Abstract
The Neurospora crassa cps-1 gene encodes a polysaccharide synthase with homology to the Cryptococcus neoformans hyaluronic acid synthase Cps1p . Homologs of the cps-1 gene are found in the genomes of many fungi. Loss of CPS-1 results in a cell wall defect that affects all stages of the N. crassa life cycle, including vegetative growth, protoperithecia (female mating structure) development, and conidia (asexual spore) development. The cell wall of cps-1 deletion mutants is sensitive to cell wall perturbation reagents. Our results demonstrate that CPS-1 is required for the incorporation of cell wall proteins into the cell wall and plays a critical role in cell wall biogenesis. We found that the N. crassa cell wall is devoid of hyaluronic acid, and conclude that the polysaccharide produced by the CPS-1 is not hyaluronic acid.
Keywords: fungal cell wall, cell wall polysaccharide, Neurospora crassa, cell wall biogenesis, polysaccharide synthase, cell wall glucan
1. Introduction
The cell wall is an important organelle for bacteria, archaea, fungi, and plant cells. Cell walls protect cells from environment stress and play a critical role in the interactions of cells with the environment and with other cells. Fungal cell walls consist of a cross-linked matrix of glucans, chitin, and cell wall proteins (Free, 2013; Gastebois et al., 2009; Klis et al., 2006; Klis et al., 2001; Latge, 2007). Research has shown that cell wall polysaccharides are needed for the formation and functionality of the fungal cell wall. Mutants affected in chitin synthases, glucan synthases, and in the post-translational modification of cell wall proteins have shown the importance of these elements for cell wall biogenesis. Fungi contain multiple chitin synthase genes, and the mutational loss of chitin synthase genes can affect the ability of the fungus to generate a normal cell wall (Free, 2013; Klis et al., 2006; Latge, 2007). Fungal cell walls have been shown to contain a variety of glucans. The most abundant glucans are β-1,3-glucan, β-1,6-glucan, and α-1,3-glucan. Most fungi have a single β-1,3-glucan synthase, and the β-1,3-glucan synthase gene is needed for cell wall synthesis. Cell wall β-1,6-glucans are found in yeast cell walls, but are absent from filamentous fungi. In the yeasts, β-1,6-glucans are used to cross-link cell wall elements together in generating a cell wall matrix (Kapteyn et al., 1996; Kollar et al., 1997; Lu et al., 1995). Cell wall α-1,3-glucans are found in many cell types and their role varies from being a critical component in Schizzosacchromyces pombe and Cryptococcus neoformans to being a cell-type specific cell wall component in N. crassa (Free, 2013; Grun et al., 2005; Hochstenbach et al., 1998; Reese et al., 2007). The glucans and chitins are cross-linked together into a cell wall matrix by cell wall proteins having glucanase and chitinase activity. Presumably, these enzymes cleave the polymers (forward reaction) and then attach the cleaved polymers to other cell wall polymers (reverse reaction) to generate a cross-linked matrix (Cabib et al., 2007; Goldman et al., 1995; Mouyna et al., 2000). The cell wall proteins have associated O-linked and N-linked mannans or galactomannans. These polysaccharides are post-translationally added to the proteins as the proteins pass through the secretory pathway. The post-translationally added N-linked galactomannan has been shown to be needed for the incorporation of cell wall proteins into the N. crassa cell wall (Maddi and Free, 2010). The dcw-1 and dfg-5 mannanases have been shown to function in cross-linking the cell wall protein galactomannan into the glucan/chitin matrix (Maddi et al., 2012).
Cryptococcus neoformans, the causative agent of fungal meningitis, has an outer capsid that extends beyond the canonical fungal cell wall, and functions as an important virulence factor. The major constituents of the outer capsid are glucuronoxylomannan, galactomylomannan, and mannoproteins. Some of the enzymes needed for the synthesis of the capsid have been identified (Doering, 2009). The CPS1 gene encodes a hyaluronic acid synthase. Mutational and biochemical analyses of the C. neoformans CPS1 mutant showed that Cps1p was needed for the formation of the outer capsid and that purified Cps1p was capable of synthesizing hyaluronic acid (a repeating polymer of β-1,4-glucuronic acid-β-1,3-N-acetylglucosamine) using UDP-glucuronic acid and UDP-N-acetylglucosamine as substrates (Jong et al., 2007). CPS-1 is also related to the Streptococcus pneumoniae type 3 polysaccharide synthase, which directs the synthesis of the type 3 polysaccharide (Chang et al., 2006). The type 3 polysaccharide is a β-1,3-glucuronic acid-β-1,4-glucose repeating polymer (Arrecubieta et al., 1996; Cartee et al., 2005).
In screening the N. crassa single gene deletion library for mutants that were defective in the protoperithecia (female mating structure) production, we identified the Δcps-1 mutants as being unable to produce protoperithecia. Our analysis of the mutants showed that the cps-1 gene (NCU00911) plays an important role in cell wall biogenesis. In particular, we found that the incorporation of cell wall glycoprotein into the cell wall was defective in the Δcps-1. The mutant was affected in vegetative growth, and was unable to grow in the presence of cell wall perturbation reagents. In addition to being affected in vegetative growth and sexual development, the mutant was also affected in asexual development (production of conidia). We were unable to detect any hyaluronic acid in the N. crassa cell wall, and an analysis of the cell wall carbohydrates failed to detect any uronic acids. The composition of the polysaccharide produced by the CPS-1polysaccharide synthase remains to be determined.
2. Materials and Methods
2.1. Strains and Growth conditions
The Δcps-1 mutant isolates (FGSC#16861 and #16862) were taken from the single gene deletion library, which was obtained from Fungal Genetics Stock Center (Kansas City, MO). The wild type parental N. crassa strains ORS-SL6 a (FGSC#4200) and 74-OR-23-IV A (FGSC# 2489), the his-3 A mutant (FGSC#6103), and the pBM60 plasmid used in the cloning experiments were also supplied by the Fungal Genetics Stock Center. Cultures were routinely grown on either Vogel's medium with 2% sucrose or on synthetic crossing medium with 0.5% sucrose (Davis and DeSerres, 1970). When testing for growth in the presence of cell wall perturbation reagents, the cells were inoculated onto Vogel's agar medium that was supplemented with caspofungin (10 ug/ml), calcofluor white (10 mg/ml), SDS (0.01%), glycerol (2M) or NaCl (10%) as previously described (Maddi et al., 2012). The media were inoculated with conidia from Δcps-1 or wild type cultures and incubated at 30°C for 4 days. Transformation of N. crassa was carried out as described by Margolin et al. (Margolin et al., 1997).
N. crassa mating experiments were done as described by Davis and DeSerres (Davis and DeSerres, 1970). To generate a Δcps-1, his-3 strain for the N. crassa transformation experiments, the Δcps-1 a mutant (FGSC#16862) was mated with a his-3 A isolate (FGSC#6103) and individual ascospore progeny were isolated and tested for histidine auxotrophy, the Δcps-1 mutant phenotype, and for hygromycin resistance. The cps-1 deletion was created by replacing the cps-1 coding region with a hygromycin-resistance cassette (Colot et al., 2006). A Δcps-1 (hygromycin resistant and mutant phenotype), his-3 (histidine auxotroph) progeny isolate was used for the transformation experiments.
To measure vegetative hyphal growth rates, a drop of conidia was placed near the edge of a Petri dish containing Vogel's sucrose medium and the Petri dish was incubated at 30° C. The distance traveled by the growing edge of the colony was then measured as a function of time.
2.2 Cloning, RIP mutagenesis, complementation, and Western blot experiments
To clone the cps-1 gene, the genome sequences from 1538 bp upstream to 678 bp downstream of the cps-1 coding region were PCR amplified using the cps-1 5’ end forward and 3’ end reverse primers (Table 1). The PCR product was digested with SpeI and NotI and cloned into a SpeI and NotI digested pBM60 vector to create pCPS-1. The pCPS-1 plasmid contains sequences from the his-3 gene and the intergenic region 3’ of the his-3 gene. Upon transformation, homologous recombination between the vector and genome generated a his3+ allele and inserted the cps-1 gene into the intergenic region 3’ of his-3. Individual transformants were picked from unsupplemented sorbose agar plates as his3+ isolates and further characterized.
Table 1.
The sequences used in cloning the cps-1 gene and for cloning the upstream regulatory region are provided. The SpeI, NotI and XmaI sites used for cloning the cps-1 gene are all inside the amplified DNA sequences.
| Primer name | Sequence (5’ to 3’) |
|---|---|
| For cloning of cps-1 | |
| cps-1 5’ end forward primer | ATTAACTAGTCGGAGTCATCTGTCAATGCAG |
| cps-1 3’ end reverse primer | AAATGCGGCCGCCCAATCTCCTCATGCCTCTGC |
| For cps-1 RIP experiment | |
| cps-1 RIP forward primer | ATTACCCGGGACAAGGTTGCCCTTTCCTTC |
| cps-1 RIP reverse primer | GCACTAGTCAAGCATACCATATAACAATATGG |
| For cloning the HA-cps-1 | |
| HA-cps-1 forward primer | TACCCCTACGACGTCCCCGACTACGCCTAACGG TACTGGCTTGCTGCT |
| HA-cps-1 reverse primer | GGCGTAGTCGGGGACGTCGTAGGGGTAACCTTG CAGAGATGGGGGTGA |
The N. crassa RIP phenomenon can be used to create multiple mutations in a targeted gene (Selker, 1999). In the Neurospora crassa RIP process, genes that are present in two or more copies in the haploid genome receive multiple C-to-T mutations during the sexual cycle. To demonstrate that mutations in cps-1 give rise to the mutant phenotype, the 5’ end of the gene was cloned and used in a RIP experiment. For this experiment, cps-1 RIP forward and reverse primers (Table 1) were used to PCR amplify the 5’UTR, the first exon and a portion of the first intron of cps-1. The PCR product was digested with XmaI and SpeI and ligated into an XmaI and SpeI digested pBM61 vector to create pCPS1-5’. pCPS1-5’ was used to transform the his-3A mutant (FGSC#6103) to generate an isolate having two copies of the cps-1 sequences (the endogenous copy and the transforming copy at the his-3+ locus). Transformants were mated with a his-3 isolate to activate the RIP process. The his-3 progeny from this mating will contain a single copy of cps-1 at the endogenous locus. Progeny having the his-3, cps-1 mutant phenotype were isolated and the cps-1 gene sequences were amplified from the genomic DNA and sequenced.
To detect CPS-1 inside the cell, an HA-tagged CPS-1 protein was generated by inserting the HA-tag sequence YPYDVPDYA right before the stop codon. This was done in a two-step process. First, two PCR products were generated using primer pair cps-1 5’ end forward primer and HA-cps-1 reverse primer and primer pair HA-cps-1 forward primer and cps-1 3’ end reverse primer (Table 1). Second, these two PCR products were combined and used as template for a second PCR reaction using primer set cps-1 5’ end forward primer and cps-1 3’ end reverse primer. The final PCR product was digested with SpeI and NotI and cloned into SpeI and NotI digested pBM60 to create pHA-CPS1. The HA-tagged construct was then transformed into a Δcps-1, his-3 isolate. To detect HA-tagged CPS-1 protein expression, Western blot analyses were performed. The transformed cells were inoculated on cellophane filters placed on top of Vogel's sucrose agar medium plates. After 24 hours of growth at room temperature, the cellophane filters were peeled off of the agar and ground in liquid nitrogen. Protein extraction buffer (100mM Tris/HCl pH 7.4, 1% SDS, supplemented with 1X protease inhibitor cocktail (P-8340 Sigma Aldrich, St. Louis, MO)) was added to the samples and the supernatant was collected after centrifugation. Protein concentrations were determined by using the DC protein assay kit (BioRad, Hercules, CA). Samples containing 60 μg of protein were subjected to SDS-PAGE and transferred to nitrocellulose membranes. The nitrocellulose membranes were subjected to Ponceau S red (Sigma Aldrich, MO) staining to verify equal loading of the different protein samples. Mouse monoclonal anti-HA (Covance, Princeton, NJ) and Rabbit anti-mouse IgG-HRP (Sigma Aldrich) were used to assess the levels of HA-CPS-1 protein expression. The ChemiDoc XRS+ chemiluminescent detection system was used for the Western blots and the images were analyzed with Bio Rad Image Lab software.
2.3 Analysis of cell wall protein secretion
Wild type and Δcps-1 cells were grown in Vogel's liquid medium for 24 hours and the cells were harvested by filtration on a Buchner funnel. The secreted proteins were subjected to trichoroacetic acid precipitation and cell extracts were prepared described by Maddi and Free (Maddi and Free, 2010). Samples of cell extracts containing 30 ug of protein and secreted protein samples corresponding to 30 ug of cell extract were subjected to SDS PAGE and stained with Coomassie Brilliant Blue. For Western blot analysis of the major cell wall protein, ACW-1, the procedure described by Bowman et al. was followed (Bowman et al., 2006).
2.4. Coomassie Brilliant blue dye binding assay
The Coomassie Brilliant blue dye binding assay was used to assess the amounts of protein within purified cell walls and was used as previously described (Maddi et al., 2012). The assay consists of incubating purified cell wall samples with a solution of Coomassie Brilliant blue and allowing the dye to bind to cell wall protein. The samples were then subjected to a centrifugation step to remove the cell wall and the amount of Coomassie Brilliant blue remaining was determined as OD465. The assay was performed in triplicate.
2.5. Testing for hyaluronic acid
To determine if wild type and Δcps-1 cells release hyaluronic acid into the growth medium, aliquots of Vogel's medium in which the cells had grown were tested for hyaluronic acid using the hyaluronic acid test kit from Corgenix Inc. (Denver, CO). The test kit is an ELISA-based assay system in which hyaluronic acid binding protein is attached to the wells of microtiter plates and was used as described in the kit assay instructions. Aliquots of the growth medium were added to the wells and allowed to incubate for 60 minutes at room temperature to allow any hyaluronic acid in the medium to bind to the wells. The wells were then washed three times with buffer, and horseradish peroxidase-conjugated hyaluronic acid-binding protein was added to the wells and the plates were incubated at room temperature for 60 minutes. The wells were then washed and the chromogenic peroxidase substrates, tetramethylbenzidine and hydrogen peroxide, were added. The presence of hyaluronic acid was assessed as OD450.
To test for the presence of hyaluronic acid in the cell wall, wild type and Δcps-1 conidia were used to inoculate Vogel's sucrose liquid medium and allowed to grow for 16 hours. The cells were collected by centrifugation (6,000 g for 5 min), washed with ice-cold PBS and resuspended in ice-cold PBS. Cell walls were prepared as described by Maddi et al. (Maddi et al., 2012). HRP–conjugated hyaluronic acid-binding protein (Corgenix Inc., Denver, CO) was added to 200 ug samples of purified cell wall. After a 60 minute incubation at 25°C, the cell walls were then collected by centrifugation and washed four times with ice-cold PBS. The HRP substrates were then added to the washed cell walls and the presence of hyaluronic acid was assessed with a spectrometer at OD450.
2.6. Analysis of cell wall amino sugars and uronic acids
Wild type and Δcps-1 purified cell wall samples were prepared as previously described (Maddi et al., 2009). The samples were sent to the Complex Carbohydrate Research Center (Athens, GA) for carbohydrate analyses of neutral sugars, amino sugars, and uronic acids.
FITC-tagged lectins were also used to assess the presence of sugars in the purified cell walls. The FITC-tagged lectins were purchased from Sigma Aldrich Corp. (St. Louis, MO). Lectins were added to the cell wall samples in PBS and, after 30 minutes of incubation, the samples were examined with a fluorescence microscope to assess whether the lectins had bound to the cell walls. Aprotinin has been shown to bind to hyaluronic acid (Stoddart and Kiernan, 1973). Aprotinin was fluorescently tagged as described by Kiernan and Stoddart (Kiernan and Stoddart, 1973) and used with the same protocol as the lectins to label purified cell walls.
3. Results
3.1. Isolation and phenotypic characterization of Δcps-1
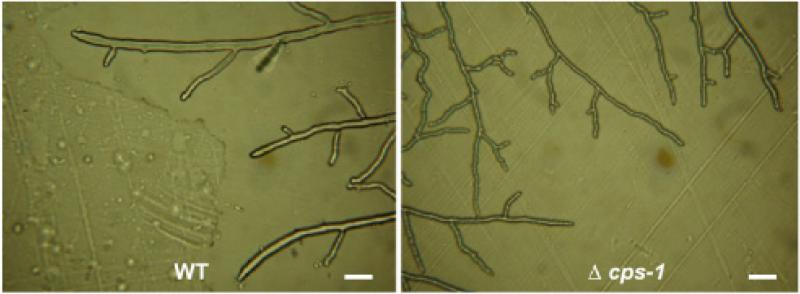
The Δcps-1 mutant was initially identified in a screening of the Neurospora crassa single gene deletion library for mutants that were unable to produce protoperithecia when grown on synthetic crossing medium. In addition to being protoperithecia-defective, other phenotypic alterations were clearly obvious. The Δcps-1 cells grew slower than wild type cells. On Vogel's sucrose medium, Δcps-1 had a growth rate of 1.0 mm/hr as compared to the wild type measured growth rate of 3.1 mm/hr. An examination of the vegetative hyphae at the edge of the growing colony shows that the Δcps-1 hyphae were abnormal (Fig. 1). The Δcps-1 hyphae were clearly smaller in diameter and had a slightly uneven, bumpy surface. The mutant did not produce the abundance of aerial hyphae and conidia (asexual spores) characteristic of the wild type (Fig. 2). When grown in liquid Vogel's medium, the mutant grew in a semi-colonial manner instead of growing with the long hyphal elements typical of wild type cells. Thus, all aspects of the Neurospora life cycle (vegetative growth, asexual development, and female development) are affected in Δcps-1.
Fig. 1.
The Δcps-1 mutant is affected in hyphal cell morphology. Pictures of the growth at the edge of wild type and Δcps-1 colonies were taken with a Cannon Power Shot A620 camera with a microscope adaptor. Both pictures were taken with at 200X magnification. The white bar represents a distance of 20 μm.
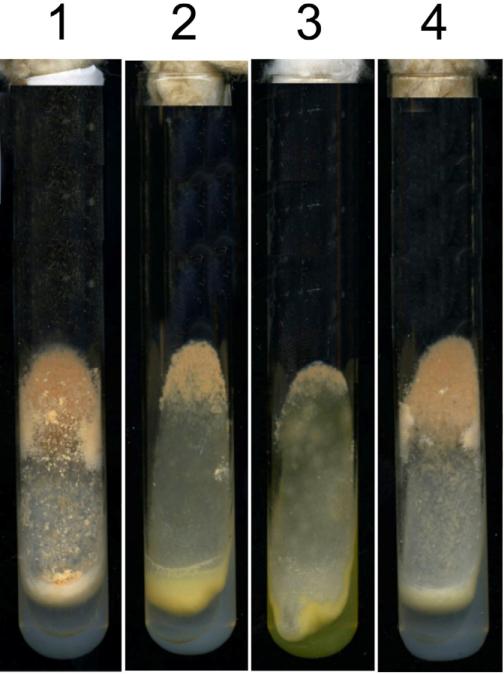
Fig. 2.
RIP and complementation experiments demonstrate that loss of cps-1 is responsible for the mutant phenotype. Slants containing 1) WT, 2) Δcps-1, 3) cps-1RIP1, and 4) Δcps-1 being complemented by a wild type copy of cps-1 are shown. The slants show 4 day-old cultures grown at room temperature.
Since the mutants in the single gene deletion library frequently have other mutations in addition to the deletion mutation (Fu et al., 2011), we verified that the cps-1 deletion mutation was responsible for the mutant phenotypes by doing co-segregation, RIP, and complementation experiments. To demonstrate that the cps-1 deletion mutation co-segregated with the mutant phenotypes, Δcps-1 was mated with the wild type strain. Twenty four progeny ascospores were isolated and tested for hygromycin resistance and for the presence of the mutant phenotypes. In constructing the mutant library, the deleted genes were replaced by a hygromycin resistance cassette to facilitate the isolation of the deletion mutants. Thus hygromycin resistance can be used as a marker for the deletion mutation. We found that in all of the progeny, the mutant phenotypes co-segregated with hygromycin resistance, strongly suggesting that the cps-1 deletion was responsible for the mutant phenotypes.
To ask whether the mutations in the cps-1 gene would reproduce the phenotypes seen in Δcps-1, we generated additional mutants using the RIP procedure (see Materials and Methods). We found that these mutants had the same phenotype as the Δcps-1 mutants (Fig. 2). For two of these RIP mutants, the cps-1 gene was PCR amplified and sequenced. We found that both mutants have several mutations in cps-1, and both DNAs contained a mutation creating a stop codon at amino acid 11 in the coding region (a TGG codon mutated to TAG). We conclude that mutations to the cps-1 gene generate the mutant phenotypes found in the Δcps-1 mutant.
A complementation experiment was also used to verify that the cps-1 deletion mutant was responsible for the mutant phenotypes. A wild type copy of the cps-1 gene was cloned into the pBM60 vector as described in Materials and Methods. Transformation of the Δcps-1 mutant with the wild type copy of the gene rescued all of the mutant phenotypes (Figure 2). Based on the co-segregation data, the additional cps-1 mutants generated by RIP, and the complementation experiment, we conclude that all of the mutant phenotypes were due to the loss of the CPS-1 polysaccharide synthase activity.
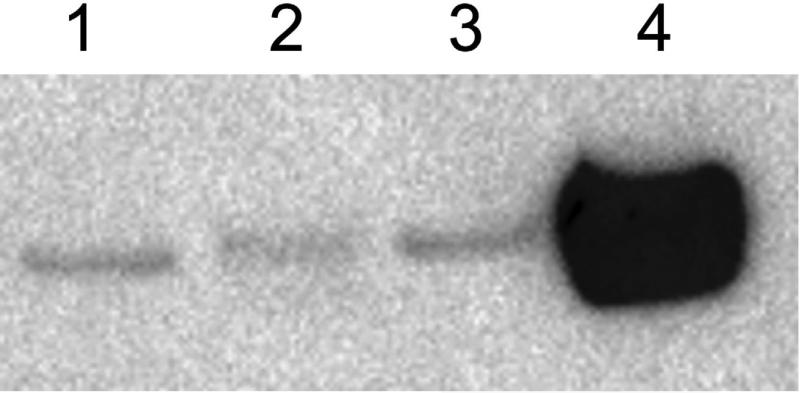
An HA-tagged version of CPS-1was generated as described in Materials and Methods. The HA-tagged version of CPS-1 gave a partial restoration of the growth and conidiation phenotypes. Cellular extracts from transformants growing on Vogel's sucrose medium were used for a Western blot analysis. As shown in Fig. 3, the transformant extract contained a 58 kD protein that was recognized by the HA antibody, and the protein was absent from the wild type extract. The observed size of the HA-tagged CPS-1 was in agreement with the predicted molecular weight of 58.3 kD.
Fig. 3.
CPS-1 is a 58 kD protein. A HA-tagged version of CPS-1 was prepared as described in Materials and Methods and used to transform a Δcps-1; his-3 isolate. The presence of HA-tagged CPS-1in a cellular extract was assessed by Western blot analysis with anti-HA antibody. Lane 1) Transformant cell extract. Lane 2) wild type cell extract.
Because the cps-1 gene encodes a polysaccharide synthase with homology to plasma membrane synthases that extrude polysaccharides into the cell wall space during synthesis, we examined the effects of cell wall perturbation reagents on Δcps-1 growth. Wild type and Δcps-1 conidia were used to inoculate Vogel's medium containing cell wall perturbation reagents (Maddi et al., 2012). We found that Δcps-1 was unable to grow in the presence of 10 ug/ml caspofungin (an inhibitor of β-1,3-glucan synthase), 10 mg/ml calcofluor white (an inhibitor of chitin synthesis), 10% NaCl (osmotic stress), 2 M glycerol (osmotic stress) or 0.01 % SDS (general stress). We conclude that the structure or integrity of the cell wall was affected by the loss of the polysaccharide polymer produced by CPS-1.
3.2. CPS-1 is needed for the incorporation of cell wall protein into the cell wall matrix
The colonial manner in which Δcps-1 grows in liquid was reminiscent of the phenotypes seen in the Δoch-1 mutant and the Δdcw-1, Δdfg-5 mutant (Maddi and Free, 2010; Maddi et al., 2012). These mutants have been previously characterized and shown to be defective in the incorporation of cell wall proteins into the cell wall matrix. To examine the possibility that Δcps-1 was affected in the incorporation of cell wall proteins into the cell wall matrix, we grew mutant and wild type cells in Vogel's liquid medium for 24 hours. The cells were collected on a Buchner funnel, and the growth medium was saved. The proteins that had been released into the growth medium were precipitated with trichloroacetic acid and subjected to SDS PAGE. As shown in Fig. 4, the wild type cells released minimal amounts of protein into the medium while the Δcps-1 cells released a substantial amount of protein into the medium. The staining patterns for the secreted proteins and cell extract proteins were distinctly different (Fig. 4), suggesting that the proteins seen in the medium do not come from cell lysis.
Fig. 4.
Δcps-1 releases large amounts of cell wall protein into the growth medium. Wild type and Δcps-1 were grown for 24 hours in liquid Vogel's medium and harvested. Cell extracts and samples of the growth medium were subjected to SDS-PAGE and stained with Coomassie Blue. Lane M) molecular weight markers. Lane 1) 30 ug of wild type cell extract. Lane 2) Secreted wild type proteins corresponding to 30 ug of cell extract. Lane 3) 30 ug of Δcps-1 cell extract. Lane 4) Secreted proteins corresponding to 30 ug of Δcps-1 cell extract.
To definitively ascertain whether cell wall proteins were being released into the growth medium, we did a Western blot assay with antibody directed against ACW-1, which has been previously shown to be a major GPI-anchored cell wall protein in N. crassa (Bowman et al., 2006; Maddi et al., 2009). As shown in Fig. 5, the growth medium from Δcps-1 contained large amounts of ACW-1, while the growth medium from the wild type cell had a negligible amount of ACW-1. The release of large amounts of the cell wall ACW-1 by Δcps-1 demonstrates that Δcps-1 isn't effectively incorporating cell wall proteins into the cell wall matrix. This is in stark contrast with the wild type cell, in which the ACW-1 is being efficiently cross-linked into the cell wall and not being released into the medium (Fig. 5). We conclude that the incorporation of cell wall proteins is defective in Δcps-1. The defect in incorporating cell wall proteins into the cell wall could explain all of the phenotypic characteristics we observed in Δcps-1.
Fig. 5.
Δcps-1 releases large amounts of ACW-1, a known cell wall protein, to the growth medium. Proteins were loaded on an SDS gel as shown in Fig. 4 and subjected to Western blot analysis for ACW-1. Lane 1) 30 ug of wild type cell extract. Lane 2) Secreted wild type proteins corresponding to 30 ug of cell extract. Lane 3) 30 ug of Δcps-1 cell extract. Lane 4) Secreted proteins corresponding to 30 ug of Δcps-1 cell extract.
To further verify that the incorporation of cell wall protein into the wall was compromised in Δcps-1, we carried out a Coomassie Brilliant Blue dye binding assay (Fig. 6). In this assay, the ability of the cell wall to bind the dye was assessed by quantifying the amount of dye bound by increasing amounts of purified cell wall. Although the assay does not give a linear relationship between the amount of dye bound and the amount of cell wall material, Fig. 6 clearly shows that the Δcps-1 cell wall does not bind as much Coomassie dye as the wild type cell wall. The results demonstrate that the Δcps-1 cell wall contains less protein than the wild type cell wall, and are consistent with the finding that cell wall proteins are not being effectively cross-linked into the cell wall matrix in the mutant (Fig. 4 and Fig. 5).
Fig. 6.
The Δcps-1 cell wall is deficient in cell wall proteins. The Coomassie Brilliant Blue dye binding assay was used to assess the levels of cell wall proteins in purified wild type and Δcps-1 cell walls. Increasing amounts of cell wall proteins were incubated with the dye and the amount of dye binding to cell wall proteins was determined.
3.3. Analyses of the cell wall polysaccharides in Δcps-1
The C. neoformans CPS1-encoded polysaccharide synthase has been shown to function in the synthesis of hyaluronic acid, a polysaccharide with repeating disaccharide units of glucuronic acid (β-1,3) and N-acetylglucosamine (β-1,4) (Jong et al., 2007). Jong et al. demonstrated that the synthase, when expressed in either E. coli or in S. cerevisiae, was capable of making a polymer recognized by the bovine hyaluronic acid-binding protein. They also showed that a purified his6-tagged version of the synthase could be used to synthesize hyaluronic acid using UDP-glucuronic acid and UDP-N-acetylglucosamine as substrates. To determine whether N. crassa CPS-1 could synthesize hyaluronic acid, we asked whether the bovine hyaluronic acid-binding protein could bind to purified Δcps-1 and wild-type cell walls (see Materials and Methods). We found that the bovine hyaluronic acid-binding protein did not show significant binding to either wild type or mutant cell wall, suggesting that CPS-1 does not synthesize a polymer recognized by the binding protein.
We also used the hyaluronic acid test kit to determine whether the growth medium from Δcps-1 or wild type cells contained hyaluronic acid (see Materials and Methods). We found no evidence of hyaluronic acid in either growth medium. Together with the data on the cell wall samples, this suggests that the N. crassa CPS-1 synthesizes a polymer that is not recognized by the bovine hyaluronic acid binding protein, and that CPS-1 does not encode a hyaluronic acid synthase.
Carbohydrate analyses of the Δcps-1 and wild type vegetative cell walls were carried out to identify the neutral sugars present and the linkages between these sugars (Table 2). Table 2 showed that the Δcps-1 cell wall was deficient in mannose and galactose residues, and had more glucose than the wild type cell wall. Mannose and galactose residues made up 5.8% of the Δcps-1 cell wall and 20.2% of the wild type cell wall. Most of the mannose and galactose residues in the N. crassa cell wall are found as post-translational modifications on the cell wall glycoproteins (Maddi and Free, 2010; Maddi et al., 2012). The reduced level of mannose and galactose in the Δcps-1 cell wall provides additional evidence that cell wall proteins are not effectively cross-linked into the mutant cell wall. The increase in the percentile of cell wall neutral sugar represented by glucose in the Δcps-1 sample (Table 2) reflects the paucity of mannose and galactose in the mutant cell wall.
Table 2.
Cell wall samples were subjected to glycosyl linkage analysis. The samples were permethylated, depolymerized, reduced and acetylated before being analyzed by gas chromatography-mass spectrometry.
| Linkage type | % of total carbohydrate | |
|---|---|---|
| Wild type | Δcps-1 | |
| Terminal glucopyranose | 4.5% | 5.6% |
| 2-linked glucopyranose | n.d. | 4.0% |
| 3-linked glucopyranose | 47.1% | 39.9% |
| 4-linked glucopyranose | 19.7% | 37.3% |
| 6-linked glucopyranose | 1.9% | n.d. |
| 2,3-linked glucopyranose | 2.4% | 3.6% |
| 3,4-linked glucopyranose | L°% | 0.7% |
| 3,6-linked glucopyranose | 1.6% | 1.0% |
| 4,6-linked glucopyranose | 0.7% | 1.3% |
| 2,3,4-linked glucopyranose | 0.6% | 0.8% |
| 2,3,4,6-linked glucopyranose | 0.3% | n.d. |
| Total glucose | 79.8% | 94.2% |
| Terminal mannopyranose | 1.7% | 0.7% |
| 2-linked mannopyranose | 4.5% | n.d. |
| 2,3-linked mannopyranose | 1.3% | 0.8% |
| 2,6-linked mannopyranose | 3.7% | 1.6% |
| 2,3,6-linked mannopyranose | 0.3% | n.d. |
| Total mannose | 11.5% | 3.1% |
| Terminal galactopyranose | 0.7% | n.d. |
| 4-linked galactopyranose | 1.4% | 1.6% |
| Terminal galactofuranose | 3.0% | 1.1% |
| 2-linked galactofuranose | 1.5% | n.d. |
| 3-linked galactofuranose | 2.1% | n.d. |
| Total galactose | 8.7% | 2.7% |
In Table 2, there are seven sugar linkages found in the wild type cell wall but not in the Δcps-1 cell wall (6-linked glucopyranose, 2,3,4,6-linked glucopyranose, 2-linked mannopyranose, 2,3,6-linked mannopyranose, terminal galactopyranose, 2-linked galactofuranose, and 3-linked galactofuranose). The mannose and galactose sugar linkages could be associated with the N-linked galactomannan used to cross-link proteins into the wall (Maddi et al., 2012). The lack of these sugar linkages in the mutant cell wall may reflect the problem in cross-linking glycoproteins into the wall. The 6-linked glucopyranose was found as 1.9% of the wild type cell wall total carbohydrate, and was absent from the Δcps-1 cell wall. The absence of 6-linked glucose in the mutant cell wall suggests that CPS-1 may synthesize a polymer containing 6-linked glucose residues. We also noted that there was an increase in 2-linked glucose in the mutant cell wall (Table 2). We hypothesize that Δcps-1 experiences cell wall stress and that the increased levels of 2-linked glucose residues in the mutant cell wall may reflect the activation of the cell wall stress response pathway.
An analysis that was specifically designed to identify the presence of glucosaminoglycans and similar uronic acid-containing polysaccharides was carried out (Table 3). The levels of glucose, mannose, and galactose in this analysis did not closely agree with the analysis shown in Table 2. However, the digestion conditions used to release amino sugars and uronic acids destroy some of the neutral sugars. In the analysis for uronic acids only 60% of the total mass for the wild type cell wall and 36.8% of the total mass for the Δcps-1 cell wall were recovered. The difference between the levels of glucose, mannose, and galactose in the two analyses likely reflects the loss of these sugars in the uronic acid analysis (Table 3). Most importantly, the uronic acid analysis failed to identify any uronic acids, and provides additional and compelling evidence that CPS-1 does not synthesize hyaluronic acid.
Table 3.
Wild type and Δcps-1 cell walls were subjected to a monosaccharide composition analysis designed to identify uronic acids and amino sugars in the cell wall samples.
| Sugar residue | % of total cell wall mass | % of total cell wall mass |
|---|---|---|
| Wild type | Δcps-1 | |
| Residues | ||
| Glucose | 41.3% | 17.7% |
| Mannose | 9.8% | 6.5% |
| Galactose | 5.9% | 5.6% |
| N-acetyl-galactosamine | 2.2% | 6.0% |
| N-acetyl-glucosamine | 0.8% | 0.9% |
| xylose | n.d. | 0.1% |
| Glucuronic acid | n.d. | n.d. |
| Iduronic acid | n.d. | n.d. |
| % of total cell wall mass | 60.0% | 36.8% |
We also used lectins to examine the sugars present in wild type and Δcps-1 cell walls. Purified cell walls were incubated with FITC-tagged lectins as described in Materials and Methods. Table 4 shows the results of these lectin binding experiments. The experiments showed that the cell walls contained N-acetylglucosamine, glucose, mannose, galactose, and perhaps N-acetylgalactosamine, and confirmed the data we obtained from the carbohydrate analysis. The mutant cell wall was indistinguishable from the wild type cell wall with regard to the intensity of cell wall staining by the lectins (Table 4). We were unable to find any lectins that recognize hyaluronic acid, but aprotinin has been shown to have an affinity for hyaluronic acid and fluorescein-tagged aprotinin has been used to visualize this interaction (Kiernan and Stoddart, 1973; Stoddart and Kiernan, 1973). Using the methods described by Kiernan and Stoddard, we prepared fluorescein-tagged aprotinin and used it to stain cell walls. The tagged-aprotinin did not stain either the wild type or the Δcps-1 cell wall. This provides further evidence for a lack of hyaluronic acid in the N. crassa cell wall. Unfortunately, the lectin and aprotinin staining experiments did not provide any indication of what type of polymer CPS-1 produces.
Table 4.
Wild type and Δcps-1 cell wall preparations were incubated with FITC-labelled lectins and the binding of the lectins to the cell walls was monitored by fluorescent microscopy. The lectins used in the analysis are listed along with their binding specificities. The fluorescent signals observed on the cell wall samples were graded as highly fluorescent (++), fluorescent (+) or not fluorescent (-).
| lectin | Lectin specificity | Wt cell wall | Δcps-1 cell wall |
|---|---|---|---|
| Phytolacca Americana | GlcNac | ++ | ++ |
| Dolichos Bifloras | GalNac | - | - |
| Lens Culinaris | a-mannan, a-glucan | + | + |
| Sophora Japonica | Galactose, GalNac, lactose | - | + |
| Erythrina Corralodendron | Gal(D)Nac, Galactose | + | + |
| Glycine Max | Gal(D)Nac | + | + |
| Bauhinia Purpurea | GlcNac, glucose, sucrose, fucose | + | + |
| Concanavilin A | Terminal a-mannan/a-glucan | + | + |
| Aprotinin | Glucuronic/sialic acids | - | - |
4. Discussion
The cell wall is a critical organelle for fungal growth and survival, and several anti-fungal reagents target its biosynthesis. Fungal cell walls have been shown to contain chitin and a variety of glucans, including β-1,3-glucan, β-1,6-glucan, α-1,3-glucan, and a mixed β-1,3-/β-1,4-glucan. A number of cell wall proteins with glucanase and chitinase activities are thought to function as cross-linking enzymes by using a “forward reaction” to cleave the polysaccharides and then using a “reverse reaction” to cross-link the chitin and glucan polymers together (Free, 2013; Klis et al., 2006; Latge, 2007). The cell wall also contains a number of proteins with N-linked and O-linked mannans and galactomannans that are covalently cross-linked into the cell wall. Evidence from N. crassa shows that the N-linked oligosaccharides can be used to cross-link glycoproteins into the cell wall, and two mannanases have been shown to function in cross-linking the proteins into the cell wall matrix (Maddi and Free, 2010; Maddi et al., 2012).
In this report, we demonstrate that CPS-1 functions in the biosynthesis of the N. crassa cell wall. We show that loss of CPS-1 affects the growth and morphology of the fungus. All aspects of the N. crassa life cycle are affected in Δcps-1, suggesting that the polysaccharide produced by CPS-1 is an important cell wall component.
The N. crassa CPS-1 has homology to the C. neoformans Cps1p, a hyaluronic acid synthase responsible for generating cell wall hyaluronic acid. Hyaluronic acid is a component of the C. neoformans cell wall and capsid structure (Chang et al., 2006; Jong et al., 2007). We were unable to demonstrate the presence of hyaluronic acid in the N. crassa cell wall or in the growth medium with the bovine hyaluronic acid binding protein assay system, and we were unable to detect hyaluronic acid in cell wall samples using the fluorescein-tagged aprotinin assay. Furthermore, we were unable to detect any uronic acids in N. crassa cell wall with a carbohydrate analysis. It is worth noting that CPS-1 is also related to the S. pneumoniae type 3 polysaccharide synthase, which directs the synthesis of a β-1,3-glucuronic acid-β-1,4-glucose repeating polymer (Arrecubieta et al., 1996; Cartee et al., 2005). Thus, there is precedence for the synthesis of polymers other than hyaluronic acid by synthases related to CPS-1. Type 3 polysaccharide synthase and hyaluronic acid synthases generate 1,4-linkages, and the carbohydrate analysis of the N. crassa cell wall shows that it contains 1,4-linked glucose (Table 2). One possibility that we have considered is that CPS-1 might be involved in the synthesis of a polymer containing β-1,4-linked glucose. However, if CPS-1 makes a polymer with β-1,4-linked glucose residues, there must be other polysaccharide synthases producing 1-4 linked glucose units in addition to CPS-1, because the Δcps-1 cell wall contains 1,4-linked glucose. The monosaccharide linkage analysis for the Δcps-1 cell wall did show that the mutant was lacking in 1,6-linked glucose residues, which suggests that CPS-1 may synthesize a polymer containing a 1,6-linked glucose. Although we haven't identified the polymer produced by CPS-1, the research clearly demonstrates that CPS-1 plays an important role in the formation of the N. crassa cell wall. Closely related homologs of cps-1 are found in the genomes of filamentous fungi, suggesting that a CPS-1 generated polysaccharide is likely to be a component of other fungal cell walls.
One important aspect of cell wall biogenesis that is affected by the loss of CPS-1 is that cell wall proteins are not being effectively incorporated into the cell wall. We present four different lines of evidence to demonstrate that CPS-1 is needed for the incorporation of cell wall proteins into the cell wall. First, we demonstrate that Δcps-1 releases large amount of protein into the medium, and that the released proteins have a different SDS-PAGE staining pattern than cytosolic proteins (Fig. 4). Second, we show by Western blot analysis that the major cell wall protein, ACW-1, is released into the growth medium by Δcps-1 but not by wild type cells (Fig. 5). Third, a Coomassie Brilliant Blue dye binding assay was used to demonstrate that the mutant cell wall has less protein than the wild type cell wall (Fig. 6). Fourth, the analysis of the sugars in the Δcps-1 cell wall demonstrates that the wall is deficient in mannose and galactose, the sugars that are incorporated into the wall as post-translational modifications on cell wall proteins (Table 2). Our analyses clearly demonstrate that the cross-linking of cell wall proteins into the wall is compromised in the absence of CPS-1.
There are at least two plausible explanations for why the loss of a CPS-1-generated polysaccharide from the cell wall would affect the incorporation of proteins into the wall. One possibility is that polysaccharide might be used to cross-link the proteins into the matrix. The β-1,6-glucan is used to cross-link proteins and cell wall polymers together in the S. cerevisiae and C. albicans cell wall(Kapteyn et al., 1996; Kollar et al., 1997; Lu et al., 1995). The CPS-1 generated polysaccharide might play a similar role for the cell wall in N. crassa and perhaps other filamentous fungi. Interestingly, the S. cerevisiae and C. albicans genomes have the enzymes for β-1,6-glucan synthesis and lack a cps-1 homolog, while the genomes of the filamentous fungi lack the enzymes for β-1,6-glucan synthesis and have cps-1 homologs. A second possibility is that the CPS-1 generated polysaccharide is simply an integral cell wall polysaccharide component and that its loss causes a major change in cell wall glucan organization. The defect in incorporating cell wall proteins into the wall could be an indirect consequence of the altered glucan organization. Further experimentation will be needed to determine why the loss of the polysaccharide affects the cross-linking of cell wall glycoproteins into the cell wall.
Highlights.
CPS-1 plays an important role in cell wall biogenesis in Neurospora crassa.
All stages of the life cycle are affected in the Δcps-1 mutant.
CPS-1 is needed for the incorporation of cell wall proteins into the cell wall matrix.
Acknowledgement
This research was supported by grant R03-Al103897 from the National Institutes of Health and funds from the University at Buffalo Foundation. The carbohydrate analyses were supported in part by the Department of Energy-funded (DE-FG02-09ER-20097) Center for Plant and Microbial Complex Carbohydrates. Funding for the creation of the single gene deletion library was provided by the grant PO1 GM068087. We are grateful to James Stamos for help in preparing the images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrecubieta C, Lopez R, Garcia E. Type 3-specific synthase of Streptococcus pneumoniae (Cap3B) directs type 3 polysaccharide biosynthesis in Escherichia coli and in pneumococcal strains of different serotypes. J Exp Med. 1996;184:449–55. doi: 10.1084/jem.184.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SM, Piwowar A, Al Dabbous M, Vierula J, Free SJ. Mutational analysis of the glycosylphosphatidylinositol (GPI) anchor pathway demonstrates that GPI-anchored proteins are required for cell wall biogenesis and normal hyphal growth in Neurospora crassa. Eukaryot Cell. 2006;5:587–600. doi: 10.1128/EC.5.3.587-600.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E, Blanco N, Grau C, Rodriguez-Pena JM, Arroyo J. Crh1p and Crh2p are required for the cross-linking of chitin to beta(1-6)glucan in the Saccharomyces cerevisiae cell wall. Mol Microbiol. 2007;63:921–35. doi: 10.1111/j.1365-2958.2006.05565.x. [DOI] [PubMed] [Google Scholar]
- Cartee RT, Forsee WT, Yother J. Initiation and synthesis of the Streptococcus pneumoniae type 3 capsule on a phosphatidylglycerol membrane anchor. J Bacteriol. 2005;187:4470–4479. doi: 10.1128/JB.187.13.4470-4479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Jong A, Huang S, Zerfas P, Kwon-Chung KJ. CPS1, a homolog of the Streptococcus pneumoniae type 3 polysaccharide synthase gene, is important for the pathobiology of Cryptococcus neoformans. Infect Immun. 2006;74:3930–8. doi: 10.1128/IAI.00089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A. 2006;103:10352–7. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RH, DeSerres FJ. Genetic and microbiological research techniques for Neurospora crassa. Meth. Enzymol. 1970;27:79–143. [Google Scholar]
- Doering TL. How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu Rev Microbiol. 2009;63:223–47. doi: 10.1146/annurev.micro.62.081307.162753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free SJ. Fungal cell wall organization and biosynthesis. Adv Genet. 2013;81:33–82. doi: 10.1016/B978-0-12-407677-8.00002-6. [DOI] [PubMed] [Google Scholar]
- Fu C, Iyer P, Herkal A, Abdullah J, Stout A, Free SJ. Identification and characterization of genes required for cell-to-cell fusion in Neurospora crassa. Eukaryot Cell. 2011;10:1100–9. doi: 10.1128/EC.05003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastebois A, Clavaud C, Aimanianda V, Latge JP. Aspergillus fumigatus: cell wall polysaccharides, their biosynthesis and organization. Future Microbiol. 2009;4:583–95. doi: 10.2217/fmb.09.29. [DOI] [PubMed] [Google Scholar]
- Goldman RC, Sullivan PA, Zakula D, Capobianco JO. Kinetics of beta-1,3 glucan interaction at the donor and acceptor sites of the fungal glucosyltransferase encoded by the BGL2 gene. Eur J Biochem. 1995;227:372–8. doi: 10.1111/j.1432-1033.1995.tb20399.x. [DOI] [PubMed] [Google Scholar]
- Grun CH, Hochstenbach F, Humbel BM, Verkleij AJ, Sietsma JH, Klis FM, Kamerling JP, Vliegenthart JF. The structure of cell wall alpha-glucan from fission yeast. Glycobiology. 2005;15:245–57. doi: 10.1093/glycob/cwi002. [DOI] [PubMed] [Google Scholar]
- Hochstenbach F, Klis FM, van den Ende H, van Donselaar E, Peters PJ, Klausner RD. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc Natl Acad Sci U S A. 1998;95:9161–6. doi: 10.1073/pnas.95.16.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A, Wu CH, Chen HM, Luo F, Kwon-Chung KJ, Chang YC, Lamunyon CW, Plaas A, Huang SH. Identification and characterization of CPS1 as a hyaluronic acid synthase contributing to the pathogenesis of Cryptococcus neoformans infection. Eukaryot Cell. 2007;6:1486–96. doi: 10.1128/EC.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapteyn JC, Montijn RC, Vink E, de la Cruz J, Llobell A, Douwes JE, Shimoi H, Lipke PN, Klis FM. Retention of Saccharomyces cerevisiae cell wall proteins through a phosphodiester-linked beta-1,3-/beta-1,6-glucan heteropolymer. Glycobiology. 1996;6:337–45. doi: 10.1093/glycob/6.3.337. [DOI] [PubMed] [Google Scholar]
- Kiernan JA, Stoddart RW. Fluorescent-labelled aprotinin: a new reagent for the histochemical detection of acid mucosubstances. Histochemie. 1973;34:77–84. doi: 10.1007/BF00304309. [DOI] [PubMed] [Google Scholar]
- Klis FM, Boorsma A, De Groot PW. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23:185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- Klis FM, de Groot P, Hellingwerf K. Molecular organization of the cell wall of Candida albicans. Med Mycol. 2001;39(Suppl 1):1–8. [PubMed] [Google Scholar]
- Kollar R, Reinhold BB, Petrakova E, Yeh HJ, Ashwell G, Drgonova J, Kapteyn JC, Klis FM, Cabib E. Architecture of the yeast cell wall. Beta(1-->6)-glucan interconnects mannoprotein, beta(1-->)3-glucan, and chitin. J Biol Chem. 1997;272:17762–75. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- Latge JP. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol. 2007;66:279–90. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Lu CF, Montijn RC, Brown JL, Klis F, Kurjan J, Bussey H, Lipke PN. Glycosyl phosphatidylinositol-dependent cross-linking of alpha-agglutinin and beta 1,6-glucan in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1995;128:333–40. doi: 10.1083/jcb.128.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddi A, Bowman SM, Free SJ. Trifluoromethanesulfonic acid-based proteomic analysis of cell wall and secreted proteins of the ascomycetous fungi Neurospora crassa and Candida albicans. Fungal Genet Biol. 2009;46:768–81. doi: 10.1016/j.fgb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddi A, Free SJ. alpha-1,6-Mannosylation of N-linked oligosaccharide present on cell wall proteins is required for their incorporation into the cell wall in the filamentous fungus Neurospora crassa. Eukaryot Cell. 2010;9:1766–75. doi: 10.1128/EC.00134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddi A, Fu C, Free SJ. The Neurospora crassa dfg5 and dcw1 genes encode alpha-1,6-mannanases that function in the incorporation of glycoproteins into the cell wall. PLoS One. 2012;7:e38872. doi: 10.1371/journal.pone.0038872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin BS, Frietag M, Selker EU. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa. Fungal Genet. Newsl. 1997;44:34–36. [Google Scholar]
- Mouyna I, Fontaine T, Vai M, Monod M, Fonzi WA, Diaquin M, Popolo L, Hartland RP, Latge JP. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J Biol Chem. 2000;275:14882–9. doi: 10.1074/jbc.275.20.14882. [DOI] [PubMed] [Google Scholar]
- Reese AJ, Yoneda A, Breger JA, Beauvais A, Liu H, Griffith CL, Bose I, Kim MJ, Skau C, Yang S, Sefko JA, Osumi M, Latge JP, Mylonakis E, Doering TL. Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol Microbiol. 2007;63:1385–98. doi: 10.1111/j.1365-2958.2006.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker EU. Gene silencing: repeats that count. Cell. 1999;97:157–60. doi: 10.1016/s0092-8674(00)80725-4. [DOI] [PubMed] [Google Scholar]
- Stoddart RW, Kiernan JA. Aprotinin, a carbohydrate-binding protein. Histochemie. 1973;34:275–80. doi: 10.1007/BF00306299. [DOI] [PubMed] [Google Scholar]