prior week. In session 2, patients wrote about their eating behaviors during the current day and reviewed their eating over recent days. In session 3, patients wrote about physical activity behaviors during the past week. In session 4, patients detailed their sleep over the past week (i.e., when they went to sleep, hours they slept, if they woke up in the night). Patients were instructed to write about only the facts of their behaviors but not their thoughts, feelings, or opinions.
Training Conditions
Immediately after the 1-week writing period, each patient was contacted by his / her assigned therapist (CST) or health educator (arthritis education) to schedule the first training session. Both training conditions were conducted individually rather than in group format, were manualized, and used highly structured procedures (e.g., a posted flowchart of session content for CST, and slides / handouts for arthritis education). Both conditions lasted for eight sessions, which were 75 minutes long and held weekly. We used in-person sessions when possible, and always for the first session, so that the patient and therapist / educator could develop an alliance; however, telephone sessions were used as an alternative for any subsequent session for patients who were unable to meet in person due to distance, transportation problems, weather, or disease status. Our use of telephone sessions, as needed, was expected to increase intervention participation and study retention, and studies have demonstrated the efficacy of telephone administered CBT protocols for various health problems, particularly chronic, non-life-threatening conditions like RA (Cox et al., 2012; Muller & Yardley, 2011).
CST was administered by advanced doctoral students or post-doctoral fellows in clinical psychology, and arthritis education was administered by people with training in health education, primarily nurses. Although crossing therapists over training conditions has some advantages, as discussed by Elkin (1999), there were several reasons that we nested therapists / educators with training condition and had different professionals lead each training condition. First, ecological validity and competence is increased because in clinical practice, psychologists are likely to conduct CST, and health educators such as nurses conduct health education. Second, the two conditions could be presented with greater fidelity, rather than risk the confounding of protocols that sometimes occurs when one person administers multiple protocols. Third, each set of providers could present their condition with full commitment to and belief in its efficacy, thus avoiding the bias that can stem from having psychologists be more committed to CST than to the education control condition.
Initial training of the therapists or educators was provided at both study sites during 2-day, intensive workshops designed specifically for CST or arthritis education. In these workshops, study leaders discussed and demonstrated each session’s content, had the therapists / educators role play each session, and provided feedback and troubleshot difficulties. To further ensure treatment competence, fidelity, and adherence to training protocols, sessions were audio recorded and regularly reviewed by a doctoral level psychologist supervisor at each site. Weekly supervision, using the session recordings, was conducted by the same psychologist with therapists or educators.
Coping skills training (CST)
We adapted previously validated cognitive-behavioral CST protocols (Carson et al., 2006; Keefe et al., 1990) for the 8-session format and structure required by this study. The following rationale was provided: “Research studies suggest that how you cope with your arthritis influences how much pain, disability, and mood problems you experience. You can learn specific techniques or skills to help you cope with and manage your arthritis pain and disability.” In session 1, therapists provided the rationale for CST, including the gate-control theory of pain, and patients learned progressive muscle relaxation. Session 2 reviewed relaxation and taught pleasant activity scheduling. Session 3 introduced assertive communication skills through role-playing, modeling, and feedback, and taught the use of applied relaxation (“mini-practice”) in daily life. Session 4 continued communication skills training and introduced cognitive restructuring, particularly identifying maladaptive thoughts. Session 5 continued cognitive restructuring, with a focus on changing cognitions, and also covered activity-rest cycling, which is designed to interrupt pain cycles and help patients gradually increase their activity level. Session 6 reviewed activity-rest cycling and taught distraction skills (pleasant imagery, focal point, auditory stimuli). In session 7, patients reviewed all of the skills and learned problem solving, including how to apply the skills to problem situations. Session 8 addressed relapse prevention by having patients develop a written maintenance plan that included coping skills to deal with anticipated setbacks and pain flares.
Arthritis education control training
This condition has been used in other clinical trials to control for a host of non-specific factors, including having an equivalent rationale for change, having equal time with and attention from a therapist, and actively attending and engaging in sessions (Barsky et al., 2010; Knittle, Maes, & de Gucht, 2010). In this study, the rationale that we provided was that “RA is a complex disease that requires both medical care and self-care. Knowledgeable and educated patients can make better medical decisions and take care of themselves more effectively. Therefore, you can benefit by learning the most recent information about this disease and its treatments.” Session 1 covered basic facts and characteristics of RA; session 2, joint anatomy and physiology, and signs and symptoms of RA; session 3, diagnosis and prognosis of RA, goals of treatment, overview of all treatments; session 4, the immune system and RA-specific medications (steroids, DMARDS, biological response modifiers); session 5, pain assessment, analgesics, and other pain medications; session 6, complementary and alternative medicine interventions, dietary supplements, nutrition, weight control, exercise; session 7, surgery, physical modalities, physical and occupational therapy, adaptive devices; and session 8, shared management of RA, clinical trials for RA, resources for patients, including internet skills.
Measures
There were two primary outcomes, one for each intervention: disease activity for WED, and pain for CST. There also were several secondary outcomes covering a range of constructs of relevance to RA.
Disease activity
A rheumatologist, kept blind to patients’ treatment conditions, conducted a physical examination and joint assessment. The physician evaluated the presence or absence of tenderness and swelling in each of 16 joints bilaterally (5 interphalangeal and 5 metacarpal phalangeal joints in addition to shoulder, elbow, wrist, knee ankle and metatarsals, for a total of 32 joints; Felson et al., 1993). The physician then provided a global rating of the patient’s disease activity (Anderson, Felson, Meenan, & Williams, 1989) on a 100-mm visual analog scale, with anchors of “no disease activity” to “most disease activity.” Because joint “tenderness” requires patients to report their pain (which was assessed with other measures) we used only the swollen joint count, which is more objective and not dependent on patient report. As expected, the physician’s count of the number of swollen joints and the physician’s rating of disease activity were highly correlated (r = .67), so these two measures were composited (each converted to z-scores and averaged) to yield a single index of disease activity. Higher scores indicate greater disease activity.
Pain
Patients completed the self-report Arthritis Impact Measurement Scales-2 (AIMS2; Meenan, Jason, Anderson, Guccione, & Kazis, 1992), which assesses several domains of functioning during the past month. This measure is a psychometrically-improved version of the original AIMS, which has been well validated and is sensitive to clinical changes (Anderson, Firschein, & Meenan, 1989). The 5-item pain subscale of the AIMS2 served as a primary outcome. In addition, secondary outcomes were the sensory and affective dimensions of pain as assessed with the McGill Pain Questionnaire (MPQ; Melzack, 1975), a checklist of 102 adjectives divided into 20 categories. Research supports its reliability and validity (Melzack & Katz, 1992). Higher scores on these measures indicate greater pain.
Physical disability
This was assessed with a composite of the six AIMS2 subscales (mobility, walking/bending, hand/finger function, arm functioning, household tasks, and self-care). Higher mean scores indicate greater disability.
Psychological symptoms
This was assessed with a composite of two AIMS2 subscales (tension and mood). Higher scores indicate more psychological symptoms, particularly of anxiety and depression.
Walking speed
A blinded rheumatologist or technician had patients walk down a 50-foot hallway “as quickly as possible, but safely.” The time to walk the 50 feet was recorded in seconds. Higher values indicate slower walking (i.e., poorer functioning).
Inflammatory activity
Patients had blood serum drawn and assayed for C-reactive protein (CRP), yielding levels measured in mg/liter. CRP is a more specific measure of systemic inflammatory activity than erythrocyte sedimentation rate; higher levels of CRP indicate greater inflammatory activity.
Data Analyses
Power analyses and sample size determination
The targeted sample size was determined a priori by these factors: a) meta-analytic reviews (see Introduction) of the effects of both WED and cognitive-behavioral protocols, which show that each intervention, when conducted separately, has at least small effects (0.20 to 0.30 SD) across the range of outcome measures used in this study; b) our expectation that the combined intervention (WED + CST) would yield larger effects than either intervention alone or neither intervention; and c) the presence of a baseline covariate (assumed to correlate r = .5 with the outcome). To obtain a power of .80, a sample size of 60 patients per intervention combination (i.e., 240 total patients) would be needed to detect an effect size of d = .36. Accounting for expected attrition (estimated at 10% from randomization to the last follow-up, we planned to randomize 268 patients, and we were able to randomize 264 patients. Note that this sample size also provided more than adequate power to detect small effects for the planned main effect analyses (WED vs. control writing, and CST vs. control training), which essentially doubled the sample size, averaging 132 patients per condition.
Preliminary analyses
Distributions of variables were examined. Walking time had a few high outliers, so these distributions were winsorized (high outliers were reduced to 4 SDs above the mean; i.e., to 26 seconds) to bring them to normality. Also, C-reactive protein was highly positively skewed, which was corrected with log transformation, but the transformation did not change the results of statistical analyses, so only untransformed CRP values are presented. We next used t-tests or chi-squares to examine the success of randomization in creating equivalence on demographics and baseline levels of outcome measures among all four condition combinations, and also between the two main effect conditions: WED versus control writing, and CST versus control training. Finally, attrition analyses compared those who completed the 12-month follow-up to those who did not.
Primary analyses
The hypotheses were tested using two complimentary approaches: hierarchical linear modeling (HLM) of condition by time interaction effects over the three follow-up assessments, followed by analyses of covariance (ANCOVA), comparing conditions at each follow-up time point separately. The HLM approach, which is a form of multilevel modeling, is best suited for determining trends across multiple points; it estimates condition differences in slopes or rates of change from the baseline through the three follow-up periods for each outcome measure. We used HLM v7.0 (Raudenbush, Bryk, & Congdon, 2011). Such HLM analyses have limitations, however. In particular, subsequent testing is needed to determine the specific time points of condition effects, and if effects are limited to just certain time points, then omnibus HLM may not detect them. Thus, we also conducted ANCOVAs (covarying the baseline level of the outcome measure) on each outcome at the 1-month, 4-month, and 12-month follow-up points separately, using the General Linear Model, Univariate program of SPSS version 20. When between-condition effects were found, we conducted paired t-tests within each condition to determine how the measure changed over time. All analyses were conducted on the intent-to-treat sample of all 264 randomized participants. This is done by default in HLM, which estimates missing outcome values; but for the ANCOVAs, we replaced those few missing outcome values with the last available value, which in most cases was the baseline value (Shao & Zhong, 2003).
Given the 2 × 2 design, both the HLM and ANCOVA models first tested the hypothesis that the combined WED + CST condition would have greater benefits beyond that expected from main effects alone; analyses examined presence of interactions between writing (WED vs. control writing) and training (CST vs. control training). If there was no significant interaction, we proceeded to test the hypotheses that WED would yield better outcomes than control writing, and CST would yield better outcomes than control training. Writing main effects compared all patients who engaged in WED to all patients who engaged in control writing, regardless of whether the patients subsequently engaged in CST or control training. Similarly, training main effects compared all patients who received CST to those who received control training, regardless of the prior writing condition. Note that such main effect comparisons are not confounded by the presence or absence of the other intervention, given the independence of the two interventions in this random assignment experiment. Nonetheless, main effects were tested in statistical models that also included the other main effect.
Effect sizes
We present several types of effect sizes. For between-condition effects, we present the effect size (ES), which is defined as intervention group change (follow-up minus baseline) minus control group change (follow-up minus baseline) / pooled SD of the change scores. Positive values of this ES indicate greater improvements for the intervention than the control condition. We also present within-condition effect sizes (d), calculated as follow-up minus baseline, divided by the SD of the change scores for that condition. Values ES or d of 0.20, 0.50, and 0.80 are often considered to be small, medium, and large, respectively (Cohen, 1988). We also present the partial eta squared (pη2) statistic from the ANCOVAs that we conducted, which represents the proportion of variance in the outcome related to the group factor while holding constant baseline scores. Values of .01, .06, and .14 correspond to small, medium, and large effects, respectively (Cohen, 1988).
Ancillary analyses
To determine whether the results were biased by inclusion of all randomized participants, including those who completed less than the full interventions, a final set of ANCOVAs was conducted on protocol adherent patients, defined as those who completed all four of the writing sessions and all eight of the training sessions.
Results
Baseline Comparisons / Randomization Check
Table 1 presents demographic and medical history data for each of the four condition combinations and the results of analyses comparing the conditions. The four conditions did not differ on age, gender, education, marital status (partnered or not), RA duration, medication, or race (White vs. other). Table 2 presents data on outcome measures for all four condition combinations at all four assessment points (baseline and 1-, 4-, and 12-month follow-ups). The four condition combinations did not differ on baseline levels of outcome measures (all p > .31). Similarly, there were no differences at baseline on outcome measures between WED and control writing (all p > .36, but walking speed: p = 07), and between CST and control training (all p > .10). Thus, randomization was successful at generating conditions that were equivalent.
Table 1.
Demographic and Medical History (Mean and SD, or n and %) for the Four Intervention Combinations of Writing and Training, and Comparisons Among Them
| Variable | WED + CST (n = 67) |
WED + Control Training (n = 69) |
Control Writing + CST (n = 63) |
Control Writing + Control Training (n = 65) |
ANOVA or Chi-square p-value |
|---|---|---|---|---|---|
| Age | 56.0 (10.4) | 55.2 (12.3) | 54.0 (13.7) | 55.3 (11.9) | .81 |
| Education | 14.9 (2.8) | 14.6 (2.3) | 14.3 (2.4) | 14.9 (2.5) | .52 |
| RA duration (yrs) | 11.9 (11.5) | 12.6 (10.9) | 14.4 (11.4) | 13.7 (11.2) | .61 |
| Gender: | .88 | ||||
| Female | 54 (80.6%) | 56 (81.2%) | 53 (84.1%) | 51 (78.8%) | |
| Male | 13 (19.4%) | 13 (18.8%) | 10 (15.9%) | 14 (21.5%) | |
| Race: | .27 | ||||
| White | 43 (64.2%) | 43 (62.3% | 44 (69.8%) | 50 (76.9%) | |
| Black | 21 (31.3%) | 24 (34.8%) | 18 (28.6%) | 11 (16.9%) | |
| Other | 3 (4.5%) | 2 (2.9%) | 1 (1.6%) | 4 (6.2%) | |
| Marital status: | .85 | ||||
| Married/partner | 46 (68.7%) | 44 (63.8%) | 41 (65.1%) | 42 (64.6%) | |
| Single/widow | 21 (31.3%) | 25 (36.2%) | 22 (34.9%) | 23 (35.4%) | |
| Medications: | |||||
| Biologicals | 31 (46.3%) | 34 (49.3%) | 34 (54.0%) | 32 (49.2%) | .85 |
| DMARDS | 50 (74.6%) | 48 (69.6%) | 41 (65.1%) | 40 (61.5%) | .41 |
| Steroids | 31 (46.3%) | 23 (33.3%) | 29 (46.0%) | 34 (52.3%) | .16 |
| Opioids | 20 (29.9%) | 15 (20.5%) | 15 (23.8%) | 23 (35.4%) | .29 |
| NSAIDS | 45 (67.2%) | 42 (60.9%) | 47 (74.6%) | 40 (61.5%) | .32 |
Note. WED = Written Emotional Disclosure; CST = Coping Skills Training; DMARDS = Disease modifying antirheumatic drugs; NSAIDS = Nonsteroidal anti-inflammatory drugs
Table 2.
Baseline and Follow-up Data (M, SD) for Each Outcome Measure for the Four Combinations of Writing and Training
| Outcome measure Time point |
WED + CST (n = 67) |
WED + Control Training (n = 69) |
Control Writing + CST (n = 63) |
Control Writing + Control Training (n = 65) |
|---|---|---|---|---|
| Disease activity | ||||
| Baseline | 0.11 (0.86) | −0.02 (0.91) | −0.02 (0.83) | −0.09 (1.03) |
| 1-month | 0.09 (0.80) | −0.21 (0.71) | 0.03 (0.90) | 0.09 (1.09) |
| 4-month | 0.11 (0.86) | −0.06 (0.83) | −0.11 (0.79) | 0.05 (1.09) |
| 12-month | 0.10 (0.87) | −0.09 (0.75) | −0.05 (0.80) | 0.04 (1.03) |
| AIMS2 Pain | ||||
| Baseline | 3.06 (1.00) | 2.87 (1.02) | 2.96 (0.96) | 2.74 (1.02) |
| 1-month | 2.89 (1.00) | 2.78 (1.09) | 2.60 (0.97) | 2.67 (1.02) |
| 4-month | 2.87 (0.99) | 2.81 (0.96) | 2.61 (0.86) | 2.55 (0.98) |
| 12-month | 2.97 (1.02) | 2.88 (1.08) | 2.59 (0.95) | 2.74 (1.03) |
| Affective pain | ||||
| Baseline | 0.44 (0.46) | 0.43 (0.50) | 0.42 (0.41) | 0.41 (0.41) |
| 1-month | 0.37 (0.45) | 0.41 (0.48) | 0.36 (0.43) | 0.46 (0.53) |
| 4-month | 0.36 (0.43) | 0.38 (0.51) | 0.34 (0.41) | 0.46 (0.53) |
| 12-month | 0.36 (0.48) | 0.51 (0.56) | 0.34 (0.41) | 0.46 (0.51) |
| Sensory pain | ||||
| Baseline | 1.35 (0.75) | 1.34 (0.80) | 1.26 (0.71) | 1.26 (0.75) |
| 1-month | 1.22 (0.80) | 1.37 (0.84) | 1.20 (0.77) | 1.35 (0.84) |
| 4-month | 1.32 (0.85) | 1.29 (0.88) | 1.33 (0.72) | 1.26 (0.77) |
| 12-month | 1.24 (0.76) | 1.43 (0.88) | 1.18 (0.75) | 1.38 (0.77) |
| Physical disability | ||||
| Baseline | 1.93 (0.64) | 1.88 (0.69) | 1.97 (0.63) | 1.85 (0.70) |
| 1-month | 1.78 (0.52) | 1.73 (0.59) | 1.88 (0.66) | 1.84 (0.72) |
| 4-month | 1.83 (0.61) | 1.74 (0.53) | 1.87 (0.67) | 1.76 (0.76) |
| 12-month | 1.83 (0.59) | 1.75 (0.56) | 1.82 (0.65) | 1.79 (0.71) |
| Psychological symptoms | ||||
| Baseline | 2.18 (0.68) | 2.15 (0.67) | 2.20 (0.72) | 2.12 (0.66) |
| 1-month | 1.98 (0.64) | 2.12 (0.65) | 2.09 (0.74) | 2.19 (0.76) |
| 4-month | 2.01 (0.67) | 2.12 (0.70) | 2.10 (0.70) | 2.04 (0.69) |
| 12-month | 1.96 (0.60) | 2.10 (0.71) | 2.06 (0.70) | 2.15 (0.70) |
| Walking speed | ||||
| Baseline | 11.71 (3.28) | 12.36 (3.72) | 12.75 (4.00) | 12.76 (5.01) |
| 1-month | 11.84 (3.17) | 11.97 (4.06) | 12.46 (3.92) | 12.42 (4.91) |
| 4-month | 11.95 (3.07) | 11.79 (3.42) | 12.40 (3.76) | 12.22 (4.24) |
| 12-month | 12.05 (3.17) | 12.00 (3.57) | 12.58 (3.97) | 12.29 (4.36) |
| Inflammation (CRP) | ||||
| Baseline | 8.07 (10.6) | 8.72 (12.9) | 9.48 (13.5) | 9.38 (17.8) |
| 1-month | 5.65 (10.2) | 4.64 (9.66) | 5.43 (13.8) | 6.45 (11.8) |
| 4-month | 6.14 (12.8) | 4.22 (9.10) | 6.07 (16.1) | 6.35 (12.1) |
| 12-month | 6.56 (14.1) | 5.25 (10.3) | 4.60 (9.51) | 6.54 (12.2) |
Note: Any missing follow-up data was replaced by the last value carried forward; thus, sample sizes are identical across the four time points within each condition.
Participation and Attrition Analyses
For the 4-session writing protocol, fully 239 patients (90.5% of the sample, including 89.0% of WED and 92.2% of control writing) completed and returned all four writings, and another 11 patients (4.2%) completed three of the four. Only 14 patients (9 in WED and 5 in control writing) did less than two writings. Patients recorded the duration of writing at home for sessions 2 through 4, and the mean writing duration was just over 20 minutes for each session for both writing conditions.
For the 8-session course of training, fully 238 of the patients (90.2% overall; 86.9% CST and 93.3% control training) completed all 8 sessions, and another 15 patients (3.0%) completed from four to seven sessions; only 11 patients (5 in CST and 6 in control training) completed less than two sessions. Regarding in-person vs. telephone training sessions, following the initial mandatory in-person meeting for both training conditions, 63.3% of patients had at least one phone session (M = 2.32 phone sessions per patient, SD = 2.26), and the two training conditions did not differ on the percentage of patients who had phone sessions.
We obtained some follow-up data on 249 of the 264 patients (94.3%), losing only 3 to 5 patients from each of the four combination conditions. Fully 237 patients (89.8%) were assessed at the final, 12-month follow-up; the four combination conditions did not differ on attrition at 12 months (from 7.7% to 11.9%), nor did the two study sites (Detroit: 12.1% of 124 total randomized patients; North Carolina: 8.6% of 140 total patients). The 27 patients who dropped before 12 months did not differ from the 237 patients who completed the study on age, education, or RA duration (all p > .15); but those who dropped were more likely to be male (p =.04), non-White (p = .055), and had greater psychological symptoms (p = .04) than those who completed.
Preliminary Outcome Analyses: Interactions Between WED and CST
We first tested whether the combination of WED and CST was uniquely effective. None of the HLM writing × training × time interactions was significant (all p > .20 except CRP: p = .09). Similarly, the ANCOVAs revealed no writing × training interactions on any outcome at any point. Thus, the combined WED and CST did not differ significantly from the other condition combinations. Subsequent analyses, therefore, examined only the main effects of WED versus control writing, and of CST versus control training.
Main Effects of WED vs. Control Writing
Table 3 presents data for each outcome at each time point for WED and control writing. The HLM analyses indicated no significant writing × time effects, suggesting no overall difference between WED and control writing over the entire follow-up period. We note, however, the HLM model revealed a non-significant trend in the unexpected direction for AIMS2 pain over the follow-up period (b = −0.05, p = .10), which is examined below.
Table 3.
Baseline and Follow-up Data for Each Outcome Measure for Written Emotional Disclosure (WED) and Control Writing, along with Hierarchical Linear Modeling of Writing × Time Effects over all Follow-up Points, and ANCOVAs at each Follow-up Time Point
| Outcome Time point |
WED (n = 136) |
Control Writing (n = 128) |
HLM | ANCOVA | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | b | p | F | p | pη2 | |
| Disease Activity | −0.00 | .94 | |||||||
| Baseline | 0.05 | (0.89) | −0.05 | (0.93) | |||||
| 1-month | −0.06 | (0.77) | 0.06 | (1.00) | 4.78 | .03 | .018 | ||
| 4-month | 0.02 | (0.85) | −0.03 | (0.95) | 0.02 | .90 | .000 | ||
| 12-month | 0.00 | (0.82) | −0.00 | (0.92) | 0.38 | .54 | .001 | ||
| AIMS2 Pain | −0.05 | .10 | |||||||
| Baseline | 2.96 | (1.01) | 2.85 | (0.99) | |||||
| 1-month | 2.83 | (1.04) | 2.64 | (0.99) | 1.54 | .22 | .006 | ||
| 4-month | 2.84 | (0.97) | 2.58 | (0.92) | 5.27 | .023 | .020 | ||
| 12-month | 2.93 | (1.04) | 2.66 | (0.99) | 3.86 | .05 | .015 | ||
| Affective pain | −0.01 | .71 | |||||||
| Baseline | 0.44 | (0.48) | 0.41 | (0.41) | |||||
| 1-month | 0.39 | (0.46) | 0.41 | (0.48) | 0.75 | .39 | .003 | ||
| 4-month | 0.37 | (0.47) | 0.40 | (0.47) | 0.81 | .37 | .003 | ||
| 12-month | 0.44 | (0.53) | 0.40 | (0.46) | 0.11 | .74 | .000 | ||
| Sensory pain | 0.01 | .74 | |||||||
| Baseline | 1.34 | (0.77) | 1.26 | (0.73) | |||||
| 1-month | 1.29 | (0.82) | 1.28 | (0.81) | 0.26 | .61 | .001 | ||
| 4-month | 1.30 | (0.86) | 1.29 | (0.75) | 0.33 | .57 | .001 | ||
| 12-month | 1.34 | (0.83) | 1.28 | (0.77) | 0.01 | .92 | .000 | ||
| Physical disability | −0.00 | .78 | |||||||
| Baseline | 1.90 | (0.66) | 1.91 | (0.67) | |||||
| 1-month | 1.75 | (0.56) | 1.86 | (0.69) | 6.14 | .014 | .023 | ||
| 4-month | 1.79 | (0.57) | 1.82 | (0.71) | 0.31 | .58 | .001 | ||
| 12-month | 1.79 | (0.57) | 1.81 | (0.68) | 0.01 | .76 | .000 | ||
| Psych symptoms | 0.01 | .49 | |||||||
| Baseline | 2.16 | (0.68) | 2.16 | (0.69) | |||||
| 1-month | 2.05 | (0.64) | 2.14 | (0.75) | 2.95 | .09 | .011 | ||
| 4-month | 2.07 | (0.69) | 2.07 | (0.70) | 0.01 | .93 | .000 | ||
| 12-month | 2.03 | (0.66) | 2.10 | (0.70) | 1.86 | .17 | .007 | ||
| Walking speed | −0.09 | .35 | |||||||
| Baseline | 12.04 | (3.51) | 12.75 | (4.53) | |||||
| 1-month | 11.91 | (3.63) | 12.44 | (4.43) | 0.14 | .71 | .001 | ||
| 4-month | 11.87 | (3.24) | 12.31 | (4.00) | 0.19 | .66 | .001 | ||
| 12-month | 12.03 | (3.37) | 12.43 | (4.16) | 0.24 | .63 | .001 | ||
| Inflammation (CRP) | −0.05 | .51 | |||||||
| Baseline | 8.40 | (11.8) | 9.43 | (15.8) | |||||
| 1-month | 5.14 | (9.91) | 5.95 | (12.8) | 0.04 | .84 | .000 | ||
| 4-month | 5.17 | (11.1) | 6.21 | (14.1) | 0.19 | .66 | .001 | ||
| 12-month | 5.90 | (12.3) | 5.58 | (10.9) | 0.31 | .58 | .001 | ||
Note: Any missing follow-up data was replaced by the last value carried forward; thus, sample sizes are identical across the four time points within each condition.
As shown in Table 3, ANCOVAs at each follow-up point revealed several significant effects of WED compared to control writing. For the primary outcome of disease activity, there was a significant main effect of WED at 1-month, ES = 0.30. The WED group decreased somewhat in disease activity from baseline to 1-month, t(135) = 1.57, p = .12, d = 0.13; whereas control writing increased, t(127) = −1.77, p = .08, d = −0.16, although neither change was statistically significant. WED did not affect disease activity at 4- or 12-month follow-ups.
On secondary outcomes, there was a significant main effect of writing on physical disability at 1-month, ES = 0.28. The WED group improved significantly in functioning from baseline to 1-month, t(135) = 4.78, p < .001, d = 0.41, whereas control writing did not change, t(127) = 1.62, p = .11, d = 0.14. There were no benefits of WED on these measures at the 4- or 12-month follow-ups, nor did WED improve any other outcome (pain, psychological symptoms, walking speed, inflammatory activity) at any time point.
Regarding the above-noted non-significant HLM trend for WED to lead to greater AIMS2 pain over the follow-up period, ANCOVAs showed that WED led to significantly greater pain than control writing at both 4 months, ES = −0.21, and 12 months, ES = −0.18. Although AIMS2 pain decreased significantly after WED from baseline to 4 months, t(135) = 2.05, p = .04, d = 0.18; and did not change from baseline to 12 months, t(135) = 0.59, p = .55, d = 0.05; the control writing group had a larger decrease in pain at 4 months, t(127) = 42.9, p < .001, d = 0.38; and 12 months, t(127) = 2.37, p = .02, d = 0.21.
Main Effects of CST vs. Control Training
Table 4 presents data for each outcome at each time point for CST and control training. As shown in Table 4, HLM analyses revealed significant training × time effects for the primary outcomes of AIMS2 pain and McGill affective pain, as well as the secondary outcome of AIMS2 psychological symptoms. Thus, CST significantly improved pain and psychological symptoms over the follow-up 12 months, relative to control training. HLM modeling was not significant for the other outcomes.
Table 4.
Baseline and Follow-up Data for Each Outcome Measure for Coping Skills Training (CST) and Arthritis Education Control Training, along with Hierarchical Linear Modeling of Training × Time Effects over all Follow-up Points, and ANCOVAs at each Follow-up Time Point
| Outcome measure Time point |
CST (n = 130) |
Control Training (n = 134) |
HLM | ANCOVA | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | b | p | F | p | pη2 | |
| Disease activity | |||||||||
| Baseline | 0.05 | (0.85) | −0.05 | (0.97) | 0.02 | .48 | |||
| 1-month | 0.06 | (0.85) | −0.06 | (0.93) | 0.49 | .49 | .002 | ||
| 4-month | 0.01 | (0.83) | −0.01 | (0.96) | 0.02 | .90 | .000 | ||
| 12-month | 0.03 | (0.84) | −0.03 | (0.90) | 0.01 | .95 | .000 | ||
| AIMS2 Pain | 0.08 | .01 | |||||||
| Baseline | 3.01 | (0.98) | 2.81 | (1.02) | |||||
| 1-month | 2.75 | (0.99) | 2.73 | (1.05) | 3.02 | .08 | .011 | ||
| 4-month | 2.75 | (0.94) | 2.68 | (0.97) | 0.97 | .33 | .004 | ||
| 12-month | 2.78 | (1.00) | 2.81 | (1.05) | 3.23 | .07 | .012 | ||
| Affective pain | 0.04 | .01 | |||||||
| Baseline | 0.43 | (0.44) | 0.42 | (0.45) | |||||
| 1-month | 0.36 | (0.44) | 0.43 | (0.50) | 2.74 | .10 | .010 | ||
| 4-month | 0.35 | (0.42) | 0.41 | (0.52) | 1.93 | .17 | .007 | ||
| 12-month | 0.35 | (0.44) | 0.49 | (0.54) | 7.59 | .006 | .028 | ||
| Sensory pain | 0.05 | .09 | |||||||
| Baseline | 1.30 | (0.73) | 1.30 | (0.77) | |||||
| 1-month | 1.21 | (0.78) | 1.36 | (0.84) | 3.79 | .05 | .014 | ||
| 4-month | 1.32 | (0.79) | 1.27 | (0.82) | 0.38 | .54 | .001 | ||
| 12-month | 1.21 | (0.75) | 1.41 | (0.83) | 7.19 | .008 | .027 | ||
| Physical disability | 0.01 | .69 | |||||||
| Baseline | 1.95 | (0.63) | 1.87 | (0.69) | |||||
| 1-month | 1.83 | (0.59) | 1.78 | (0.66) | 0.34 | .56 | .001 | ||
| 4-month | 1.85 | (0.64) | 1.75 | (0.65) | 0.47 | .50 | .002 | ||
| 12-month | 1.83 | (0.62) | 1.77 | (0.63) | 0.03 | .87 | .000 | ||
| Psych symptoms | 0.05 | .01 | |||||||
| Baseline | 2.19 | (0.70) | 2.14 | (0.67) | |||||
| 1-month | 2.04 | (0.69) | 2.15 | (0.70) | 9.05 | .003 | .034 | ||
| 4-month | 2.06 | (0.69) | 2.08 | (0.69) | 1.93 | .17 | .007 | ||
| 12-month | 2.01 | (0.65) | 2.12 | (0.70) | 8.48 | .004 | .032 | ||
| Walking speed | 0.19 | .06 | |||||||
| Baseline | 12.21 | (3.67) | 12.55 | (4.38) | |||||
| 1-month | 12.14 | (3.55) | 12.19 | (4.48) | 1.47 | .23 | .006 | ||
| 4-month | 12.16 | (3.41) | 12.00 | (3.83) | 3.70 | .06 | .014 | ||
| 12-month | 12.31 | (3.57) | 12.14 | (3.96) | 2.56 | .11 | .010 | ||
| Inflammation (CRP) | −0.01 | .76 | |||||||
| Baseline | 8.75 | (12.1) | 9.04 | (15.4) | |||||
| 1-month | 5.54 | (12.0) | 5.51 | (10.7) | 0.06 | .80 | .000 | ||
| 4-month | 6.11 | (14.4) | 5.25 | (10.7 | 0.74 | .34 | .003 | ||
| 12-month | 5.62 | (12.1) | 5.87 | (11.2) | 0.00 | .95 | .000 | ||
Note: Any missing follow-up data was replaced by the last value carried forward; thus, sample sizes are identical across the four time points within each condition.
As shown in Table 4, ANCOVAs compared CST and control training on pain measures at each follow-up point. Although the HLM model on AIMS2 pain was significant (as noted above), the effect of CST on AIMS2 pain did not reach statistical significance at any of the three separate time points. There were several effects of CST on the two McGill pain scales. Compared with control training, CST led to significantly less sensory pain at 1-month follow-up (ES = 0.23); there was some decrease in sensory pain from baseline to 1-month following CST, t(129) = 1.69, p = .09, d = 0.15; but no change following control training, t(133) = −0.94, p = .35, d = −0.08. At 12 months, CST significantly decreased both sensory pain, ES = 0.31, and affective pain, ES = 0.32. From baseline to 12 months, CST led to some reduction in sensory pain, t(129) = 1.71, p = .09, d = 0.15; and affective pain, t(129) = 2.06, p = .04, d = 0.18; whereas control training was followed by some increases in sensory pain, t(133) = −1.87, p = .06, d = −0.16; and affective pain, t(133) = −1.65, p = .10, d = −0.14.
On secondary outcomes, CST affected only psychological symptoms at 1 month, ES = 0.37, and 12 months, ES = 0.36. Psychological symptoms significantly reduced after CST from baseline to 1 month, t(129) = 3.60, p < .001, d = 0.32; and 12 months, t(129) = 4.35, p < .001, d = 0.38; but did not change after control training, t(133) = −0.49, p = .63, d = −0.04; and t(133) = 0.30, p = .77, d = 0.03. There were no benefits of CST on these measures at the 4-month follow-up, nor did CST influence any other outcome at any time point.
Ancillary Analyses of Protocol Adherent Patients
ANCOVA analyses on the subsample of patients who completed all four writings and all eight training sessions (n = 223; 84.4% of the full sample) were similar to, or more robust than, those found on the full sample. Again, there were no writing × training interactions. Compared with control writing, WED led to significantly less disease activity (p = .03) and physical disability (p = .05) at 1 month, but to more AIMS2 pain at 4 months (p = .01) and 12 months (p = .04). Compared with control training, CST led to significantly less psychological symptoms at 1 month (p = .006), 4 months (p = .04), and 12 months (p = .003); less sensory pain at 1 month (p = .05) and 12 months (p = .005); and less affective pain at 1 month (p = .04), 4 months (p = .05), and 12 months (p < .001).
Discussion
This randomized clinical trial tested the independent and combined effects of two distinct interventions for patients with rheumatoid arthritis: written emotional disclosure and cognitive behavioral coping skills training. Although we had hypothesized that the combination of WED and CST would yield the greatest improvement in health status, this did not occur. Instead, each intervention had independent and time-specific effects. First, compared with control writing, WED led to early, albeit temporary, reductions in disease activity and self-reported physical disability, but there also was some evidence of increased pain after WED after 4 and 12 months. Second, compared with control training, CST led to less pain and psychological symptoms, which lasted for the full year post-treatment. These effects were found for the full randomized sample as well as for those patients who completed all of the sessions for both interventions.
The benefits of CST are the clearest. The 8-session course of individually administered CST improved several subjective outcomes—pain (on two different measures) and psychological symptoms—although CST did not improve disease activity, inflammation, disability, or walking time. The benefits of CST for pain and psychological symptoms were long lasting, out to the 12-month post-treatment assessment point. The between-group effect sizes of this intervention on these outcomes ranged from 0.23 to 0.37 SD, which is small to medium in size and generally consistent with other reports in the literature (Astin et al., 2002; Dixon et al., 2007). Yet, it should be remembered that these somewhat small effects of CST are in comparison to an active control training experience, arthritis education, which was comparable to CST in format, controlled for various non-specific factors (individualized attention and teaching from a dedicated educator, the effects of repeated testing and passage of time), and may have had some benefits of its own. It is likely that CST would have even stronger effects when compared to only medical treatment as usual, which is the usual decision facing clinicians who are considering implementing or recommending CST.
The outcomes for WED were less clear and consistent, however. Compared to control writing, WED led to improvements in the primary outcome, rheumatologist-rated disease activity, and a secondary outcome, patient-reported physical disability. These effects were positive but small in magnitude (0.28 to 0.30 SD), which is consistent with reviews of the WED literature (Frattaroli, 2006; Frisina et al., 2004). Yet, the effects were found only with analyses that examined follow-up time points separately, but not with omnibus testing of WED’s general effects over the year. This was likely due to the short-lived nature of the benefits, occurring only at the 1-month follow-up point (which actually was about 3 months after writing ended, given the subsequent 8-week training phase before the first follow-up). In addition, only measures of disease activity and physical functioning improved after WED, but not the subjective measures of pain or psychological symptoms. Other research has shown that WED has somewhat larger effects on physiological than subjective outcomes (Frisina et al., 2004; Smyth, 1998), perhaps due to direct emotion regulation influences on physiology and the tendency for WED to create upsetting subjective experiences. Finally, and importantly, WED led to worse pain on the AIMS2 than control writing at 4-month and 12-month follow-ups. This unexpected negative finding, however, was not supported by data from the McGill Pain Questionnaire or other outcome measures, and was due to reduced pain after control writing rather than increased pain after WED. Although this delayed negative effect of WED on pain gives us pause, its reliability needs to be evaluated. We also note that our WED instructions differed somewhat from many prior studies. We enhanced them by providing more guidance throughout the writing process, but it is possible that this added complexity or demand somehow interfered with effective emotional processing. Also, our control writing condition focused on various health behaviors, which appears face valid for improving health and might have had some unexpected efficacy, thereby reducing the relative efficacy of WED.
Although we hypothesized that the combination of WED followed by CST would lead to the best health outcomes and largest effects, this did not occur. Rather, the two interventions influenced different outcomes at different time points, which limited the possibility of finding an interactive benefit to the unique combination of these two interventions. It also is possible that the two interventions were not well combined. Perhaps it would have been more effective to have WED follow CST, or have a WED session occur with each CST session, so that the patients could better integrate WED and CST. Or perhaps CST could be modified so that it would map specific coping skills to the specific needs of the patients, as reflected in their disclosure writings. We encourage further research on ways to integrate emotional processing and skills training approaches for people who have chronic pain.
There are a number of strengths of this study. We used a factorial design that allowed us to test main effects of WED and CST as well as their combination, and we used comparably active control conditions to control for many non-specific effects of each intervention. We had a socio economically and ethnically diverse sample recruited from communities and clinics in different parts of the country, and a much larger sample than prior studies of either WED or CBT for people with RA, resulting in quite reliable estimates of effect sizes and adequate statistical power to detect even small effects. We had high participation in the interventions—over 90% of patients completed every session of each intervention, and 84.4% of patients fully completed both interventions—and low attrition over a 12-month follow-up; only 5.7% of patients provided no follow-up data, and only 10.2% were lost at the 12-month follow-up. We assessed a range of subjective, physiological, and functional outcomes and report on all of them, even those that did not show effects of the interventions, so that readers have full disclosure about the effects of each intervention.
Some of our methodological decisions increased the feasibility and ecological validity of the study, but at the cost of internal validity or experimental control. For example, WED was conducted primarily at home because having patients come to the clinic or lab daily to write for 20 minutes would be excessively burdensome, result in highly selected patients or greater attrition, and not reflect how WED is conducted in real life. Regarding the training conditions, having psychologists conduct CST and health educators conduct arthritis education increased the ecological validity of the study and the presumed competence and fidelity of the delivery of the conditions, but having two sets of providers with different professional backgrounds increased the possibility of therapist effects contributing to differences between CST and arthritis education. Our design was also somewhat unbalanced because we had doctoral psychologists provide the training and supervision for both training conditions, rather than a doctoral health educator for arthritis education. Although both CST and arthritis education were highly standardized and scripted, and the supervisors and providers monitored adherence to the protocol, we did not have an independent, blinded assessment of provider competence and fidelity to the protocols, nor did we assess patient adherence to homework. In addition, our version of CST was conducted individually for eight, 75-minute sessions, and for some patients, some sessions were done over the telephone. Furthermore, other studies of cognitive behavioral interventions for RA have been conducted in groups, with a different number and timing of sessions, and without the possibility of telephone or make-up sessions. Thus, our approach was somewhat less standardized than some others that have been conducted, and direct comparisons with those studies are hindered. However, our high intervention completion and study retention rates suggest the advantages of some of the design and procedural choices that we made.
Finally, we note that, although our analyses were hypothesis-driven, we tested a number of effects and conducted analyses that were both relatively conservative (HLM over all three follow-up time-points) and relatively liberal (ANCOVA at each follow-up time point), which raises concerns about chance findings. We also have not yet examined the content of the WED and control writings or systematically reviewed the process of the CST or education sessions, which would permit us to better understand the mechanisms underlying change.
In summary, our findings regarding CST indicate that patients with RA—and likely other chronic pain disorders—benefit in both the short and long-term from learning skills such as relaxation, cognitive reappraisal, assertive communication, distraction, increasing pleasant activities, and problem solving. We recommend this intervention be offered clinically, but because its effects are modest and patients typically remain symptomatic, it should be considered an adjunct to medical or pharmacological therapies. Regarding WED, however, this study generally supports previous findings (Lumley, Sklar, & Carty, 2012) indicating that WED is quite weak for people with RA, yielding small and temporary benefits on scattered outcomes, and usually no improvement in pain. The limited magnitude, scope, and duration of WED’s benefits for RA, and the fact that it usually generates a temporary negative mood (Lumley et al., 2011), are drawbacks that may not be balanced by the fact that WED can be disseminated widely and implemented at little or no cost professional cost. Thus, the potential use of WED in clinical practice needs to be thoughtfully evaluated.
There remain unanswered questions that call for further study. First, there likely are substantial differences among individuals or disorders in response to both of these interventions. In particular, we hypothesize that WED is more effective for those conditions marked by central nervous system sensitization or augmentation and elevated life stress and emotion regulation difficulties, such as fibromyalgia, irritable bowel syndrome, and chronic pelvic pain, than for conditions with substantial peripheral disease processes and less unresolved life stress, such as RA (Broderick, Junghaenel, & Schwartz, 2005; Walker et al., 1997). Second, the mechanisms by which these interventions operate remain unclear. Although the control conditions we used indicate that the mechanisms are more than non-specific effects, process research is needed for both of these interventions. We anticipate that future analyses of the data from this trial will illuminate moderators and mediators. Future research is needed to develop and test enhancements of both interventions, such as increasing patient motivation to disclose and process unresolved stress in WED, or to engage in skills training for CST, extending the number of writing or training sessions, and providing more guidance or feedback for people engaging in WED. We hope, however, that the continued development and integration of therapies that target the full range of processes underlying pain and functioning—behavioral, cognitive, emotional, and interpersonal—will result in more patients experiencing greater benefits.
Acknowledgments
This research was funded by award AR049059 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, and also supported by awards, AR057808 and AR057047. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Mark A. Lumley, Department of Psychology, Wayne State University
Francis J. Keefe, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center
Angelia Mosley-Williams, Department of Medicine, Wayne State University.
Daphne McKee, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center.
Sandra J. Waters, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center
R. Ty Partridge, Department of Psychology, Wayne State University.
Jennifer N. Carty, Department of Psychology, Wayne State University
Ainoa M. Coltri, Department of Psychology, Wayne State University
Anita Kalaj, Department of Psychology, Wayne State University.
Jay L. Cohen, Department of Psychology, Wayne State University
Lynn C. Neely, Department of Psychology, Wayne State University
Jennifer K. Pahssen, Department of Social Work, Wayne State University
Mark A. Connelly, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center
Yelena B. Bouaziz, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center
Paul A. Riordan, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center
References
- Affleck G, Urrows S, Tennen H, Higgins P. Daily coping with pain from rheumatoid arthritis: patterns and correlates. Pain. 1992;51:221–229. doi: 10.1016/0304-3959(92)90263-B. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Felson DT, Meenan RF, Williams HJ. Which traditional measures should be used in rheumatoid arthritis clinical trials? Arthritis and Rheumatism. 1989;32:1093–1099. doi: 10.1002/anr.1780320907. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Firschein HE, Meenan RF. Sensitivity of a health status measure to short term clinical changes in arthritis. Arthritis and Rheumatism. 1989;32:844–850. [PubMed] [Google Scholar]
- Applebaum KA, Blanchard EB, Hickling EJ, Alfonson M. Cognitive-behavioral treatment of a veteran population with moderate to severe rheumatoid arthritis. Behavior Therapy. 1988;19:489–502. [Google Scholar]
- Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: A meta-analysis of randomized controlled trials. Arthritis Care & Research. 2002;47:291–302. doi: 10.1002/art.10416. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Ahern DK, Orav EJ, Nestoriuc Y, Liang MH, Berman IT, Wilk KG. A randomized trial of three psychosocial treatments for the symptoms of rheumatoid arthritis. Seminars in Arthritis and Rheumatism. 2010;40:222–232. doi: 10.1016/j.semarthrit.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LA, Young LD, Anderson JO, Turner RA, Agudelo CA, McDaniel LK, Morgan TM. Effects of psychological therapy on pain behavior of rheumatoid arthritis patients: treatment outcome and six-month follow-up. Arthritis and Rheumatism. 1987;30:1105–1114. doi: 10.1002/art.1780301004. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Junghaenel DU, Schwartz JE. Written emotional expression produces health benefits in fibromyalgia patients. Psychosomatic Medicine. 2005;67:326–334. doi: 10.1097/01.psy.0000156933.04566.bd. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Stone AA, Smyth JM, Kaell AT. The feasibility and effectiveness of an expressive writing intervention for rheumatoid arthritis via home-based videotaped instructions. Annals of Behavioral Medicine. 2004;27:50–59. doi: 10.1207/s15324796abm2701_7. [DOI] [PubMed] [Google Scholar]
- Cameron LD, Nicholls G. Expression of stressful experiences through writing: effects of a self-regulation manipulation for pessimists and optimists. Health Psychology. 1998;17:84–92. doi: 10.1037//0278-6133.17.1.84. [DOI] [PubMed] [Google Scholar]
- Carson JW, Keefe FJ, Affleck G, Rumble ME, Caldwell DS, Beaupre PM, Weisberg JN. A comparison of conventional pain coping skills training and pain coping skills training with a maintenance training component: A daily diary analysis of short- and long-term treatment effects. The Journal of Pain. 2006;7:615–625. doi: 10.1016/j.jpain.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc., Publishers; 1988. [Google Scholar]
- Cox CE, Porter LS, Hough CL, White DB, Kahn JM, Carson SS, Keefe FJ. Development and preliminary evaluation of a telephone-based coping skills training intervention for survivors of acute lung injury and their informal caregivers. Intensive Care Medicine. 2012;38:1289–1297. doi: 10.1007/s00134-012-2567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danoff–Burg S, Agee JD, Romanoff NR, Kremer JM, Strosberg JM. Benefit finding and expressive writing in adults with lupus or rheumatoid arthritis. Psychology Health. 2006;21:651–665. [Google Scholar]
- Dissanayake RK, Bertouch JV. Psychosocial interventions as adjunct therapy for patients with rheumatoid arthritis: a systematic review. International Journal of Rheumatic Diseases. 2010;13:324–334. doi: 10.1111/j.1756-185X.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- Dixon KE, Keefe FJ, Scipio CD, Perri LM, Abernethy AP. Psychological interventions for arthritis pain management in adults: a meta-analysis. Health Psychology. 2007;26:241–250. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]
- Elkin I. A major dilemma in psychotherapy outcome research: Disentangling therapists form therapies. Clinical Psychology: Science and Practice. 1999;6:10–32. [Google Scholar]
- Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain. 2002;100:141–153. doi: 10.1016/s0304-3959(02)00274-9. [DOI] [PubMed] [Google Scholar]
- Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, Wolfe F. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis and Rheumatism. 1993;36:729–740. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- Frattaroli J. Experimental disclosure and its moderators: a meta-analysis. Psychological Bulletin. 2006;132:823–865. doi: 10.1037/0033-2909.132.6.823. [DOI] [PubMed] [Google Scholar]
- Freeman K, Hammond A, Lincoln NB. Use of cognitive-behavioural arthritis education programmes in newly diagnosed rheumatoid arthritis. Clinical Rrehabilitation. 2002;16:828–836. doi: 10.1191/0269215502cr565oa. [DOI] [PubMed] [Google Scholar]
- Frisina PG, Borod JC, Lepore SJ. A meta-analysis of the effect of written disclosure on the health outcomes of clinical populations. Journal of Nervous and Mental Disease. 2004;192:629–634. doi: 10.1097/01.nmd.0000138317.30764.63. [DOI] [PubMed] [Google Scholar]
- Germond S, Schomer H, Meyers O, Weight L. Pain management in rheumatoid arthritis: a cognitive-behavioural intervention. South African Journal of Psychology. 1993;23:1–9. [Google Scholar]
- Gidron Y, Peri T, Connolly JF, Shalev AY. Written disclosure in posttraumatic stress disorder: Is it beneficial for the patient? Journal of Nervous and Mental Disease. 1996;184:505–507. doi: 10.1097/00005053-199608000-00009. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Harris AH. Does expressive writing reduce health care utilization? A meta-analysis of randomized trials. Journal of Consulting and Clinical Psychology. 2006;74:243–252. doi: 10.1037/0022-006X.74.2.243. [DOI] [PubMed] [Google Scholar]
- Harris ML, Loxton D, Sibbritt DW, Byles JE. The relative importance of psychosocial factors in arthritis: findings from 10,509 Australian women. Journal of Psychosomatic Research. 2012;73:251–256. doi: 10.1016/j.jpsychores.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Stone JH. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis and Rheumatism. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA. Why most published research findings are false. PLoS Medicine. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM, Karoly P. Coping with chronic pain: a critical review of the literature. Pain. 1991;47:249–283. doi: 10.1016/0304-3959(91)90216-K. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37:51–56. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Caldwell DS, Williams DA, Gil KM, Mitchell D, Robertson C, Helms M. Pain coping skills training in the management of osteoarthritic knee pain: A comparative study. Behavior Therapy. 1990;21(1):49–62. http://dx.doi.org/10.1016/S0005-7894(05)80188-1. [Google Scholar]
- Keefe FJ, Anderson T, Lumley M, Caldwell D, Stainbrook D, McKee D, Uhlin BD. A randomized controlled trial of emotional disclosure in rheumatoid arthritis: Can clinician assistance enhance the effects? Pain. 2008;137:164–172. doi: 10.1016/j.pain.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JE, Lumley MA, Leisen JCC. Health effects of emotional disclosure in rheumatoid arthritis patients. Health Psychology. 1997;16:1–10. doi: 10.1037//0278-6133.16.4.331. [DOI] [PubMed] [Google Scholar]
- Kennedy-Moore E, Watson JC. Expressing emotion: Myths, realities, and therapeutic strategies. New York: Guilford; 1999. [Google Scholar]
- Knittle K, Maes S, de Gucht V. Psychological interventions for rheumatoid arthritis: Examining the role of self-regulation with a systematic review and meta-analysis of randomized controlled trials. Arthritis Care & Research. 2010;62:1460–1472. doi: 10.1002/acr.20251. [DOI] [PubMed] [Google Scholar]
- Kraaimaat FW, Brons MR, Geenen R, Bijlsma JWJ. The effect of cognitive behavior therapy in patients with rheumatoid arthritis. Behaviour Research and Therapy. 1995;33:487–495. doi: 10.1016/0005-7967(94)00094-z. [DOI] [PubMed] [Google Scholar]
- Leibing E, Pfingsten M, Bartmann U, Rueger U, Schuessler G. Cognitive-behavioral treatment in unselected rheumatoid arthritis outpatients. The Clinical Journal of Pain. 1999;15:58–66. doi: 10.1097/00002508-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Lumley MA, Leisen JCC, Partridge RT, Meyer TM, Radcliffe AM, Macklem DJ, Granda JL. Does emotional disclosure about stress improve health in rheumatoid arthritis? Randomized, controlled trials of written and spoken disclosure. Pain. 2011;152:866–877. doi: 10.1016/j.pain.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley MA, Sklar ER, Carty JN. Emotional disclosure interventions for chronic pain: from the laboratory to the clinic. Translational Behavioral Medicine. 2012;2:73–81. doi: 10.1007/s13142-011-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley MA, Tojek TM, Macklem DJ. The effects of written emotional disclosure among repressive and alexithymic people. In: Lepore SJ, Smyth JM, editors. The writing cure: How expressive writing promotes health and emotional well-being. Washington, DC: American Psychological Association; 2002. pp. 75–95. [Google Scholar]
- Meenan RF, Jason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2: The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis and Rheumatism. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- Meenan RF, Yelin EH, Nevitt M, Epstein WV. The impact of chronic disease: a sociomedical profile of rheumatoid arthritis. Arthritis and Rheumatism. 1988;25:544–548. doi: 10.1002/art.1780240315. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;30:191–197. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Melzack R, Katz J. The McGill Pain Questionnaire: Appraisal and current status. In: Turk DC, Melzack R, editors. The handbook of pain assessment. New York: Guilford Press; 1992. pp. 152–168. [Google Scholar]
- Muller I, Yardley L. Telephone-delivered cognitive behavioural therapy: a systematic review and meta-analysis. Journal of Telemedicine and Telecare. 2011;17:177–184. doi: 10.1258/jtt.2010.100709. [DOI] [PubMed] [Google Scholar]
- Muller R, Kallikorm R, Polluste K, Lember M. Compliance with treatment of rheumatoid arthritis. Rheumatology International. 2012;32:3131–3135. doi: 10.1007/s00296-011-2162-x. [DOI] [PubMed] [Google Scholar]
- Norcross JC, Krebs PM, Prochaska JO. Stages of change. Journal of Clinical Psychology: In Session. 2011;67:143–154. doi: 10.1002/jclp.20758. [DOI] [PubMed] [Google Scholar]
- O’Leary A, Shoor S, Lorig K, Holman HR. A cognitive-behavioral treatment for rheumatoid arthritis. Health Psychology. 1988;7:527–544. doi: 10.1037//0278-6133.7.6.527. [DOI] [PubMed] [Google Scholar]
- Parker JC, Frank R, Beck N, Smarr K, Buescher K, Phillips L, Walker SE. Pain management in rheumatoid arthritis: A cognitive-behavioral approach. Arthritis and Rheumatism. 1988;31:593–601. doi: 10.1002/art.1780310503. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Beall SK. Confronting a traumatic event: Toward an understanding of inhibition and disease. Journal of Abnormal Psychology. 1986;95:274–281. doi: 10.1037//0021-843x.95.3.274. [DOI] [PubMed] [Google Scholar]
- Potter PT, Zautra AJ. Stressful life events’ effects on rheumatoid arthritis disease activity. Journal of Cognitive Clinical Psychology. 1997;65:319–323. doi: 10.1037//0022-006x.65.2.319. [DOI] [PubMed] [Google Scholar]
- Radojevic V, Nicassio PM, Weisman MH. Behavioral intervention with and without family support for rheumatoid arthritis. Behavior Therapy. 1992;23:13–30. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [computer software] Skokie, IL: Scientific Software International, Inc.; 2011. [Google Scholar]
- Shao J, Zhong B. Last observation carry-forward and last observation analysis. Statistics in Medicine. 2003;22:2429–2441. doi: 10.1002/sim.1519. [DOI] [PubMed] [Google Scholar]
- Sharpe L, Sensky T, Timberlake N, Ryan B, Brewin C, Allard S. A blind, randomized, controlled trial of cognitive-behavioural intervention for patients with recent onset rheumatoid arthritis: preventing psychological and physical morbidity. Pain. 2001;89:275–283. doi: 10.1016/s0304-3959(00)00379-1. [DOI] [PubMed] [Google Scholar]
- Sharpe L, Sensky T, Timberlake N, Ryan B, Allard S. Long-term efficacy of a cognitive behavioural treatment from a randomized controlled trial for patients recently diagnosed with rheumatoid arthritis. Rheumatology. 2003;42:435–441. doi: 10.1093/rheumatology/keg144. [DOI] [PubMed] [Google Scholar]
- Shearn MA, Fireman BH. Stress management and mutual support groups in rheumatoid arthritis. The American Journal of Medicine. 1985;78:771–775. doi: 10.1016/0002-9343(85)90282-7. [DOI] [PubMed] [Google Scholar]
- Smyth JM. Written emotional expression: Effect sizes, outcome types, and moderating variables. Journal of Consulting and Clinical Psychology. 1998;66:174–184. doi: 10.1037//0022-006x.66.1.174. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Stone AA, Hurewitz A, Kaell A. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis. Journal of the American Medical Association. 1999;281:1304–1309. doi: 10.1001/jama.281.14.1304. [DOI] [PubMed] [Google Scholar]
- Stone AA, Smyth JM, Kaell A, Hurewitz A. Structured writing about stressful events: exploring potential psychological mediators of positive health effects. Health Psychology. 2000;19:619–624. [PubMed] [Google Scholar]
- Taal E, Riemsma RP, Brus HLM, Seydel ER, Rasker JJ, Wiegman O. Group education for patients with rheumatoid arthritis. Patient Education and Counseling. 1993;20:177–187. doi: 10.1016/0738-3991(93)90131-f. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA. Disorders of affect regulation: alexithymia in medical and psychiatric illness. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- Thomason BT, Brantley PJ, Jones GN, Dyer HR, Morris JL. The relation between stress and disease activity in rheumatoid arthritis. Journal of Behavioral Medicine. 1992;15:215–220. doi: 10.1007/BF00848326. [DOI] [PubMed] [Google Scholar]
- van Middendorp H, Geenen R, Sorbi M, van Doornen LJP, Bijlsma JWJ. Health and physiological effects of an emotional disclosure intervention adapted for application at home: A randomized clinical trial in rheumatoid arthritis. Psychotherapy and Psychosomatics. 2009;78:145–151. doi: 10.1159/000206868. [DOI] [PubMed] [Google Scholar]
- Walker EA, Keegan D, Gardner G, Sullivan M, Bernstein D, Katon WJ. Psychosocial factors in fibromyalgia compared with rheumatoid arthritis: II. Sexual, physical, and emotional abuse and neglect. Psychosomatic Medicine. 1997;59:572–577. doi: 10.1097/00006842-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Wetherell MA, Byrne-Davis L, Dieppe P, Donovan J, Brookes S, Byron M, Miles J. Effects of emotional disclosure on psychological and physiological outcomes in patients with rheumatoid arthritis: An exploratory home-based study. Journal of Health Psychology. 2005;10:277–285. doi: 10.1177/1359105305049778. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Allaire S, Michaud K. The prevalence and incidence of work disability in rheumatoid arthritis, and the effect of anti-tumor necrosis factor on work disability. The Journal of Rheumatology. 2007;34:2211–2217. [PubMed] [Google Scholar]
- Zautra AJ, Burleson MH, Matt KS, Roth S, Burrows L. Interpersonal stress, depression, and disease activity in rheumatoid arthritis and osteoarthritis patients. Health Psychology. 1994;13:139–148. doi: 10.1037//0278-6133.13.2.139. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Davis MC, Reich JW, Niccassio P, Tennen H, Finnan P, Irwin MR. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76:408–421. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Okun MA, Robinson SE, Lee D, Roth SH, Emmanual J. Life stress and lymphocyte alterations among patients with rheumatoid arthritis. Health Psychology. 1989;8:1–14. doi: 10.1037//0278-6133.8.1.1. [DOI] [PubMed] [Google Scholar]