Abstract
Objective
Behavioral economic theory predicts that reductions in consumption of highly valued commodities, such as drugs or palatable food items, are facilitated by increasing engagement in reinforcing substitutes. The current study prospectively examines changes in engagement in and enjoyment of food versus food-free activities during an 18-month behavioral weight loss intervention.
Methods
Two-hundred two overweight/obese individuals participated in an 18-month behavioral weight loss treatment and were randomly assigned to a traditional hypocaloric, low-fat diet condition or a traditional hypocaloric, low-fat diet plus a goal to limit variety in snack food consumption condition. At baseline, 6, 12, and 18 months, participants were weighed and completed a measure that assessed recent frequency of engagement in and enjoyment of a variety of both food and food-free activities.
Results
Growth models revealed a statistically significant decrease in the relative percentage of food-related reinforcement (versus food-free) over time (Reinforcement Ratio; RR), with the greatest reduction during the first 6 months of treatment. Food-related reinforcement decreased over time and food-free reinforcement increased. Additionally, the RR change predicted change in BMI from 0 to 6 months and 0 to 18 months, such that greater changes in RR were associated with greater changes in BMI.
Conclusions
Findings suggest that behavioral weight loss treatment may promote a shift away from food-related reinforcement towards food-free reinforcement, and that this change may predict BMI change. Future interventions may consider targeting increasing engagement in food-free enjoyable activities to help with long-term maintenance.
Keywords: Reinforcement Ratio, Obesity treatment, Behavioral economics, Food-related Reinforcement, Food-free Reinforcement
Obesity is a significant public health concern; over 90 million Americans are obese (Flegal, Carroll, Ogden, & Curtin, 2010; Mokdad et al., 2003) and are therefore at elevated risk for health complications such as heart disease, stroke, diabetes, hypertension, dyslipidemia, metabolic syndrome, respiratory problems, and some types of cancer (Bray, 2004; Pi-Sunyer, 2002). Most efficacious dietary interventions for obesity require the restriction of high calorie foods; the current Dietary Guidelines for Americans (2010) recommend calorie restriction in combination with physical activity for BMI reduction.
One barrier to the reduction of calories is that food is highly reinforcing; human and nonhuman animals will allocate considerable behavioral resources towards procuring food even in the face of increases in behavioral response cost (i.e., the effort or price required to obtain food) and potential adverse effects of overconsumption (Berridge, 1996; Salamone, 1994). Food is both a primary and secondary reinforcer (Del Parigi, Chen, Salbe, Reiman, & Tataranni, 2003) in that it is reinforcing without any prior learning (i.e., humans are biologically predisposed to eat when hungry), and the reinforcing value of food can increase over time when it is paired with other reinforcing events such as social activity or stress reduction.
Behavioral economic theory has been usefully applied to the analysis of factors that contribute to the onset, progression, and reduction of another highly valued reinforcer, drug use (Vuchinich & Tucker, 1988), and may also be relevant to obesity (Epstein, Salvy, Carr, Dearing, & Bickel, 2010a). Behavioral economic theory attempts to understand how individuals allocate resources (e.g., time, money, behavior) among the various activities available in their environment (Vuchinich & Heather, 2003). There is an emphasis on choice (Rachlin, 1992), with the relative value of various activities estimated from relative patterns of behavioral or resource allocation (Murphy, Correia, Colby, & Vuchinich, 2005; Murphy, MacKillop, Skidmore, & Pederson, 2009; Tucker, Roth, Vignolo, & Westfall, 2009). For example, individuals who abuse alcohol tend to value alcohol over other alternative activities, and this is reflected in their relative levels of enjoyment and participation in alcohol-related and alcohol-free activities (Correia, Carey, Simons, & Borsari, 2003; Correia, Simons, Carey, & Borsari, 1998), as well as their patterns of spending and resource allocation (Murphy et al., 2009; Tucker et al., 2009; Tucker, Vuchinich, & Rippens, 2002). Individuals with drug and alcohol addiction not only find the drug to be reinforcing, but they find it more reinforcing than other behaviors, will work harder to obtain drug reinforcers compared to other reinforcers, and generally have limited accessibility to alternative reinforcers (Heinz, Lilje, Kassel, & de Wit, 2012; Higgins, Heil, & Plebani, 2004; Murphy, Correia, & Barnett, 2007). Similarly, the reinforcing value of food is a consistent predictor of food consumption in laboratory settings: individuals who place a higher value on food ingest more of it (Epstein et al., 2007; Epstein et al., 2004). Obese individuals find food to be more reinforcing than non-obese individuals (Epstein, & Saelens, 2000; Saelens & Epstein, 1996) and gain more enjoyment from eating sweet foods versus vegetables (Epstein et al., 2010a). The restriction of calories required for BMI change is acutely associated with increases in the reinforcing value of food, possibly contributing to the difficulty of BMI reduction and maintenance, especially in individuals who place a high value on food consumption at baseline (Epstein, Truesdale, Wojcik, Paluch, & Raynor, 2003; Raynor & Epstein, 2003). In sum, holding all outside circumstances equal (e.g., price, time), individuals are more likely to engage in behaviors/activities which are relatively more reinforcing to them, and these individual differences may have a direct relation to obesity (Lichtman et al., 1992).
A key implication of behavior economic theory is that reductions in the consumption of highly valued commodities such as drugs or palatable food items are facilitated by increasing in engagement in reinforcing substitute activities (Correia, Benson, & Carey, 2005; Higgins, Heil, & Lussier, 2004; Murphy et al., 2005; Murphy et al., 2012). Murphy, Correia, Colby, and Vuchinich (2005) found that participants in a brief alcohol intervention trial who derived a smaller proportion of their total reinforcement from substance use at baseline reported lower levels of follow-up drinking, even after controlling for their baseline drinking level. Thus, even among heavy drinkers, the presence of enjoyable alternatives to drinking is associated with increased likelihood of change (for examples with other drugs see Audrain-McGovern et al., 2009; Lubman et al 2009). This study also found that participants who reduced their drinking by at least 5 drinks per week showed increased reinforcement from substance-free activities at follow up, suggesting that the change process is supported by increasing engagement in alternative reinforcing activities (Tucker et al., 2002; 2009). A follow-up study found that a behavioral economic session that attempted to increase engagement in substance-free alternatives to drinking enhanced the effects of a standard alcohol-focused intervention (Murphy et al., 2012).
The role of alternative reinforcement and the relative reinforcing value of food versus food-free activities has also received some attention in the eating behaviors and obesity literatures (Best et al., 2012; Foltin, 1991, 1992; Foltin & Fischman, 1988; Temple, Legierski, Giacomelli, Salvy, & Epstein, 2008). Saelens and Epstein (1996) gave a small sample of female college students a choice between consuming snack foods or engaging in sedentary leisure activities (e.g., reading, video games). Obese individuals were more likely to choose food as a reinforcer over engaging in an alternative food-free activity as compared to non-obese individuals, and consumed more calories during the lab task. This operant laboratory paradigm has been modified and replicated using both a hypothetical computerized lab-based task and a self-report questionnaire (Epstein et al., 2003; Goldfield & Epstein, 2002). Best and colleagues (2012) found that baseline relative reinforcing value of food was a predictor of success in a 16-week obesity treatment program in a sample of 241 children ages 7–12. Specifically, they found that children who both had a high relative reinforcing value of food combined with high impulsivity at baseline were less responsive to treatment than children without this combination of risk factors. The goal of the current study is to extend this research by assessing engagement in and enjoyment of food relative to a range of food-free activities over time in the natural environment, and in relation to BMI reduction in an adult sample.
To our knowledge, no studies to date have measured changes in the RR of food versus alternative behaviors in obese adults participating in a behavioral weight loss treatment program. In the current study, we prospectively examine changes in engagement in and enjoyment of food versus food-free activities during an 18-month behavioral weight loss intervention. Consistent with behavioral economic theory and laboratory research (Epstein et al., 2003; Foltin, 1991; Goldfield & Epstein, 2002; Saelens & Epstein, 1996), we hypothesized that: 1) the relative ratio of food-related (vs. food-free) reinforcement would decrease and food-free reinforcement would increase across time points, 2) changes in reinforcement indices would predict greater reductions in BMI over time, and 3) Lower baseline RR would be associated with greater changes in BMI over time. If these hypotheses are supported, it would provide evidence for the relevance of behavioral economic theory to BMI change process among adults and would suggest that obesity treatment programs should measure this construct and attempt to enhance the availability and relative value of food-free activities.
Method
Participants
Two-hundred two overweight/obese (body mass index [BMI] M 34.9 kg/m2; M age 51.30; 92.2% White; 57.8% female) individuals participated in an 18-month behavioral obesity intervention and were randomly assigned to either a traditional hypocaloric, low-fat (1200 – 1500 kcals/day, ≤ 30% kcals from fat) diet condition (Lifestyle) or a traditional hypocaloric, low-fat diet plus a goal to limit variety in snack food consumption (Lifestyle+LV; 60% female, M BMI = 34.91). Eligible participants had a BMI between 27 and 45 kg/m2 and were aged 21 to 65 years. Participants were excluded if they could not walk two blocks; reported heart problems; were using weight loss medications or participating in another weight loss program; had bariatric surgery; were pregnant or planning to become pregnant; were allergic to foods used in the study; and if they consumed less than 5 different types of non-nutrient dense, energy dense foods (see Raynor, Steeves, Hecht, Fava, and Wing (2012) for more details on this IRB approved clinical trial).
Intervention
The two intervention groups contained three common components: a cognitive behavioral intervention, a diet prescription (1200 – 1500 kcals/day, ≤ 30% kcals from fat), and a physical activity prescription (40 minutes/day, 5 times/week). In addition to these components, participants in the Lifestyle+LV group were also asked to reduce the number of different non-nutrient dense, energy-dense foods in their diet to two throughout the intervention period. The intervention was designed to assist participants with developing a healthy lifestyle and to achieve at least 10% weight loss. The intervention spanned 18 months, providing 48, 60-minute group meetings. The meetings occurred weekly from months 1–6, and twice a month from month 7–18.
Measures
Demographics and Anthropometrics
Basic demographic information (e.g., gender, age, education level) was obtained by self-report at baseline only. Height was measured to the nearest millimeter at entry into the trial using a stadiometer and weight was measured at 0, 6, 12, and 18 months, with participants wearing light street clothes with shoes removed using a calibrated digital scale and recorded to the nearest 0.05 kg. BMI was calculated as weight in kg/ height in m2. Percent weight loss was calculated as the following: (amount of weight loss from baseline/baseline weight) * 100.
The Activity Level Questionnaire-Eating Version (ALQ - EV)
The Activity Level Questionnaire-Eating Version (Appendix A) was adapted from reinforcement survey schedules which were originally developed to examine behavioral models of depression (MacPhillamy & Lewinsohn, 1974) and have been previously modified to study reinforcement related to substance-related and substance-free activities (Correia et al., 2005; Murphy et al., 2005). We adapted a measure developed by Sigmon (2010) and included only activities where eating would be practical (e.g., having a conversation with another person, attending a cultural event, reading, playing golf, and going to the park (Sigmon, 2010).
The ALQ-EV asks participants to rate how often they engage in and enjoy each activity both with and without food. Both frequency and enjoyment are rated on a 4-point scale [range=0 (zero times per week) to 4 (more than once per day)] and enjoyment ratings [range=0 (unpleasant or neutral) to 4 (extremely pleasant), respectively]. A cross-product, reflecting “obtained reinforcement” from each activity, was computed by multiplying the frequency and the enjoyment ratings (separately for food-related and food-free participation). The average of the food-related and food-free cross-product scores were computed to measure average food-related and food-free reinforcement for each individual. Finally, the reinforcement ratio (RR) was computed with the following formula: average food related cross product / average food related plus average food-free cross product. RR values can range from 0 (no reinforcement related to food) to 1 (100% of reinforcement related to food). For interpretive clarity, ratio values were multiplied by 100 and interpreted as percent of total reinforcement derived from food-related activities. Note that this is not a direct measure of food reinforcement but instead a proxy measure of reinforcement associated with food-related activities. Internal consistencies for the cross product with food (α = .78) and without food (α = .83) were acceptable in our sample and previous research has indicated that responses to reinforcement surveys are valid and correspond with collateral reports (MacPhillamy & Lewinsohn, 1974).
Procedure
Data were collected by trained research staff blinded to randomization assignment at baseline, 6, 12, and 18 months. At each time point, participants were weighed and completed The Activity Level Questionnaire-Eating Version.
Analytic Plan
The goal of this study was to characterize the nature of and relationships among changes in BMI, Food-Related Reinforcement (FRR), Food-Free Reinforcement (FFR), and the Reinforcement Ratio (RR) during behavioral weight loss treatment. To characterize change and to determine whether significant variability existed in participants’ rates of change and initial values, a random effects approach to growth modeling was used. All models were estimated in Mplus version 7 (Muthén & Muthén, 1998–2013), under missing data theory using all available data and robust (Full Information) maximum likelihood estimation. This strategy is a modern method of modeling with missing data that decreases bias and makes use of all available data points (Little, Jorgensen, Lang, & Moore, in press). As such, models were fit to the data using values for all 204 participants, based on information estimated from N = 204 (time 1), n =197 (6 months), n = 194 (12 months), and n = 189 (18 months). This approach when coupled with robust maximum likelihood estimation also adjusts the standard errors and scales chi-square statistics to account for non-normally distributed data.
Given what appeared to be non-linear relationships between time and all outcomes (e.g. changes from baseline to 6 months, followed either stability or a slight return toward baseline values), unconditional piecewise growth was modeled using three random parameters: intercept (reflecting individual differences in baseline values), slope 1 (reflecting individually varying rates of change during active treatment or baseline to six months), and slope 2 (reflecting individually varying rates of change from 6 to 18 months). Residual variances were constrained to be equal to ensure model identification, and freed if modification indices suggested an improved fit. Non-significant random variances were fixed to zero. Given the interest in weight loss across the entire trial, one additional model was estimated, in which the linear change in BMI from baseline to 18 months was modeled.
In order to determine whether changes in the RR, controlling for baseline BMI, gender, race, and intervention group were related to changes in BMI, three separate regression models were conducted using exported slopes and intercepts from the previous growth models. Given the possibility of a differential effect of RR change on outcomes due to initial BMI and initial RR, the baseline BMI by RR change and baseline RR by RR change interactions were included in the model. These three regression models had different dependent variables: BMI Change from 0 to 6 months, 6 to 18 months, and 0 to 18 months. This approach is equivalent to modeling change scores (e.g., the BMI change score and BMI slope 1: r = 1.0).
Results
The average percent of initial weight lost was 10.9% at 6 months and 9.7% at 18 months, with no between group differences (Raynor, Steeves, Hecht, Fava, & Wing, 2012). There was a 6% attrition rate at 18 months; analyses were run only on participants with complete data at each follow-up.
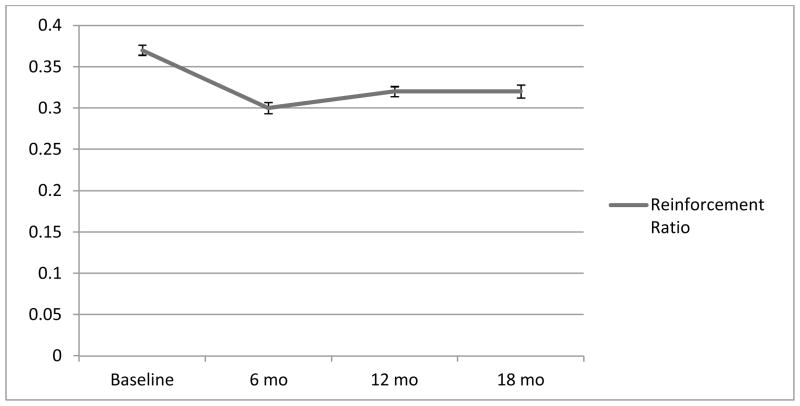
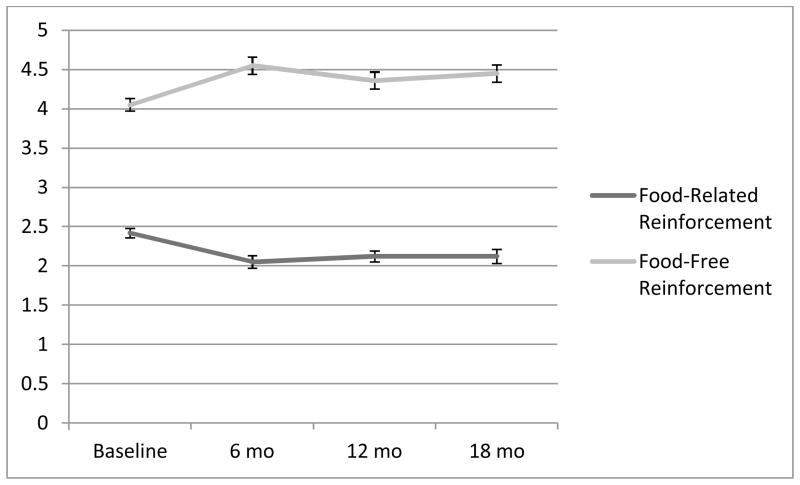
Table 1 presents the results of the growth models. BMI decreased significantly from zero to six months then increased slightly from 6 to 18 months, with significantly variability in these changes. With regard to linear BMI changes from 0 to 18 months, participants BMIs’ significantly decreased with significantly variability in these changes. The RR followed the same pattern as BMI values. More specifically, on average participants derived 37.9% of reinforcement from food-related activities at baseline (M=0.38, SD=0.10), and this decreased to 30.3% at 6 months (M=0.30, SD=0.10) before increasing slightly to 32.3% at 12 (M=0.32, SD=0.09) and 32.1% at 18 months (M=0.32, SD=0.10; Figure 1). When examining the change in the components of the reinforcement ratio (RR), food-free reinforcement (FFR) increased and food-related reinforcement (FRR) decreased significantly from zero (M=4.06, SD=1.40; M=2.50, SD=1.08, respectively) to six months (M=4.55, SD=1.43; M=2.04, SD=1.14, respectively, with significantly variability in these changes; Figure 2). Both FFR and FRR remained stable with no changes or significant variability from six (M=4.36, SD=1.46; M=2.11, SD=1.03, respectively) to 18 months (M=4.45, SD=1.50; M=2.19, SD=1.20, respectively).
Table 1.
Parameter estimates and inferential statistics for the growth models
| Model: BMI 1 | Estimate | S.E. | Est./S.E. | P-Value |
|---|---|---|---|---|
| Covariances | ||||
| Slope 1 with Intercept | −0.38 | 0.12 | −3.21 | 0.001 |
| Slope 2 with Intercept | 0.04 | 0.06 | 0.70 | 0.48 |
| Slope 1 with Slope 2 | 0.02 | 0.006 | 2.98 | 0.003 |
| Means | ||||
| Intercept | 34.92 | 0.30 | 115.49 | <0.001 |
| Slope 1 | −0.65 | 0.03 | −24.57 | <0.001 |
| Slope 2 | 0.04 | 0.01 | 2.90 | 0.004 |
| Variances | ||||
| Intercept | 18.05 | 1.45 | 12.44 | <0.001 |
| Slope 1 | 0.11 | 0.02 | 6.21 | <0.001 |
| Slope 2 | 0.02 | 0.006 | 3.66 | <0.001 |
| Residual Variances | ||||
| BMI 0, 6, 12, and 18 | 0.60 | 0.08 | 7.90 | <0.001 |
|
| ||||
| Model: BMI 2 | Estimate | S.E. | Est./S.E. | P-Value |
|
| ||||
| Covariances | ||||
| Slope 1 with Intercept | −0.48 | 0.14 | −3.45 | 0.001 |
| Slope 2 with Intercept | 0.04 | 0.06 | 0.71 | 0.48 |
| Slope 1 with Slope 2 | 0.01 | 0.01 | 0.63 | 0.53 |
| Means | ||||
| Intercept | 34.92 | 0.30 | 115.49 | <0.001 |
| Slope 1 | −0.64 | 0.03 | −22.45 | <0.001 |
| Slope 2 | 0.04 | 0.01 | 2.81 | 0.01 |
| Variances | ||||
| Intercept | 18.61 | 1.54 | 12.06 | <0.001 |
| Slope 1 | 0.13 | 0.03 | 4.78 | <0.001 |
| Slope 2 | 0.03 | 0.01 | 2.49 | 0.01 |
| Residual Variances | ||||
| BMI 0, 6, and 18 | 0.04 | 0.56 | 0.07 | 0.94 |
| BMI12B | 0.89 | 0.22 | 4.10 | 0.00 |
|
| ||||
| Model: BMI Change Baseline to 18 months | Estimate | S.E. | Est./S.E. | P-Value |
|
| ||||
| Covariances | ||||
| Slope with intercept | −0.14 | 0.06 | −2.27 | 0.02 |
| Means | ||||
| Intercept | 34.92 | 0.30 | 115.49 | <0.001 |
| Slope | −0.20 | 0.01 | −15.97 | <0.001 |
| Variances | ||||
| Intercept | 18.65 | 1.46 | 12.75 | <0.001 |
| Slope 1 | ||||
| Residual Variances | ||||
| BMI 0 and 18 (fixed to zero) | 0 | N/A | N/A | N/A |
|
| ||||
| Model: Reinforcement Ratio (RR) | Estimate | S.E. | Est./S.E. | P-Value |
|
| ||||
| Covariances | ||||
| Slope 1 with intercept | <0.001 | <0.001 | −2.36 | 0.02 |
| Slope 2 with Intercept | <0.001 | <0.001 | 2.62 | 0.01 |
| Slope 1 with Slope 2 | <0.001 | <0.001 | −3.22 | 0.001 |
| Means | ||||
| Intercept | 0.38 | 0.01 | 53.27 | <0.001 |
| Slope 1 | −0.01 | 0.001 | −9.33 | <0.001 |
| Slope 2 | 0.001 | 0.001 | 2.67 | 0.01 |
| Variances | ||||
| Intercept | 0.01 | 0.001 | 6.10 | <0.001 |
| Slope 1 | <0.001 | <0.001 | 3.24 | 0.001 |
| Slope 2 | <0.001 | <0.001 | 10.74 | <0.001 |
| Residual Variances | ||||
| RR 0, 6, 12, and 18 | 0.004 | 0.000 | 46.870 | <0.001 |
|
| ||||
| Model: Food Related Reinforcement (FRR) | Estimate | S.E. | Est./S.E. | P-Value |
|
| ||||
| Covariances | ||||
| Slope 1 with Intercept | −0.04 | 0.02 | −1.85 | 0.10 |
| Slope 2 with Intercept | 0.01 | 0.01 | 1.20 | 0.23 |
| Slope 1 with Slope 2 | −0.003 | 0.003 | −0.98 | 0.33 |
| Means | ||||
| Intercept | 2.48 | 0.08 | 32.45 | <0.001 |
| Slope 1 | −0.08 | 0.01 | −6.18 | <0.001 |
| Slope 2 | 0.01 | 0.01 | 1.88 | 0.06 |
| Variances | ||||
| Intercept | 0.87 | 0.14 | 6.23 | <0.001 |
| Slope 1 | 0.01 | 0.01 | 2.12 | 0.03 |
| Slope 2 | 0.002 | 0.002 | 1.19 | 0.24 |
| Residual Variances | ||||
| FRR 0, 6, 12, and 18 | 0.30 | 0.03 | 8.40 | <0.001 |
|
| ||||
| Model: FRR 2 | Estimate | S.E. | Est./S.E. | P-Value |
|
| ||||
| Covariances | ||||
| Slope 1 with Intercept | −0.02 | 0.02 | −0.78 | 0.43 |
| Means | ||||
| Intercept | 2.48 | 0.08 | 32.46 | <0.001 |
| Slope 1 | −0.08 | 0.01 | −6.03 | <0.001 |
| Slope 2 | 0.01 | 0.01 | 1.76 | 0.08 |
| Variances | ||||
| Intercept | 0.81 | 0.15 | 5.36 | <0.001 |
| Slope 1 | 0.01 | 0.003 | 2.22 | 0.027 |
| Slope 2 | 0.00 | 0.00 | n/a | n/a |
| Residual Variances | ||||
| FRR 0, 6, 12, and 18 | 0.36 | 0.06 | 5.71 | 0.000 |
|
| ||||
| Model: Food-Free Reinforcement (FFR) | Estimate | S.E. | Est./S.E. | P-Value |
|
| ||||
| Covariances | ||||
| Slope 1 with Intercept | −0.05 | 0.04 | −1.37 | 0.17 |
| Slope 2 with Intercept | 0.01 | 0.01 | 0.73 | 0.47 |
| Slope 1 with Slope 2 | −0.001 | 0.004 | −0.24 | 0.81 |
| Means | ||||
| Intercept | 4.03 | 0.10 | 40.78 | <0.001 |
| Slope 1 | 0.08 | 0.017 | 4.73 | <0.001 |
| Slope 2 | −0.01 | 0.007 | −1.33 | 0.19 |
| Variances | ||||
| Intercept | 1.30 | 0.243 | 5.35 | <0.001 |
| Slope 1 | 0.02 | 0.009 | 1.80 | 0.07 |
| Slope 2 | 0.001 | 0.002 | 0.33 | 0.74 |
| Residual Variances | ||||
| FFR 0, 6, 12, and 18 mo | 0.68 | 0.09 | 7.66 | <0.001 |
|
| ||||
| Model: FFR 2 | Estimate | S.E. | Est./S.E. | P-Value |
|
| ||||
| Covariances | ||||
| Slope 1 with Intercept | −0.04 | 0.03 | −1.15 | 0.25 |
| Means | ||||
| Intercept | 4.02 | 0.10 | 40.68 | <0.001 |
| Slope 1 | 0.08 | 0.02 | 4.70 | <0.001 |
| Slope 2 | −0.01 | 0.01 | −1.29 | 0.20 |
| Variances | ||||
| Intercept | 1.29 | 0.24 | 5.27 | <0.001 |
| Slope 1 | 0.02 | 0.01 | 2.38 | 0.02 |
| Slope 2 (fixed at 0) | 0.00 | 0.00 | N/A | N/A |
| Residual Variances | ||||
| FFR 0, 6, 12, and 18 mo | 0.70 | 0.09 | 8.25 | <0.001 |
Note. FFR = food-free reinforcement; FRR = food related reinforcement; Slope 1 = change from 0 to 6 months; slope 2 = change from 6 to 18 months; intercept = baseline value.
Figure 1.
Change in Reinforcement Ratio from Baseline to 18 months
Note. The Y-axis contains the average reinforcement ratio (RR) values. The RRs were computed with the following formula: average food-related cross product / average food-related plus average food-free cross product. RR values can range from 0 (no reinforcement related to food) to 1 (100% of reinforcement related to food) Error bars reflect one standard error of the mean.
Figure 2.
Change in Food and Food-Free Reinforcement from Baseline to 18 months
Note. The Y-axis contains the average food-related and food-free reinforcement values. Both frequency and enjoyment are rated on a 4 point scale [range=0 (zero times per week) to 4 (more than once per day)] and enjoyment ratings [range=0 (unpleasant or neutral) to 4 (extremely pleasant), respectively]. A cross-product, reflecting “obtained reinforcement” from each activity, was computed by multiplying the frequency and the enjoyment ratings (separately for food-related and food-free participation). The average of the food-related and food-free cross-product scores were computed to measure average food-related and food-free reinforcement for each individual. Error bars reflect one standard error of the mean.
The three regression models presented in Tables 2–4 accounted for 17.53% (F [8,195] = 5.18, p < 0.001) of the variance in BMI change from 0 to 6 months, 2.16% (F [8,195] = 0.54, p = 0.83) of BMI change from 6 to 18 months, and 8.78% (F [6,195] = 2.35, p = 0.02) of BMI change from 0 to 18 months respectively. Both initial BMI and change in the RR predicted change in BMI from 0 to 6 months, such that those with a higher initial BMI had steeper declines in BMI, and that changes in the RR moved in tandem with change in BMI (decreases in RR associated with decreases in BMI, and increases in RR associated with increases in BMI). The significant Baseline RR by Change in RR interaction revealed that the rate of change in RR was a significant predictor of BMI change for those with an initial RR one standard deviation below the mean (b = 21.84, t = 3.69, p = 0.003) and at or around the mean (b = 12.27, t = 3.07, p = 0.0025), but not for those at one standard deviation above the mean (b = 2.69, t = 0.67, p = 0.50). No predictors were found for changes in BMI from 6 to 18 months. Baseline RR alone did not predict changes in BMI at any time point.
Table 2.
Predicting BMI Change from Baseline to 6 Months
| b | SE | t | p | |
|---|---|---|---|---|
| Constant | −0.54 | 0.20 | −2.66 | 0.0084 |
| Gender | 0.05 | 0.05 | 1.39 | 0.17 |
| Race | −0.04 | 0.04 | −1.04 | 0.30 |
| Treatment Group | 0.02 | 0.05 | 0.44 | 0.66 |
| Baseline RR | 0.41 | 0.37 | 1.12 | 0.26 |
| RRΔ | 12.27 | 4.00 | 3.07 | 0.0025 |
| Baseline BMI | −0.02 | 0.01 | −4.00 | 0.0001 |
| RRΔ X Baseline BMI | 0.74 | 0.83 | 0.89 | 0.375 |
| RRΔ X Baseline RR | −147.05 | 47.35 | −3.11 | 0.0022 |
Note. RR = reinforcement ratio
Table 4.
Predicting BMI Change from Zero to 18 Months
| b | SE | t | p | |
|---|---|---|---|---|
| Constant | −0.138 | 0.101 | −1.370 | 0.172 |
| Gender | 0.007 | 0.024 | 0.294 | 0.769 |
| Race | −0.016 | 0.017 | −0.922 | 0.358 |
| Treatment Group | 0.007 | 0.023 | 0.028 | 0.977 |
| Baseline RR | 0.067 | 0.183 | 0.365 | 0.715 |
| RRΔ | 4.857 | 1.991 | 2.439 | 0.016 |
| Baseline BMI | −0.006 | 0.003 | −2.288 | 0.023 |
| RRΔ X Baseline BMI | 0.667 | 0.414 | 1.610 | 0.109 |
| RRΔ X Baseline RR | −49.460 | 23.590 | −2.097 | 0.037 |
Note. RR = reinforcement ratio
In the model examining changes across the trial (BMI Change from 0 to 18 months) a similar pattern of results was found. Both initial BMI and change in the RR predicted change in BMI from 0 to 18 months, such that those with a higher initial BMI had steeper declines in BMI, and that changes in the RR moved in tandem with change in BMI. The significant Baseline RR by Change in RR interaction revealed that the rate of change in RR was a significant predictor of BMI change for those with an initial RR one standard deviation below the mean (b = 8.08, t = 2.74, p = 0.007) and at or around the mean (b = 4.86, t = 2.44, p = 0.016), but not for those at one standard deviation above the mean (b = 1.64, t = 0.82, p = 0.41). Intervention group did not interact with (nor predict) the Rate of Change in the reinforcement ratio to predict BMI change 0 to 6 months (b = .91, t =.3, p = .90), 6 to 18 months (b = −2.69, t = −.73, p = .46), or 0 to 18 months (b = −1.24, t = −.35, p = .73).
Discussion
The current study prospectively examined changes in the relative reinforcing value of food throughout the course of an 18-month behavioral weight loss intervention (Raynor et al., 2012). As hypothesized, the relative percentage of food-related (vs. food-free) reinforcement decreased across time points, in conjunction with decreases in BMI. The change in the ratio variable reflected both a decrease in food-related reinforcement and an increase in food-free reinforcement. Thus, consistent with predictions from behavioral economic theory, the behavior of participants undergoing behavioral weight loss treatment tends to become relatively less reinforced by food-related activities, and more reinforced by food-free activities, and this shift is associated with changes in BMI. Baseline RR itself did not predict changes in BMI; however the change in the RR occurred during the first 6 months of treatment and generally persisted for the 18-month treatment period. Initial BMI and changes in the RR predicted change in BMI from 0 to 6 months and 0 to18 months, such that those with a higher BMI at baseline had a steeper decline in BMI, and these BMI changes corresponded with changes in the RR of food. In other words, greater decreases in RR were associated with greater decreases in BMI, and increases in RR were associated with increases in BMI. The significant Baseline RR by Change in RR interaction revealed that the rate of change in RR was a significant predictor of BMI change for those with an initial RR one standard deviation below the mean and at the mean, but not for those at one standard deviation above the mean. Thus, the relative value of food reinforcement compared to food-free reinforcement may be a particularly salient mechanism across the length of the entire trial for those with higher BMIs and lower or average RR at baseline. For individuals with higher BMI and a typical or low proportion of food-related reinforcement at baseline, it may be relatively feasible and especially important to develop enjoyable alternatives to eating during the course of weight loss treatment, as this practice may lead to subsequent weight loss. Change in RR may be less relevant for individuals with high relative food related reinforcement at baseline. The relative value of food may be so high that there is little meaningful variability in relative reinforcement that might predict change. The individuals may have physical limitations associated with obesity that may limit engagement in many reinforcing food-free activities that might serve as substitutes for food-related reinforcement. Or their social context may be very food-focused such that nearly all of their meaningful social/leisure activities are focused around eating. Similar to the vicious cycle evident in substance addictions wherein repeated drug use reduces the availability and potency of drug-free reinforcers (Koob, 2006; Rachlin, 1997), overeating may become a self-perpetuating cycle wherein food becomes one of the only reinforcers capable of maintaining high rates of behavior. Yet, our overall results indicate that for the majority of our sample the relative reliance on food as a reinforcer is malleable, and that an increase in participation in and enjoyment related to food-free activities may be an especially important element of BMI changes for relatively more overweight individuals participating in weight loss treatment.
The finding that changes in BMI occur in the context of changes in engagement in food-free activity participation parallels findings from the substance abuse literature indicating that successful recovery from alcohol or drug problems entails a decrease in the relative value of drug-related relative to drug free reinforcers (Audrain-McGovern et al., 2009; Lubman et al 2009; Murphy et al., 2005; 2012; Tucker et al., 2002; 2009). This is also consistent with basic laboratory research conducted with a variety of species and reinforcers (including both food and drugs) indicating that relative levels of preference for a given reinforcer depend critically on the availability of alternative reinforcers (Vuchinich & Tucker, 1988; Foltin, 1991, 1992; Herrnstein, 1990; Higgins et al., 2004). The ability to reduce food intake may be facilitated by developing other relatively pleasurable ways of spending time. In the absence of meaningful alternatives, reductions in BMI may entail a more herculean exercise in self-control that may prove difficult for many dieters (Best et al., 2012). Thus, these results extend behavioral economics, which has previously been profitably applied to laboratory research on food choice (Foltin & Fischman, 1988; Saelens & Epstein, 1996), to applied clinical research on BMI change.
It is unclear whether the shift towards greater reinforcement of food-free activities is a direct result of the intervention or part of the naturalistic process of weight loss. There was no differential impact of the three interactions included in this trial with relative food-related reinforcement. Studies of natural recovery from alcohol problems suggest that individuals who successful reduce drinking shift their behavior towards substance-free activities even without formal treatment (Tucker et al., 2004, 2005). Although the intervention did not explicitly target engagement in alternative reinforcing activities, as many addiction treatments do (Higgins et al., 2004), the program did cover cognitive-behavioral topics such as stimulus control, problem-solving, preplanning, developing social support, and relapse prevention which may have promoted a shift away from food and toward achieving a reduced BMI. Behavioral economic theory refers to this shift as behavioral substitution (Epstein, Salvy, Carr, Dearing, & Bickel, 2010b). The present results suggest that individuals who develop effective substitutes for food-related reinforcement during the first 6-months of weight loss treatment are more likely to reduce their BMI during this period. These findings may have particular implications for weight maintenance. Although intensive behavioral weight loss interventions (as implemented in this study) have been shown to result in about a 10% weight loss (Look AHEAD, 2007), maintenance beyond 6 months post-intervention remains a challenge. The effects of baseline BMI and RR reported here suggest that changes in the relative value of food reinforcement compared to total reinforcement might have important implications for individuals with higher BMIs and baseline RR over the length of longer trial. Given that food restriction tends to be associated with acute increases in the subjective value of food reinforcement post-treatment (Epstein et al., 2003; Raynor & Epstein, 2003), this might be an ideal time to intervene by targeting increased engagement in enjoyable, food-free activities. In the substance abuse treatment literature, there is evidence that brief behavioral economic interventions that attempt to increase participation in constructive and values-driven substance-free activities are more effective than standard brief interventions (Murphy et al., 2012). Dieters may benefit from a similar intervention approach that identifies and attempts to increase food-free behaviors that are associated with important goals and values. For example, targeting sedentary leisure time for more active, but equally enjoyable activities might decrease available time for snaking on high calorie dense foods.
To our knowledge, this study is the first to investigate changes in food-related and food-free reinforcement in the context of an 18-month intensive behavioral weight loss trial among adults. Our study improves upon previous behavioral economic studies in this area by assessing actual past month behavior and enjoyment of a wide range of activities in a treatment-seeking sample. Limitations of the study include its relatively homogeneous sample in terms of ethnicity and the use of a novel measure of RR. However, similar versions of this questionnaire have been used in the depression (MacPhillamy & Lewinsohn, 1974) and addiction literatures (Murphy et al., 2005) with strong validity, and the internal consistency in our sample was acceptable. Additionally, our measure of food reinforcement was not an operant measure, but rather a self-report proxy measure of the extent to which food-related activities account for a large proportion of an individual’s enjoyable activities.
Findings from the current study provide support for a behavioral economic approach to understanding obesity and also suggest that weight loss treatment may promote a shift away from food-related reinforcement towards food-free reinforcement. Future treatment interventions may consider explicitly targeting increasing engagement in food-free enjoyable activities to help reduce and maintain BMI over time.
Table 3.
Predicting BMI Change from 6 to 18 Months
| b | SE | t | p | |
|---|---|---|---|---|
| Constant | 0.063 | 0.10 | 0.61 | 0.55 |
| Gender | −0.03 | 0.02 | −1.04 | 0.30 |
| Race | 0.01 | 0.18 | 0.10 | 0.92 |
| Treatment Group | 0.01 | 0.02 | 0.05 | 0.96 |
| Baseline RR | 0.02 | 0.19 | 0.10 | 0.92 |
| RRΔ | −0.14 | 2.05 | −0.07 | 0.95 |
| Baseline BMI | 0.01 | 0.01 | −0.67 | 0.95 |
| RRΔ X Baseline BMI | 0.26 | 0.43 | 0.60 | 0.55 |
| RRΔ X Baseline RR | −31.37 | 24.27 | −1.29 | 0.20 |
Note. RR = reinforcement ratio
Acknowledgments
This research was supported by the National Institute for Diabetes and Digestive and Kidney Diseases under grant number R01 DK074721 (PI: Hollie A. Raynor) and by the National Cancer Institute under Award Number R25CA057699. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A: Activity Level Questionnaire-Eating Version
Activity Level Questionnaire-Eating Version
Instructions
The following questions are about your participation in a number of different activities over the last 28 days. We are interested in how often you participated in each activity and did not eat during the activity, and how often you participated in each activity and ate while doing the activity. We are also interested in how enjoyable or pleasant each activity has been for you over the past month. If you have experienced an activity more than once in the past month, try to rate how pleasant it was on the average. We will ask you to make separate enjoyment ratings for times you engaged in the activity without eating and times you engaged in the activity while eating, so in some cases, you may have answers in both columns. If you did not engage in the activity, you may leave the enjoyment column blank. You will make the frequency and enjoyment ratings using these ratings.
Rate the frequency and enjoyment of each item over the past 28 days
| Frequency | Enjoyment |
|---|---|
| 0 = 0 times in past month | 0 = unpleasant or neutral |
| 1 = once a week or less | 1 = mildly pleasant |
| 2 = 2–4 times per week | 2 = moderately pleasant |
| 3 = about once a day | 3 = very pleasant |
| 4 = more than once day | 4 = extremely pleasant |
| Frequency without eating | Enjoyment without eating | Frequency with eating | Enjoyment with eating | |
|---|---|---|---|---|
| 1. Having a conversation with another person | ||||
| 2. Attending a cultural event (e.g., concert, theatre, performance, art show). | ||||
| 3. Dancing | ||||
| 4. Gardening | ||||
| 5. Shopping | ||||
| 6. Performing a hobby (e.g., painting, crafts, woodwork, photography). | ||||
| 7. Reading | ||||
| 8. Driving | ||||
| 9. Watching television or a video tape/DVD | ||||
| 10. Attending a sporting event | ||||
| 11. Going to a club or bar | ||||
| 12. Meditating | ||||
| 13. Doing crosswords or other puzzles | ||||
| 14. Taking a walk | ||||
| 15. Going to a religious service/meeting | ||||
| 16. Spending time with family | ||||
| 17. Writing letters or emails | ||||
| 18. Spending time with friends | ||||
| 19. Playing card games, board games, or video games with other people | ||||
| 20. Listening to music/the radio | ||||
| 21. Going to the movies | ||||
| 22. Daydreaming | ||||
| 23. Going to a party | ||||
| 24. Sitting in the sun | ||||
| 25. Rearranging, redecorating, or doing other jobs around the house | ||||
| 26. Playing golf | ||||
| 27. Being alone | ||||
| 28. Doing housework or cleaning things | ||||
| 29. Participating in a social or civic organization or club | ||||
| 30. Talking on the phone | ||||
| 31. Cooking | ||||
| 32. Having coffee/tea | ||||
| 33. Bowling | ||||
| 34. Caring for or playing with pets | ||||
| 35. Relaxing | ||||
| 36. Being at the beach | ||||
| 37. Going to the park |
Contributor Information
Joanna Buscemi, Institute for Health Research and Policy, University of Illinois at Chicago.
James G. Murphy, Department of Psychology, The University of Memphis
Kristoffer S. Berlin, Department of Psychology, The University of Memphis and Department of Pediatrics, University of Tennessee Health Science Center
Hollie A. Raynor, Department of Nutrition, University of Tennessee
References
- Audrain-McGovern J, Stevens S, Murray PJ, Kinsman S, Zuckoff A, Pletcher J, Wileyto EP. The efficacy of motivational interviewing versus brief advice for adolescent smoking behavior change. Pediatrics. 2011;128(1):e101–e111. doi: 10.1542/peds.2010-2174. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, Wilfley DE. Behavioral economic predictors of overweight children’s weight loss. J Consult Clin Psychol. 2012;80(6):1086–1096. doi: 10.1037/a0029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89(6):2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- Correia CJ, Benson TA, Carey KB. Decreased substance use following increases in alternative behaviors: A preliminary investigation. Addict Behav. 2005;30(1):19–27. doi: 10.1016/j.addbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Correia CJ, Carey KB, Simons J, Borsari BE. Relationships between binge drinking and substance-free reinforcement in a sample of college students - A preliminary investigation. Addict Behav. 2003;28(2):361–368. doi: 10.1016/s0306-4603(01)00229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia CJ, Simons J, Carey KB, Borsari BE. Predicting drug use: application of behavioral theories of choice. Addict Behav. 1998;23(5):705–709. [PubMed] [Google Scholar]
- Del Parigi A, Chen KW, Salbe AD, Reiman EM, Tataranni PA. Are we addicted to food? Obesity Research. 2003;11(4):493–495. doi: 10.1038/oby.2003.68. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saelens BE. Behavioral economics of obesity: Food intake and energy expenditure. In: Bickel WK, Vuchinich RE, editors. Reframing health behavior change with behavioral economics. Mahwah, N.J: Lawrence Erlbaum; 2000. pp. 293–311. [Google Scholar]
- Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiology & Behavior. 2010a;100(5):438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiology & Behavior. 2010b;100(5):438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121(5):877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiology & Behavior. 2003;78(2):221–227. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wright SM, Paluch RA, Leddy J, Hawk LW, Jr, Jaroni JL, Lerman C. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiology & Behavior. 2004;81(3):511–517. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and Trends in Obesity Among US Adults, 1999–2008. Jama-Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Foltin RW. An economic analysis of “demand” for food in baboons. J Exp Anal Behav. 1991;56(3):445–454. doi: 10.1901/jeab.1991.56-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW. Economic analysis of the effects of caloric alternatives and reinforcer magnitude on “demand” for food in baboons. Appetite. 1992;19(3):255–271. doi: 10.1016/0195-6663(92)90166-4. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The effects of varying procurement costs on food intake in baboons. Physiology & Behavior. 1988;43(4):493–499. doi: 10.1016/0031-9384(88)90124-2. [DOI] [PubMed] [Google Scholar]
- Goldfield GS, Epstein LH. Can fruits and vegetables and activities substitute for snack foods? Health Psychology. 2002;21(3):299–303. [PubMed] [Google Scholar]
- Herrnstein RJ. Rational choice theory: Necessary but not sufficient. American Psychologist. 1990;45(3):356–367. [Google Scholar]
- Heinz AJ, Lilje TC, Kassel JD, de Wit H. Quantifying Reinforcement Value and Demand for Psychoactive Substances in Humans. Current Drug Abuse Reviews. 2012;5(4):257. doi: 10.2174/1874473711205040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annual Review of Psychology. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, Heymsfield SB. Discrepancy between Self-Reported and Actual Caloric-Intake and Exercise in Obese Subjects. New England Journal of Medicine. 1992;327(27):1893–1898. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]
- Little TD, Jorgensen TD, Lang KM, Moore WG. On the joys of missing data. Journal of Pediatric Psychology. doi: 10.1093/jpepsy/jst048. in press. [DOI] [PubMed] [Google Scholar]
- Look AHEAD Research Group. Reduction in weight in cardiovascular risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JWL, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: Associations with later heroin use. Archives of General Psychiatry. 2009;66 (2):205–213. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Monti PM, Ray LA, Murphy JG, Rohsenow DJ, Gwaltney CJ. Alcohol Demand, Delayed Reward Discounting, and Craving in Relation to Drinking and Alcohol Use Disorders. J Abnorm Psychol. 2010;119(1):106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhillamy DJ, Lewinsohn PM. Depression as a Function of Levels of Desired and Obtained Pleasure. J Abnorm Psychol. 1974;83(6):651–657. doi: 10.1037/h0037467. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Correia CJ, Barnett NP. Behavioral economic approaches to reduce college student drinking. Addict Behav. 2007;32(11):2573–2585. doi: 10.1016/j.addbeh.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Correia CJ, Colby SM, Vuchinich RE. Using behavioral theories of choice to predict drinking outcomes following a brief intervention. Experimental and Clinical Psychopharmacology. 2005;13(2):93–101. doi: 10.1037/1064-1297.13.2.93. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Dennhardt AA, Skidmore JR, Borsari B, Barnett NP, Colby SM, Martens MP. A Randomized Controlled Trial of a Behavioral Economic Supplement to Brief Motivational Interventions for College Drinking. J Consult Clin Psychol. 2012;80(5):876–86. doi: 10.1037/a0028763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA. Reliability and validity of a demand curve measure of alcohol reinforcement. Exp Clin Psychopharmacol. 2009;17(6):396–404. doi: 10.1037/a0017684. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén; 1998–2013. [Google Scholar]
- Pi-Sunyer FX. The medical risks of obesity. Obes Surg. 2002;12(Suppl 1):6S–11S. doi: 10.1007/BF03342140. [DOI] [PubMed] [Google Scholar]
- Rachlin H. Judgment, Decision and Choice - Rachlin,H. Journal of Mathematical Psychology. 1992;36(2):318–320. [Google Scholar]
- Rachlin H. Four teleological theories of addiction. Psychonomic Bulletin & Review. 1997;4(4):462–473. [Google Scholar]
- Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40(1):15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Steeves EA, Hecht J, Fava JL, Wing RR. Limiting variety in non-nutrient-dense, energy-dense foods during a lifestyle intervention: a randomized controlled trial. American Journal of Clinical Nutrition. 2012;95(6):1305–1314. doi: 10.3945/ajcn.111.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27(1):41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61(2):117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Sigmon ST, Schartel JG, Boulard NE, Thrope GL. Activity Level, Activity Enjoyment, and Weather as Mediators of Physical Health Risks in Seasonal and Nonseasonal Depression. Journal of Rational Emotive Behavior Therapy. 2010;28:42–56. [Google Scholar]
- Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. American Journal of Clinical Nutrition. 2008;87(5):1121–1127. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Roth DL, Vignolo MJ, Westfall AO. A behavioral economic reward index predicts drinking resolutions: moderation revisited and compared with other outcomes. J Consult Clin Psychol. 2009;77(2):219–228. doi: 10.1037/a0014968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Gladsjo JA. Environmental events surrounding natural recovery from alcohol-related problems. Journal of Studies on Alcohol. 1994;55:401–411. doi: 10.15288/jsa.1994.55.401. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Pukish MA. Molar environmental contexts surrounding recovery from alcohol problems by treated and untreated problem drinkers. Experimental and Clinical Psychopharmacology. 1995;3:195–204. [Google Scholar]
- Tucker JA, Vuchinich RE, Rippens PD. Predicting natural resolution of alcohol-related problems: A prospective behavioral economic analysis. Experimental and Clinical Psychopharmacology. 2002;10(3):248–257. doi: 10.1037//1064-1297.10.3.248. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Heather N. Choice, behavioral economics, and addiction. Amsterdam; Boston: Pergamon; 2003. [Google Scholar]
- Vuchinich RE, Tucker JA. Contributions from behavioral theories of choice to an analysis of alcohol abuse. J Abnorm Psychol. 1988;97(2):181–195. doi: 10.1037//0021-843x.97.2.181. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]