Abstract
Objective
This study examined the relation between self-reported emotional eating scores and frontolimbic brain response to palatable taste in adolescents.
Design and Methods
Participants included 162 adolescents (Mean BMI percentile = 52.7, range 3–90). Participants completed a selfreport survey assessing emotional eating and underwent functional magnetic resonance imaging (fMRI) while viewing pictures signaling subsequent delivery of a chocolate milkshake or a control taste and receiving the corresponding taste.
Results
Results revealed no significant relation between emotional eating scores and brain response to anticipation of receipt of milkshake. In response to milkshake taste receipt, emotional eating scores were negatively related to activation in the right thalamus, the left insula and orbitofrontal cortex, and bilateral putamen and caudate. These findings remained significant after controlling for body mass index and body fat percentage.
Conclusions
The current results are discussed in the context of findings of reduced reward activation to palatable taste receipt in obese adults and adolescents.
Keywords: emotional eating, obesity, fMRI, reward
Introduction
The obesity epidemic has great consequences for our society with regard to both health and economics (1). Emotional eating, a tendency to eat in response to negative emotions, predicts binge eating, which predicts obesity (2). Although not a primary factor in obesity development, emotional eating is associated with reduced weight loss (3) weight regain following bariatric surgery (4) and depression and anxiety (2, 5). Overweight and obese children score higher on emotional eating surveys than normal weight children (6), and overweight and obese adults are more likely than normal weight adults to engage in emotional eating (7), although this effect has not been found consistently (8).
Although researchers have described emotional eating as a natural response to stress, there seem to be individual differences with regard to eating in response to stress or emotions (9). Furthermore, there is no specific emotion that seems to lead to eating, and literature describes eating in response to stress and a broad range of emotions (10). While some studies report that most people tend to eat less in response to stress (11), others find that a majority eat more when stressed (12, 13). Although there may be important differences between stress-related eating and emotional eating, there is much overlap. Indeed, the biological processes that occur during stress (e.g., increases in certain hormones) are also present during emotions such as irritability and anxiety. Thus, this manuscript describes findings on both stress-related and emotional eating.
Studies have shown increased food consumption among individuals with high scores on emotional eating under conditions of negative mood or stress (e.g., 14, 15). Animal studies have also investigated the influence of stress on eating and use stress-inducing situations since emotions are not clearly understood in animals. Rats eat less in response to stress under some conditions (e.g., food restriction (16)), but increase food intake under other conditions (e.g., presence of highly palatable foods). The observed eating behaviors in rats in response to food restriction is congruent with dietary restraint models of binge eating showing that individuals who attempt to restrain their food intake generally eat more in response to stress or negative mood (17–20). Although stress in rats may not translate precisely to emotions that trigger eating in humans, this model has particular relevance for individuals at risk for obesity in today’s environment. Individuals at familial risk for obesity may have motivation to engage in restrictive diets to prevent weight gain. This could be difficult to sustain over time for some individuals because of difficulties controlling eating in the context of food restriction and stress. The reward value of food may be relevant to understand how emotions and stress lead to increased food intake.
The presence of highly palatable food significantly increased food intake after stress and food restriction in rats (21). Higher reward sensitivity among emotional eaters would implicate a higher likelihood to be tempted by attractive foods, which could lead to eating in response to emotions and stress.. Self-reported sensitivity to reward is related to emotional eating and BMI in a sample of higher weight women (22).
Research on brain function in emotional eating is minimal. One study examined brain function related to stress eating and hunger (23). Selecting food choices in a stress condition was associated with decreased activation in the putamen, orbitofrontal cortex (OFC), amygdala, cingulate cortex, and hippocampus. The sample had low emotional eating scores, so stress response could have been variable among the nine participants.
Another study examined dopamine receptor availability among ten individuals and found that emotional eating was negatively correlated with D2 receptors in the dorsal striatum (24). These findings are similar to findings of reduced dopamine receptor availability in obese individuals (25) and provide support for hypoactivation of reward circuitry in emotional eaters. In contrast, a recent study of electrocortical activity while viewing food images revealed an enhanced late positive potential (LPP) over parieto-occipital regions among high emotional eaters (26). The authors interpreted this as high motivational relevance of food to those who engage in emotional eating. This effect was present regardless of mood and therefore may reflect an underlying propensity.
We previously examined neural response to food images and tastes in emotional eaters and found differential effects dependent on mood. During a negative mood state in emotional eaters, we found heightened activations in the parahippocampal gyrus and anterior cingulate cortex (ACC) in response to anticipation of a pleasurable taste and heightened activations in the thalamus, pallidum, and ACC in response to receipt of the taste (27). Opposite effects were found for non-emotional eaters: negative mood was associated with less activation in these regions. Although this study allowed us to examine the effect of mood on reward processing, it consisted of a small sample (n=20). Additionally, it is unclear what level of reward processing in the absence of a mood induction is present for emotional eaters. It may be that a hyperactive reward circuitry may be present in emotional eaters, which explains their tendency to eat under distress, as they may perceive relatively neutral cues as more rewarding and subsequently engage in more emotional eating. This is congruent with studies of rats that show an effect of palatable food presence on stress eating, as well as enhanced LPP to food images in emotional eaters (26). It could also be that emotional eaters have hypoactive reward circuitry, as evidenced by reduced dopamine receptor availability (24) and reduced brain response to food selection (23). Thus, the present study sought to examine general reward processing related to food cues and taste in a large sample of adolescents of normal weight at high- and low-risk of developing obesity. This sample is particularly relevant to this investigation due to the potential influence of emotional eating on future weight gain. Additionally, this sample includes both girls and boys, which is important since much of the prior research on emotional eating has been conducted in women (14, 26, 27). We expect effects to be similar in boys and girls based on limited data from samples of both genders (15), although some evidence suggests a differential relation between BMI and emotional eating among men and women (28). We hypothesized that emotional eating would be positively associated with activity in regions related to reward processing, learning, and decision making, such as the ACC, amygdala, insula, accumbens, caudate, pallidum, putamen, thalamus, and OFC, in response to both anticipation of a palatable taste and receipt of the taste.
Methods and Procedures
Participants
The sample comprised 162 adolescents (82 female; mean age = 15.3, SD = 1.1; mean BMI = 20.8, SD = 1.9). The sample was 4% Hispanic, 1% Native-American, 1% Asian/Pacific Islander, 76% European-American, and 18% mixed racial heritage. Exclusion criteria included: BMI<18 or >25, current use of psychoactive medications or drugs more than once weekly, pregnancy, head injury with a loss of consciousness, significant cognitive impairment, major medical problems, or a current psychiatric disorder. One hundred twenty-five participants were at-risk for developing obesity by nature of having two obese or overweight parents (BMI > 27) and thirty-seven participants were at low-risk for developing obesity by having two lean parents (BMI < 25). Participants completed baseline, 1-year, and 2-year assessments. Only measures from the baseline assessment relevant to the hypotheses are included in this report. The study included measures and experiments in addition to those described here. Prior publications describe additional details about the study (29, 30).
Measures
Emotional Eating
Participants completed the Dutch Eating Behavior Questionnaire – Emotional Eating subscale. The scale consists of 13 questions assessing the frequency of eating in response to various negative emotions. The scale has demonstrated internal consistency (α= 0.86–097) (31).
Body Mass Index (BMI)
Body mass index was calculated as kg/m2. Height was measured to the nearest millimeter using a portable stadiometer. Participants were measured without shoes and positioned so that the heels and buttocks were against the vertical support of the stadiometer. Weight was assessed to the nearest 0.1 kg using digital scales while participants wore light clothing without shoes or coats. Two measures of height and weight were obtained and averaged before calculating BMI.
Body Fat Percentage
Air displacement plethysmography was used to assess percent body fat of participants with the Bod Pod S/T (COSMED, Pavona di Albano, Italy) with recommended procedures and age/sex-appropriate equations. Body density was calculated as body mass divided by body volume. Body fat percentage estimates show test-retest reliability (r = .92–.99) and correlate with dual energy x-ray absorptiometry and hydrostatic weighing estimates (r = .98– .99)(32).
fMRI Paradigms
Participants were asked to consume regular meals but to refrain from eating or drinking caffeinated beverages for 5 hours preceding their scan. The paradigm assessed response to receipt and anticipated receipt of chocolate milkshake. Stimuli were two images (glasses of milkshake and water) that signaled impending delivery of either .5 mL of chocolate milkshake or tasteless solution, respectively. Cues were presented for 2s and were followed by a jitter of 1–7s during which time the screen was blank. On 40% of the trials the taste was not delivered after the cue to allow investigation of the neural response to anticipation of a taste not confounded with actual receipt of the taste (unpaired). There were 30 trials of both milkshake receipt and tasteless solution receipt and 20 trials of both the unpaired milkshake cue and the unpaired tasteless cue. Tastes were delivered with programmable syringe pumps. Syringes filled with milkshake and tasteless solution were connected via Tygon tubing to a manifold that fit into participants’ mouths. Participants were instructed to swallow when the word “swallow” appeared.
fMRI Scanner and Data Acquisition
Scans were conducted on a Siemens Allegra 3T scanner. Echo planar imaging was used to measure the blood oxygen level dependent (BOLD) signal as an indication of brain activation. A T2* weighted single shot echo planar sequence was used to image the BOLD signal with TR = 2000 ms, TE = 30ms, flip angle = 80°, with an in plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192 mm2 field of view). To cover the whole brain, 32 4mm slices (interleaved acquisition, no skip) were acquired along the AC-PC transverse, oblique plane. A high resolution, T1 weighted 3D volume was acquired for each participant (MP-RAGE; matrix size of 256×256, FOV of 22cm, slice thickness of 1mm).
Data Analysis
Data were analyzed using FEAT (FMRI Expert Analysis Tool), part of the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Images were motion corrected, skull stripped, and spatially smoothed with a 5-mm full-width half-maximum Gaussian kernel. Image processing included mean-based intensity normalization of all volumes by the same factor and high-pass temporal filtering. Functional images were coregistered with structural images in native space and registered structural images to standard structural images (MNI-152).
We utilized the general linear model in analyses, with a convolution of the hemodynamic response function (HRF), its temporal derivative, and the timing of events of interest. Our paradigm had two contrasts of interest: the picture of milkshake vs. the picture of water and receipt of milkshake vs. receipt of tasteless solution. We analyzed the data with multiple regression, including scores of emotional eating as a regressor. We included body-fat percentage, BMI, and risk status in models with emotional eating to ensure that effects were not better explained by these variables. Gender was also included to examine whether effects were consistent among boys and girls given the predominance of female samples in emotional eating research.
Within and between-group comparisons were performed using a mixed-effects model to account for inter-subject variability. We thresholded Z statistic images using clusters determined by Z > 2.3 and a corrected cluster significance threshold of p < .05.1 We utilized pre-threshold masking using regions of interest (ROI) mask created by adding the Harvard-Oxford Atlas 50 percent probability masks for the ACC, amygdala, insula, accumbens, caudate, pallidum, putamen, thalamus, and OFC.2
Results
Descriptive Analyses
We measured correlations between emotional eating scores, body fat percentage, and BMI. Body fat percentage positively correlated with both emotional eating scores (r = .240, p = .002) and BMI (r = .285, p < .001), but emotional eating and BMI were not significantly correlated (r = .029).
Because of the high-risk design, we examined differences on these measures between adolescents at high- and low-risk for obesity. There was no significant difference in emotional eating scores between high- and low-risk groups. There were significant differences between the high- and low-risk groups on both BMI (t(160) = 2.51, p = .013) and body fat percentage (t(160) = 2.72, p = .007). BMI and body fat percentage were higher in the high-risk group than the low-risk group (see Table 1).
Table 1.
Means, Standard Deviations, and Range for Measures
| Full Sample (N=162) |
Boys (n=80) |
Girls (n=82) |
High-Risk (n=125) |
Low-Risk (n=37) |
|
|---|---|---|---|---|---|
| Measures | M (SD) Range | M (SD) Range | M (SD) Range | M (SD) Range | M (SD) Range |
| Emotional Eating | 1.86 (.69) 1–3.92 | 1.61 (.62) 1–3.69 | 2.10 (.67) 1–3.92 | 1.85 (.67) 1–3.85 | 1.86 (.76) 1–3.92 |
| Body Fat % | 18.44 (7.72) .28–34.43 | 12.80 (5.34) .28–26.33 | 23.94 (5.34) 7.63–34.43 | 19.32 (7.79) .28–34.43 | 15.47 (6.76) 4–29.05 |
| Body Mass Index | 20.83 (1.92) 16.66–26.14 | 20.71 (1.99) 17.49–26.14 | 20.94 (1.85) 16.66–24.77 | 21.03 (1.93) 16.66–26.14 | 20.14 (1.75) 17.49–23.65 |
We also evaluated gender differences. Emotional eating scores were higher in females than males (t(160) = 4.87, p < .001). Body fat percentage was also higher in females than males (t(160) = 13.27, p < .001). There were no significant gender differences in BMI.
Anticipation of Taste
We investigated the relation between brain activation in response to anticipating receipt of chocolate milkshake (> anticipating receipt of the tasteless solution) and emotional eating scores. We used unpaired trials to ensure that neural response to taste did not influence the BOLD response. There was no significant relation between emotional eating scores and brain response to anticipation of milkshake receipt.
Taste Receipt
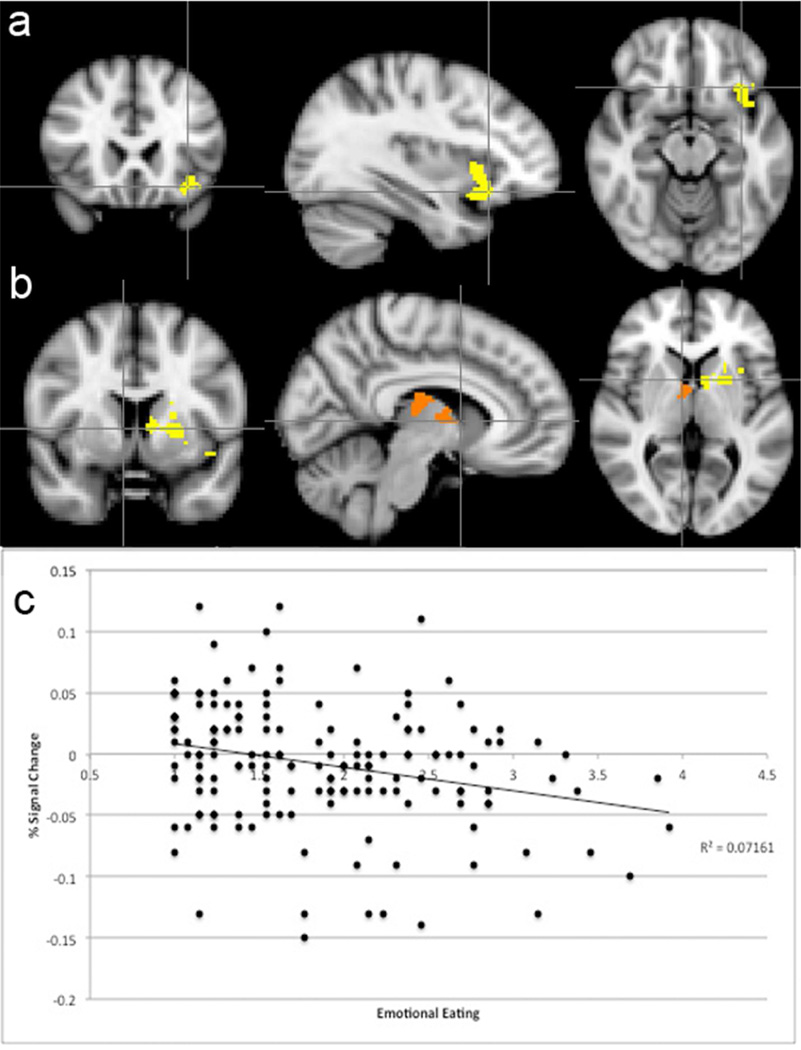
We also investigated the relation between emotional eating and neural response to milkshake receipt (> tasteless solution receipt). Emotional eating scores were negatively related to activation in the right thalamus, putamen, and caudate, and the left caudate, putamen, insula, and orbitofrontal cortex (see Table 2). In follow-up analyses, we added BMI and body fat percentage in separate models, and the relation between emotional eating scores and neural response to milkshake receipt remained significant. Interestingly, there was no significant relation between neural response and BMI or body fat percentage when emotional eating was included in the model. Figure 1 shows the brain regions with significant relation between emotional eating and response to milkshake taste, as well as a scatterplot of this averaged effect. All significant regions followed this pattern. Percentage signal change in these brain regions was not significantly different between high- and low-risk groups or between genders.
Table 2.
Brain regions related to emotional eating (MNI coordinates of cluster peaks) and corresponding Z-score
| x | y | z | Max Z | Brain Region |
|---|---|---|---|---|
| −34 | 18 | −16 | 3.38 | Left Orbitofrontal Cortex |
| −42 | 10 | −10 | 3.19 | Left Insula |
| −8 | 6 | 4 | 2.98 | Left Caudate |
| −20 | 4 | 2 | 2.93 | Left Putamen |
| −34 | 14 | 0 | 2.88 | Left Insula |
| −34 | 18 | −4 | 2.74 | Left Insula |
| 8 | −20 | 14 | 3.26 | Right Thalamus |
| 12 | −6 | 20 | 3.01 | Right Caudate |
| 10 | −4 | 8 | 3.00 | Right Thalamus |
Figure 1.
Negative correlation between emotional eating and signal change in (Panel A) the left insula and orbitofrontal cortex and (Panel B) right thalamus, putamen, and caudate and the left caudate and putamen. Images are in radiological view on the MNI-152 standard brain, with the left hemisphere displayed on the right in axial and coronal slices. Panel C: Scatterplot reveals percentage signal change averaged across the significant clusters as function of emotional eating. The overall pattern shown in the scatterplot was present for individual peaks, as well.
Discussion
Results from the present study show that adolescents with higher scores of emotional eating exhibited less activation in the thalamus, putamen, caudate, insula, and orbitofrontal cortex in response to receipt of chocolate milkshake. The thalamus and insula have been implicated in taste processing (33), while the caudate and putamen are implicated in reward learning (34), and the OFC in reward valuation (35). Thus, these findings may reflect differential functioning in various processes related to taste-related reward. The results were not better explained by BMI or body fat percentage. This is incongruent with our hypothesis of enhanced reward response to anticipated and actual taste receipt. Additionally, these findings are in line with evidence of decreased putamen activity in a stress condition while choosing foods (23) and evidence of fewer dopamine D2 receptors in the dorsal striatum in emotional eaters (24).
A prior study of brain response in emotional eaters showed greater activation of related brain regions (ACC, thalamus, pallidum) in an induced negative mood condition (27). Thus, it appears that those with low emotional eating scores respond as expected to the milkshake taste (with greater activation of reward circuitry), but individuals with high emotional eating scores only do so when in a negative mood state.
It is possible that food restriction and dieting could lead to negative mood states, resulting in enhanced reward response to foods in emotional eaters during that state. This enhanced reward could lead to overeating in that mood. Although participants completed a measure of dietary restraint (31), there were not enough participants endorsing restrained eating to evaluate the influence of restraint on these findings. Prior research found a relation between high dietary restraint scores and greater brain activation in response to taste in the right OFC and bilateral dorsolateral prefrontal cortex (36).
The present study did not include a mood induction. Participants were presumed to be in a neutral mood on average during the fMRI experiment, although there was likely variation across the sample during the experiment. Some individuals may have been anxious about the study, although efforts were made to help participants relax, as we have noticed reduced head motion when participants feel comfortable and calm. This study improves upon the prior study by including a larger and broader sample of 162 participants and including males.
Unlike the prior study, there were no significant effects of emotional eating on brain response to anticipation of chocolate milkshake taste, which could be due to different data analysis methods or less robust effects. Alternatively, the lack of a mood induction in the present study may have limited our ability to detect this effect.
The caudate and putamen (dorsal striatum) are involved in reward-related learning. Intracaudate injections of amphetamine enhance memory in rats (37), and diminished activity could result in deficient memory updating. This is important because positive effects of food intake on mood are often short-lived (38), and updated memory of lack of reward could potentially prevent subsequent emotional eating. Thus, deficient activity could reflect a maintenance mechanism of emotional eating.
These findings from a predominantly high-risk sample are interesting in the context of literature showing hypoactivation of reward circuitry in some obese individuals (25, 39). However, hyperactivation of the striatum predicted weight gain in individuals at low genetic risk of reduced dopamine receptor availability (39), suggesting differential processing of taste (too little or too much) that could lead to obesity (40). Body fat percentage and BMI did not explain the relation between emotional eating and brain response to milkshake. Thus, emotional eating may have a unique effect on brain response to taste, which could relate to future obesity. Perhaps enhanced brain response to taste during negative mood leads to weight gain over time through emotional eating, making emotional eating one individual factor that could differentiate obese individuals with hyperactivation in response to palatable taste from those with hypoactivation. This hypothesis was not possible to explore given the normal weights of the current sample. For obese individuals who engage in emotional eating, current mood state may be particularly important when interpreting the results of brain imaging studies.
Although the present study addresses limitations of prior work that consisted of small samples and excluded males, there are a number of important limitations. First, mood was not measured or manipulated. Prior research showed a differential response to palatable taste in a negative mood, so it is possible that different results would be found for different moods. Although we presume a neutral mood on average for this sample, it may be that some adolescents were more depressed or anxious during their fMRI scan than others, which could diminish our ability to detect an effect. Measurement of negative affect may have allowed us to examine the interaction between mood and emotional eating scores on brain response to taste. Additionally, including a mood induction would allow us to experimentally examine the effect of mood in this group. This study was originally designed to examine reward processing and related factors in adolescents at high- and low-risk for developing obesity. Including a mood induction would have resulted in great participant burden. Second, the range of emotional eating scores was somewhat constrained. In our prior study of emotional eating, the high emotional eating group scored higher than 3.6 on the same measure utilized in this study. The range for the present study was 1–3.92, with few participants scoring between 3.6 and 3.92. The highest possible score for the measure is a 5. A sample with more participants scoring at the high end of emotional eating would increase our confidence in the effect. Third, although the effect is interesting in the context of a growing literature on brain function and obesity, it is a small effect (R2=0.07). Indeed, the effects were not detected in whole brain analyses and required ROI analyses, which increase power and allow for detection of smaller effects. Thus, it is likely only one of many factors influencing brain response to palatable tastes and others must be considered in addition to emotional eating. Fourth, the majority of the sample (n=125) had two obese parents, which could affect results. However, this sample is at higher risk for the development of obesity, so results are interesting in this context.
In sum, this study provides evidence of a negative relation between emotional eating and brain response in the thalamus, caudate, putamen, insula, and orbitofrontal cortex in response to palatable taste receipt. Individuals with low emotional eating scores respond as expected to a palatable taste, but those with high emotional eating scores may only respond to taste reward during negative emotion. Future research should measure and experimentally manipulate mood in individuals at risk for developing obesity and with a broad range of scores of emotional eating in order to tease apart the effects under different moods on subsequent weight gain.
What is already known about this subject?
emotional eating predicts binge eating and obesity
differential reward response to taste is implicated in obesity
stress eating in rats is more likely to occur in the presence of palatable foods
What does this study add?
emotional eating is associated with diminished reward response to tastes in the absence of a mood induction
emotional eating status may be an important differentiating factor for inconsistent findings of reward response to taste in obesity
mood is likely an important factor to consider when interpreting results of reward processing study in obesity
Acknowledgements
I would like to thank Dr. Eric Stice for access to his dataset for the analyses presented in this manuscript. Data described in this paper were collected through support from a grant to Dr. Stice from NIDDK (DK080760). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest
The author has no competing interests.
FSL utilizes the Gaussian Random Field theory in determining contiguous clusters of voxels that pass this threshold.
Utilizing ROI analyses allowed us to examine brain differences related to emotional eating in regions functionally related to various aspects of reward processing, rather than focusing on areas revealed in main effects analyses, which may not include these regions. Regions were selected from prior research on taste reward, as well as identification of regions functionally relevant to reward-related decision-making, reward learning, reward evaluation, and sensation. ROI analyses limit the number of voxels included in the analyses, which reduces the number of comparisons, thus reducing the need for overly stringent corrections. For reference and meta-analytic purposes, whole brain comparisons were conducted on overall main effects of anticipation of taste and taste receipt for the full sample, as well as the relation between brain activity and emotional eating. Analyses utilized FSL FEAT cluster thresholding at Z=2.3 and p<.05. Significant main effects for anticipation of milkshake taste (> anticipation of tasteless control solution) were found in the right lateral occipital cortex, inferior division and bilateral occipital fusiform and occipital pole. Inverse effects (anticipation of tasteless solution > anticipation of milkshake) were found in the right insula, planum temporale, central opercular cortex, superior temporal gyrus, angular gyrus, supramarginal gyrus, posterior division, lateral occipital cortex, superior division, left Heschl’s gyrus, insula, postcentral gyrus, and bilateral precentral gyrus. Main effects for receipt of milkshake (> tasteless solution receipt) were found in the bilateral cerebellum and postcentral gyrus. Inverse effects (receipt of tasteless solution > receipt of milkshake) were found in the bilateral OFC and ACC, and right frontal pole. Although these main effects seem to suggest that milkshake is not generally rewarding, prior evidence of effects of BMI on taste reward, as well as the fact that this sample is predominantly atrisk for developing obesity makes these main effects difficult to interpret. This task has been utilized in a number of studies has activated reward circuitry in healthy adolescents and adults. Furthermore, self-reported ratings of taste tests of the chocolate milkshake by study participants revealed that participants liked the milkshake and had a desire for more. Anecdotally, participants often report enjoying the study because of the milkshake taste rewards. Whole brain tests of the relation between emotional eating and brain response to anticipation revealed no significant effects. Emotional eating was negatively related to brain activiation in response to milkshake receipt (> tasteless solution receipt) in the right lateral occipital cortex: superior division, left angular gyrus, supramarginal gyrus: posterior division, and bilateral superior parietal lobule, and middle frontal gyrus.
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: a 2-year prospective investigation. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2002;21:131–138. [PubMed] [Google Scholar]
- 3.Canetti L, Berry EM, Elizur Y. Psychosocial predictors of weight loss and psychological adjustment following bariatric surgery and a weight-loss program: The mediating role of emotional eating. Int J Eat Disord. 2009;42:109–117. doi: 10.1002/eat.20592. [DOI] [PubMed] [Google Scholar]
- 4.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 5.Konttinen H, Männistö S, Sarlio-Lähteenkorva S, Silventoinen K, Haukkala A. Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite. 2010;54:473–479. doi: 10.1016/j.appet.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Braet C, Van Strien T. Assessment of emotional, externally induced and restrained eating behaviour in nine to twelve-year-old obese and non-obese children. Behav Res Ther. 1997;35:863–873. doi: 10.1016/s0005-7967(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 7.Lowe MR, Fisher EB. Emotional reactivity, emotional eating, and obesity: A naturalistic study. J Behav Med. 1983;6:135–149. doi: 10.1007/BF00845377. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen-Rodriguez ST, Chou C-P, Unger JB, Spruijt-Metz D. BMI as a moderator of perceived stress and emotional eating in adolescents. Eat Behav. 2008;9:238–246. doi: 10.1016/j.eatbeh.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greeno CG, Wing RR. Stress-induced eating. Psychol Bull. 1994;115:444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- 10.Macht M, Simons G. Emotional Eating. In: Nyklíček I, Vingerhoets A, Zeelenberg M, editors. Emotion Regulation and Well-Being. New York: Springer; 2011. pp. 281–295. [Google Scholar]
- 11.Stone AA, Brownell KD. The stress-eating paradox: Multiple daily measurements in adult males and females. Psychol Health. 1994;9:425–436. [Google Scholar]
- 12.Macht M. How emotions affect eating: A five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav. 1999;66:511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- 14.Van Strien T, Herman CP, Anschutz DJ, Engels RCME, de Weerth C. Moderation of distress-induced eating by emotional eating scores. Appetite. 2012;58:277–284. doi: 10.1016/j.appet.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Sproesser G, Schupp HT, Renner B. The Bright Side of Stress-Induced Eating Eating More When Stressed but Less When Pleased. Psychol Sci. 2013 doi: 10.1177/0956797613494849. 0956797613494849. [DOI] [PubMed] [Google Scholar]
- 16.Macht M, Krebs H, Weyers P, Janke W. Effect of stress on feeding behavior in rats: individual differences. Personal Individ Differ. 2001;30:463–469. [Google Scholar]
- 17.Baucom DH, Aiken PA. Effect of depressed mood on eating among obese and nonobese dieting and nondieting persons. J Pers Soc Psychol. 1981;41:577–585. doi: 10.1037//0022-3514.41.3.577. [DOI] [PubMed] [Google Scholar]
- 18.Cools J, Schotte DE, McNally RJ. Emotional arousal and overeating in restrained eaters. J Abnorm Psychol. 1992;101:348–351. doi: 10.1037//0021-843x.101.2.348. [DOI] [PubMed] [Google Scholar]
- 19.Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84:666–672. [PubMed] [Google Scholar]
- 20.Ruderman AJ. Restraint, obesity and bulimia. Behav Res Ther. 1985;23:151–156. doi: 10.1016/0005-7967(85)90023-3. [DOI] [PubMed] [Google Scholar]
- 21.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 22.Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Born JM, Lemmens SGT, Rutters F, et al. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes. 2009;34:172–181. doi: 10.1038/ijo.2009.221. [DOI] [PubMed] [Google Scholar]
- 24.Volkow ND, Wang G-J, Maynard L, et al. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord. 2003;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Wang G-J, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. NeuroImage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blechert J, Goltsche JE, Herbert BM, Wilhelm FH. Eat your troubles away: Electrocortical and experiential correlates of food image processing are related to emotional eating style and emotional state. Biol Psychol. 2014;96:94–101. doi: 10.1016/j.biopsycho.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Bohon C, Stice E, Spoor S. Female emotional eaters show abnormalities in consummatory and anticipatory food reward: A functional magnetic resonance imaging study. Int J Eat Disord. 2009;42:210–221. doi: 10.1002/eat.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaven PCL, Mulligan K, Merrilees R, Woods T, Fairooz Y. Neuroticism and conscientiousness as predictors of emotional, external, and restrained eating behaviors. Int J Eat Disord. 2001;30:161–166. doi: 10.1002/eat.1068. [DOI] [PubMed] [Google Scholar]
- 29.Burger KS, Stice E. Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream-based milkshake. Am J Clin Nutr. 2012;95:810–817. doi: 10.3945/ajcn.111.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at Risk for Obesity Show Greater Activation of Striatal and Somatosensory Regions to Food. J Neurosci. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5:295–315. [Google Scholar]
- 32.Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- 33.Kinomura S, Kawashima R, Yamada K, et al. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res. 1994;659:263–266. doi: 10.1016/0006-8993(94)90890-7. [DOI] [PubMed] [Google Scholar]
- 34.Haruno M, Kawato M. Different Neural Correlates of Reward Expectation and Reward Expectation Error in the Putamen and Caudate Nucleus During Stimulus-Action-Reward Association Learning. J Neurophysiol. 2006;95:948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- 35.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 36.Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. NeuroImage. 2011;55:233–239. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packard MG, Teather LA. Amygdala Modulation of Multiple Memory Systems: Hippocampus and Caudate-Putamen. Neurobiol Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 38.Macht M, Dettmer D. Everyday mood and emotions after eating a chocolate bar or an apple. Appetite. 2006;46:332–336. doi: 10.1016/j.appet.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Stice E, Spoor S, Bohon C, Small DM. Relation Between Obesity and Blunted Striatal Response to Food Is Moderated by TaqIA A1 Allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoeckel L. The Goldilocks Principle of Obesity: Scientific American. 2010 [Google Scholar]