Abstract
Ion channels are essential for the regulation of neuronal functions. The significance of plasma membrane, mitochondrial, endoplasmic reticulum, and lysosomal ion channels in the regulation of Ca2+ is well established. In contrast, surprisingly less is known about the function of ion channels on the nuclear envelope (NE). Here we demonstrate the presence of functional large-conductance, calcium-activated potassium channels (BK channels) on the NE of rodent hippocampal neurons. Functionally blockade of nuclear BK channels (nBK channels) induces NE-derived Ca2+ release, nucleoplasmic Ca2+ elevation, and cAMP response element binding protein (CREB)-dependent transcription. More importantly, blockade of nBK channels regulates nuclear Ca2+-sensitive gene expression and promotes dendritic arborization in a nuclear Ca2+-dependent manner. These results suggest that nBK channel functions as a molecular linker between neuronal activity and nuclear Ca2+ to convey the signals from synapse to nucleus and is a new modulator for synaptic activity-dependent neuronal functions at the NE level.
Keywords: nuclear ion channel, excitation-transcription coupling, nuclear Ca2+ signaling, BK channel
As transmembrane proteins, ion channels are present not only on the plasma membrane but also on intracellular membrane-bound organelles. Much is known regarding plasma membrane ion channels controlling action potential firing, synaptic transmission, and gene expression through the regulation of membrane potential and Ca2+ influx1. In addition, the significance of mitochondrial2, lysosomal3,4, and endoplasmic reticulum (ER)5 ion channels in regulating ATP production, stress response and apoptosis via the control of Ca2+ signals has been well established. In contrast, despite the obvious structural and functional importance of the nucleus in gene expression and regulation, the functions of ion channels on the NE (nuclear ion channels) remain largely unknown, especially in neurons.
Recent studies have shown that the NE is a key source of nucleoplasmic Ca2+: Ca2+ in perinuclear space, the lumen between the outer and the inner nuclear membrane of NE, can be released into the nucleoplasm via nuclear inositol 1,4,5-trisphosphate receptors (IP3R) and ryanodine receptors (RyR)6–10. Therefore, the NE is a functional perinuclear Ca2+ store and is independent from the ER and cytoplasm in regulating the nucleoplasmic Ca2+ concentration ([Ca2+]nu )6–10. Meanwhile, numerous studies have demonstrated that nucleoplasmic Ca2+ is one of the most important signals in mediating neuronal activity-dependent gene expression11,12, and is critical for several long lasting adaptive responses, including modulation of synapse formation and dendritic complexity13, regulation of synaptic plasticity and memory consolidation13,14, and activity-dependent acquired neuroprotection12,15.
As a key signal in neuronal functions, numerous fundamental questions remain such as how this NE-derived Ca2+ release regulated in neurons, whether it can be regulated by nuclear ion channels through changing the NE transmembrane potential, similar to the regulation of Ca2+ fluxes in the plasma membrane and mitochondria, which type of nuclear ion channel governs this transmembrane potential change, and whether this nuclear ion channel also regulate transcription factor activation and gene expression induced by neuronal activity. To address these questions, we applied a combination of biochemical and molecular biological methods, together with calcium imaging, voltage imaging, and patch clamp techniques in wild-type and gene knockout mice to study the function of the nuclear ion channel. We found that functional BK channels are present on the NE of hippocampal neurons. Pharmacological or genetic blockade of nBK channels directly regulates the NE-derived release of Ca2+, CREB phosphorylation, gene expression, and dendritic arborization, suggesting that nBK channels play a key role in neuronal activity-dependent functions and serve as new molecular link between neuronal activities, nuclear Ca2+ signaling, and gene expression at the NE level.
RESULTS
BK channels are expressed on the NE
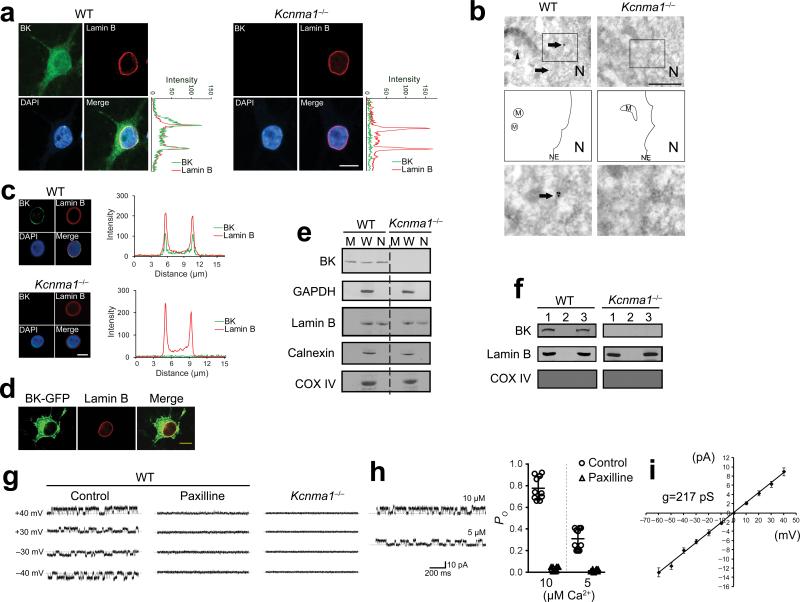
To investigate the subcellular localization of BK channels in intact hippocampal neurons, we used a monoclonal antibody for the α subunit of the BK channel in immunocytochemistry16. The confocal optical sections demonstrated an intracellular ring-like labeling around the nucleus (Fig. 1a). Analysis of intensity profiles indicated that the labeling was co-localized with the NE marker lamin B, suggesting that the BK channels are localized to the NE (Fig. 1a). To confirm that this observation did not result from non-specific binding of the BK antibody, we conducted the same experiment in the neurons from BK channel α subunit-null mice (Kcnma1–/–) mice. As shown in Fig. 1a, the BK channel antibody failed to detect any signal in the neurons from Kcnma1–/– mice, validating its specificity. Furthermore, we used immunoelectron microscopy to investigate BK channel localization at high spatial resolution. As shown in Fig. 1b, gold particles signaling BK channels were localized on the NE (Fig. 1b), further confirming the NE localization of the BK channels. Consistent with previous findings17,18, we also observed the mitochondrial localization of the BK channels (Fig. 1b). The negative labeling in Kcnma1–/– mice once again validated the specificity of the BK antibody (Fig. 1b). To further verify the nuclear localization of the BK channels, we isolated nuclei from cultured hippocampal neurons. A ring-like labeling of BK channel immunofluorescence was detected in the isolated nuclei from wild-type mice (Fig. 1c). Analysis of intensity profiles of the isolated nuclei indicated that BK channel were co-localized with lamin B (Fig. 1c). The BK channel antibody failed to detect any signal in the nucleus from Kcnma1–/– mice (Fig. 1c). Additionally, we transfected neurons with a green fluorescent protein (GFP)-tagged BK channel construct. As shown in Fig. 1d, green fluorescence was detected around the nucleus and co-localized with lamin B, once again suggesting the NE localization of the BK channels. This interpretation was further supported by subcellular fractionation and immunoblot analysis: BK channels were detected on the nuclear fraction, as well as the plasma membrane fraction and the whole cell lysate (Fig. 1e). The purity of the nuclear fraction was validated by the presence of lamin B, and the absence of cytosolic marker GAPDH, ER marker calnexin and mitochondrial marker COX IV (Fig. 1e). The presence of immunoreactivity in the NE fraction, but not in the denuded nuclei (NE-depleted nuclei) fraction further verified the localization of the BK channel on NE (Fig. 1f). The absence of the immunobands in Kcnma1–/– mice confirmed that the immunoreactivity arose from authentic BK channels (Fig. 1e,f).
Figure 1. BK channels expression on the NE of hippocampal neurons.
(a) The confocal immunofluorescence of BK channel and lamin B in cultured hippocampal neurons. Analysis of intensity profiles of the vertical line across the center of the nucleus was shown on the right. Over 85% of the neurons (>100 neurons) have the positive BK channel staining in NE. (b) Immunoelectron microscopy images of BK channel localization in cultured hippocampal neurons (Top). Gold particles signaling BK channels were localized on NE (arrows) and mitochondria (M) (arrowhead) in wild-type mice. Forty-six immunoelectron microscopy images were taken totally. NE–localized gold particles were observed in all the images from the wild-type mice, and there is no signal in Kcnma1–/– mice. Scale bar represents 1 μm. Middle, schematic diagram of the top images. Bottom, an enlargement of a square region indicated in the top images. (c) Left, immunofluorescence of BK channels and lamin B in isolated nuclei. Right, analysis of intensity profiles of the horizontal line across the center of the isolated nuclei. (d) Immunofluorescence of BK-GFP and lamin B. (e) Western blot results of the subcellular localization of the BK channel in the membrane fraction (M), the whole cell lysate (W), and the nuclear fraction (N). (f) Western blot results of the BK channel expression in intact isolated nuclei (1), denuded nuclei (2) and NE (3). (g) Single channel activities of the BK channel in isolated nuclei with 10 μM Ca2+ at different voltage in control group (left), with paxilline treatment (10 μM, middle), and in the nuclei from Kcnma1–/– mice (right) (n = 6). (h) Left, single channel activities recorded in the solutions with 10 μM or 5 μM Ca2+ at 40 mV. Right, BK channel open probability (PO) recorded in the solutions with 10 μM (n = 10 for each group, unpaired t-test, P = 2.30 × 10–14, t18 = 21.71 between control and paxilline) or 5 μM Ca2+ (n = 10 for each group, unpaired t-test, P = 1.80 × 10–8, t18 = 9.55, between control and paxilline) at 40 mV (error bars are mean ± s.d.). (i) I-V curve of the channel activity. For single channel recording, BK channel activity was recorded from 15 of the 20 successful patching of the nuclei from wild-type mice. All the data are from 3 independent cultures from at least 3 litters. All the experiments of a–f were successfully repeated for at least 3 times. WT, wild-type. Scale bars in a,c,d represent 10 μm. The full-length blots for e and f are presented in Supplementary Figure 7.
Besides the pore-forming α subunit, BK channels also contain auxiliary β subunits. The brain-specific auxiliary subunits β4 were also observed and co-localized with lamin B (Supplementary Fig. 1), suggesting that a fully assembled BK channel complex is localized on NE. To further determine whether the nuclear BK channels (nBK channels) are functional and exhibit similar electrophysiological properties to the plasma membrane BK channels (pmBK channel), we performed single channel recordings with patch clamp on isolated nuclei. We recorded a voltage-dependent and Ca2+ -dependent K+ channel activity with a conductance of approximately 220 ps, similar to that of neuronal pmBK channel19–22 (Fig. 1g–i). The blockade of the channel activity with paxilline, a BK channel-specific blocker, and the absence of the channel activity in Kcnma1–/– mice further confirmed that the channel activity arose from BK channels (Fig. 1g,h). Taken together, these observations demonstrate that BK channels are present and functional on the NE of hippocampal neurons.
nBK channels regulate nuclear Ca2+ concentrations
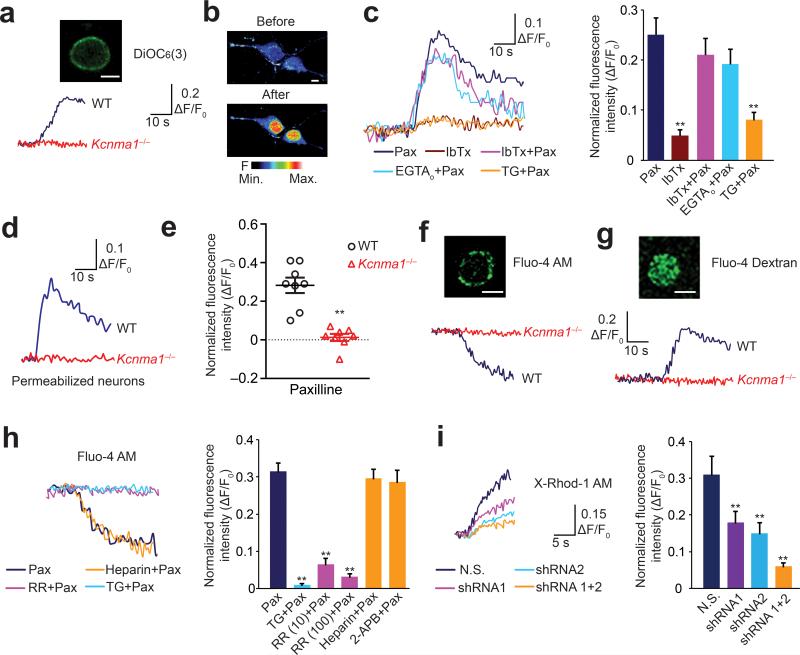
pmBK channels exhibit significant abilities in controlling the plasma membrane potential and accordingly regulating the voltage-dependent calcium influx19–22. nBK channels, with similar electrophysiological properties to pmBK channels, may also regulate the nuclear transmembrane potential (ΔΨn), thereby regulating nuclear calcium concentration. To address this hypothesis, we first examined whether nBK channels could regulate the ΔΨn. Isolated nuclei were loaded with DiOC6(3), which has been validated as a potentiometric probe in NE23,24. Paxilline elicited a consistent increase in DiOC6(3) fluorescence (0.28 ± 0.02 ΔF/F0 (mean ± s.e.m.), n = 20), indicating that the perinuclear lumen became more negative23,24 (Fig. 2a). Consistently, the paxilline-induced ΔΨn was completely absent in the nuclei from Kcnma1–/– mice (0.02 ± 0.01 ΔF/F0 (mean ± s.e.m.), n = 31, unpaired t-test, P = 2.81 × 10–13, t49 = 9.90, compared with wild-type group) (Fig. 2a). These results suggest that nBK channels can regulate ΔΨn.
Figure 2. nBK channels regulate the nuclear transmembrane potential and nuclear Ca2+ concentration in intact neurons and isolated nuclei.
(a) Fluorescence intensity changes of DiOC6(3)-loaded isolated nuclei (insert) upon addition of paxilline. (b) Confocal nuclear calcium imaging with Fluo-4/AM of the mid-nuclear region in hippocampal neurons. (c) The changes of fluorescence intensity in nuclear calcium imaging of intact neurons after indicated treatments (left). Right, summary of the data. One-way ANOVA (n = 15 for each group, P = 4.70 × 10–7, F4,70 = 11.15) and post hoc test. (d) Fluorescence intensity changes in the nuclei of digitonin-permeabilized neurons after paxilline treatment. (e) Paxilline-induced fluorescence intensity changes in intact neurons from wild-type and Kcnma1–/– mice (n = 8 for each group, unpaired t-test, P = 2.56 × 10–5, t14 = 6.14, points with error bars represent the mean ± s.e.m.). Paxilline-induced changes in Fluo-4/AM (f) or Fluo-4/dextran (g) fluorescence intensity in the isolated nuclei. (h) The changes of fluorescence intensity in isolated nuclei after indicated treatments (left). Right, summary of the data. One-way ANOVA (n = 11, 8, 12, 8, 10, 10, respectively, P = 2.50 × 10–13, F5,53 = 26.55) and post hoc test. (i) The changes of fluorescence intensity in isolated nuclei after knockdown of RyRs (left). Right, summary of the data. One-way ANOVA (n = 5, 6, 6, 5, respectively, P = 0.0016, F3,18 = 7.72) and post hoc test. Fisher's least significant difference test was used for post hoc test in one-way ANOVA. Error bars represent the mean ± s.e.m.. All the data are from 3 independent cultures from at least 3 litters. All the experiments were successfully repeated for at least 3 times. **P < 0.01. WT, wild-type; Pax, paxilline; RR, ruthenium red; TG, thapsigargin; shRNA, RyRs-specific shRNAs; N.S., non-silencing shRNAs. Scale bars in a,b,f,g represent 5 μm.
Next, we investigated whether nBK channels can regulate [Ca2+]nu in hippocampal neurons. Paxilline is membrane permeable and inhibits BK channels on both the plasma membrane and intracellular organelles. IbTx, a membrane-impermeant BK channel blocker, only inhibits pmBK channels on the plasma membrane. Calcium imaging of the confocal mid-nuclear section (Fig. 2b) demonstrated that paxilline induced [Ca2+]nu elevation in intact neurons, whereas IbTx lacked an influence on [Ca2+]nu (Fig. 2c). Moreover, paxilline elicited [Ca2+]nu elevation even after complete blockade of the pmBK channels with a high concentration of IbTx (100 μM)20 (Fig. 2c), which suggests that the effect of paxilline was mainly mediated by intracellular BK channels, but not pmBK channels. This idea was further supported by the results that paxilline induced [Ca2+]nu elevation in digitonin-permeabilized neurons with a Ca2+-buffered intracellular medium (Fig. 2d). Paxilline-induced [Ca2+]nu elevation was largely suppressed by prior depletion of intracellular Ca2+ store with thapsigargin, but not by EGTA-buffered extracellular Ca2+ (Fig. 2c), further suggesting that the nucleoplasmic Ca2+ transient is likely due to direct Ca2+ release from NE8, 24. Importantly, neurons from Kcnma1–/– mice were completely devoid of paxilline-induced [Ca2+]nu elevation (Fig. 2e). Taken together, these results strongly supported the idea that the intracellular BK channels and perinuclear Ca2+ store are responsible for eliciting nuclear Ca2+ signals.
To further verify that the mediators of the paxilline-induced [Ca2+]nu elevation are the nBK channels and perinuclear Ca2+ store, isolated nuclei from cultured hippocampal neurons were loaded with either the Ca2+ probe Fluo-4/AM or Fluo-4/dextran. Fluo-4/AM, a membrane-permeant probe, preferentially accumulated in the NE (Fig. 2f), whereas Fluo-4/dextran, a membrane-impermeant probe, was distributed uniformly in the nucleoplasm8,10,24(Fig. 2g). Confocal microscopy revealed that paxilline produced opposite Ca2+ changes in the NE and the nucleoplasm. Application of paxilline led to a decrease of NE Ca2+ (Fig. 2f), which paralleled a transient Ca2+ increase in the nucleoplasm (Fig. 2g). The paxilline-induced Ca2+ transient was largely diminished by knockdown of BK channel with lentiviral shRNAs (Supplementary Fig. 2), and was completely abolished in neurons from Kcnma1–/– mice (Fig. 2f,g, Fluo-4/AM intensity, 0.29 ± 0.03 ΔF/F0 in wild-type group, 0.01 ± 0.02 ΔF/F0 for Kcnma1–/– group, n = 25 for each group, unpaired t-test, P = 1.16 × 10–10, t48 = 8.19; Fluo-4/dextran intensity, 0.33 ± 0.04 ΔF/F0 in wild-type group, 0.03 ± 0.02 ΔF/F0 for Kcnma1–/– group, n = 22 for each group, unpaired t-test, P = 2.12 × 10–8, t42 = 6.89, data is presented as mean ± s.e.m.). Depletion of the perinuclear Ca2+ store with thapsigargin completely blocked paxilline-induced Ca2+ transient (Fig. 2h), suggesting that the perinuclear Ca2+ store are responsible for eliciting the paxilline-induced nuclear Ca2+ signals.
Numerous studies have demonstrated that IP3R and RyR, located on the inner membrane of the NE, mediate the release of Ca2+ from the perinuclear space into the nucleoplasm6,10. Therefore, we used specific IP3R inhibitors (heparin and 2-APB) and a RyR inhibitor (ruthenium red) to test which receptor is responsible for the nBK channel-regulated NE Ca2+ release. The paxilline-induced Ca2+ release was attenuated by 10 μM ruthenium red and was completely blocked by 100 μM ruthenium red (Fig. 2h). Conversely, results with the IP3R blockers indicated that neither heparin (50 μM) nor 2-APB (100 μM) affected paxilline-induced Ca2+ release (Fig. 2h). To further confirm that RyRs mediate paxilline-induced Ca2+ release, we designed two independent shRNAs to knock down RyRs expression in neurons25. RyRs expression level was reduced to 56.4% and 51.5% of control by using the shRNA individually, and was reduced to 25.5% when both shRNAs were used together (Supplementary Fig. 3). As shown in Fig. 2i, knockdown of RyRs significantly compromised paxilline-induced [Ca2+]nu elevation in intact neurons, providing direct evidence that RyRs mediate the paxilline-induced Ca2+ release. Collectively, these results indicate that blockade of nBK channel induces NE-derived Ca2+ release and [Ca2+]nu elevation via RyRs.
nBK channels regulate nuclear Ca2+ signaling
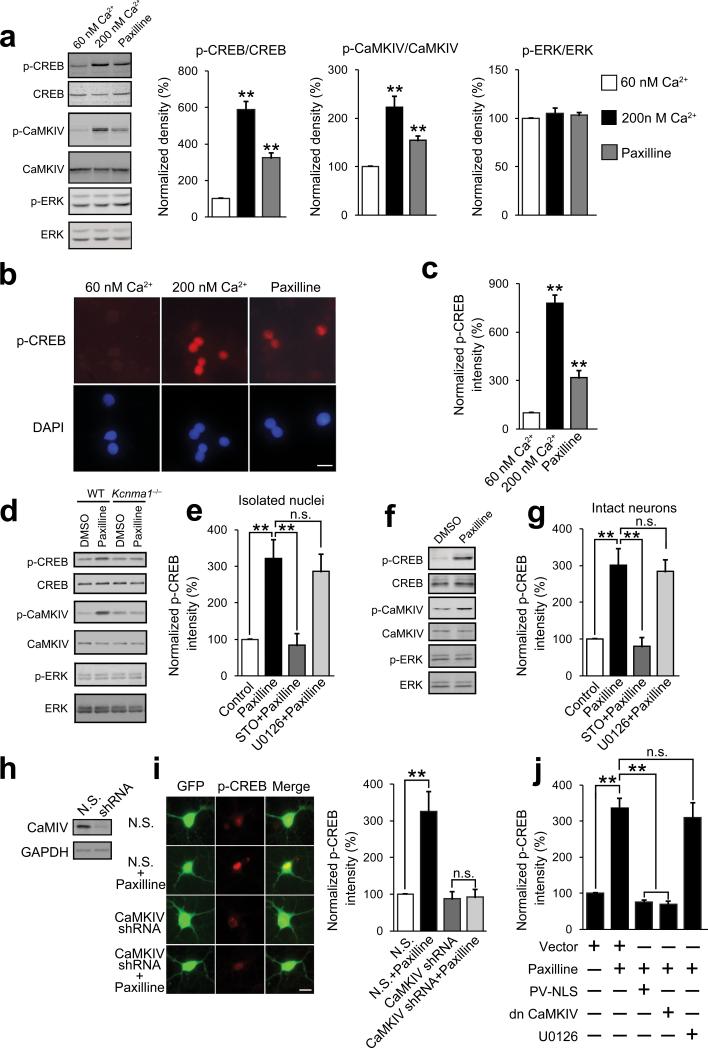
Activity-dependent activation of CREB is heavily implicated in neuronal development and plasticity, long-term memory formation, and cell survival12,15,26,27. Elevation of [Ca2+]nu results in the phosphorylation of CREB on its activator site serine 133, which is often regarded as a marker for CREB-mediated transcription28,29. Because blockade of the nBK channels induced [Ca2+]nu elevation, we therefore further investigated whether the nBK channels could also induce CREB phosphorylation in isolated nuclei and intact neurons.
Using immunoblot in isolated functional nuclei, we observed that paxilline induced the phosphorylation of CREB, similar to the response to elevate free Ca2+ (Fig. 3a). The same results were also observed in immunofluorescence experiments (Fig. 3b,c). The role of the nBK channels in regulating CREB phosphorylation was further verified in isolated nuclei from wild-type and Kcnma1–/– mice. As shown in Fig. 3d, paxilline induced CREB phosphorylation only in nuclei from wild-type mice, but not in nuclei from Kcnma1–/– mice.
Figure 3. Blockade of nBK channels induces CREB phosphorylation in isolated nuclei and intact neurons.
(a) Immunoblots of isolated functional nuclei after stimulation as indicated. One-way ANOVA (n = 4 for each group; P = 1.75 × 10–5, F2,9 = 46.81 for p-CREB; P = 0.0018, F2,9 = 13.91 for p-CaMKIV; P = 0.87, F2,9 = 0.14 for p-ERK) and post hoc test. (b) Immunofluorescence of CREB phosphorylation. (c) Statistic results of b. One-way ANOVA (N = 5 for each group, P = 3.89 × 10–6, F2,12 = 41.85) and post hoc test. (d,f) Immunoblots of isolated functional nuclei (d) and intact neurons (f) after paxilline treatment. (e,g) Immunofluorescence intensity of CREB phosphorylation in isolated functional nuclei (e) and intact neurons (g) after treatments as indicated. One-way ANOVA (N = 5 for each group; P = 0.0093, F3,16 = 5.40 for data in e; P = 0.00096, F3,16 = 9.08 for data in g) and post hoc test. (h) CaMKIV expression after shRNA transfection. (i) Left, paxilline-induced CREB phosphorylation in the neurons transfected with GFP-tagged plasmids encoding CaMKIV-specific shRNAs or non-silencing shRNAs (N.S.), respectively. Right, summary of the data. One-way ANOVA (N = 5 for each group, P = 0.0021, F3,16 = 7.67) and post hoc test. (j) Paxilline-induced CREB phosphorylation after treatments as indicated. One-way ANOVA (N = 5 for each group, P = 5.64 × 10–6, F4,20 = 15.76) and post hoc test. Fisher's least significant difference test was used for post hoc test in one-way ANOVA. Error bars represent the mean ± s.e.m.. All the data in a are from 3 independent cultures from at least 3 litters. All the other data are from 5 coverslips of cells from 3 independent cultures from at least 3 litters. All the experiments were successfully repeated for at least 3 times. **P < 0.01; n.s., no significant difference. WT, wild-type; N.S., non-silencing shRNAs. Scale bars in b,h represent 10 μm. The full-length blots for a, d, f and h are presented in Supplementary Figure 7.
CREB is a target for two Ca2+-regulated signaling pathways, the Ca2+/calmodulin (CaM)-dependent protein kinases, in particular the nuclear CaM kinase IV (CaMKIV)28,29, and the MAP kinase/extracellular signal-regulated kinase (ERK) cascade30. As shown in Fig. 3a and d, the nuclear CaMKIV were activated (phosphorylation in Thr196) by paxilline, as well as by the increase of the free Ca2+ concentration, suggesting the involvement of CaMKIV in paxilline-induced CREB phosphorylation (Fig. 3a). In contrast, due to the lack of cytoplasmic and plasma membrane-associated proteins in isolated nuclei, ERK1/2 were not activated by paxilline (Fig. 3a,d). In addition, paxilline-induced CREB phosphorylation in isolated nuclei was blocked by the selective CaMKIV pathway (CaM kinase kinase (CaMKK)) inhibitor STO-609, but not by the ERK pathway inhibitor U0126 (Fig. 3e). These results indicate that paxilline-induced CREB phosphorylation is likely catalyzed by nuclear CaM kinases, but not ERK, which is consistent with studies showing that CaMKIV mediates [Ca2+]nu elevation-induced CREB phosphorylation31.
To further confirm the roles of nuclear Ca2+ and CaMKIV, we conducted similar experiments in intact hippocampal neurons. Similar to the results in isolated nuclei, paxilline induced CREB phosphorylation even after pmBK channel blockade by IbTx pretreatment (Fig. 3f and Supplementary Fig. 4). This event was counteracted by STO-609, but not by U0126 (Fig. 3g). Additionally, to provide the direct evidence for the role of CaMKIV, we used shRNAs to knock down the CaMKIV expression (Fig. 3h). As shown in Fig. 3i, CaMKIV-specific shRNAs significantly inhibited paxilline-induced CREB phosphorylation, further confirming the critical role of CaMKIV in paxilline-induced pCREB. Furthermore, we specifically blocked nuclear Ca2+ signaling by overexpressing the nuclear-targeted Ca2+ buffering protein parvalbumin (PV-NLS)32 and kinase-inactive form of CaMKIV (CaMKIVK75E), which functions as a dominant negative mutant of CaMKIV (dnCaMKIV)12,13. As shown in Fig. 3j, the induction of CREB phosphorylation by paxilline was reduced in hippocampal neurons transfected with PV-NLS or dnCaMKIV, but not in U0126-treated neurons. Collectively, these results indicate that blockade of nBK channels induces CREB phosphorylation in a nuclear Ca2+/CaMKIV-dependent manner.
nBK channels regulate activity-evoked gene expression
Synaptic excitation on the dendritic spines of pyramidal neurons induces transcription factor activation and gene expression in the nucleus. This excitation-transcription (E-T) coupling is critically important for a broad range of processes and behaviors, including neuronal development, learning and memory, and cell survival12,29,31. To identify the role of nBK channels in E-T coupling, we investigate the effects of the nBK channels on the regulation of synaptic activity-evoked CREB phosphorylation and gene expression.
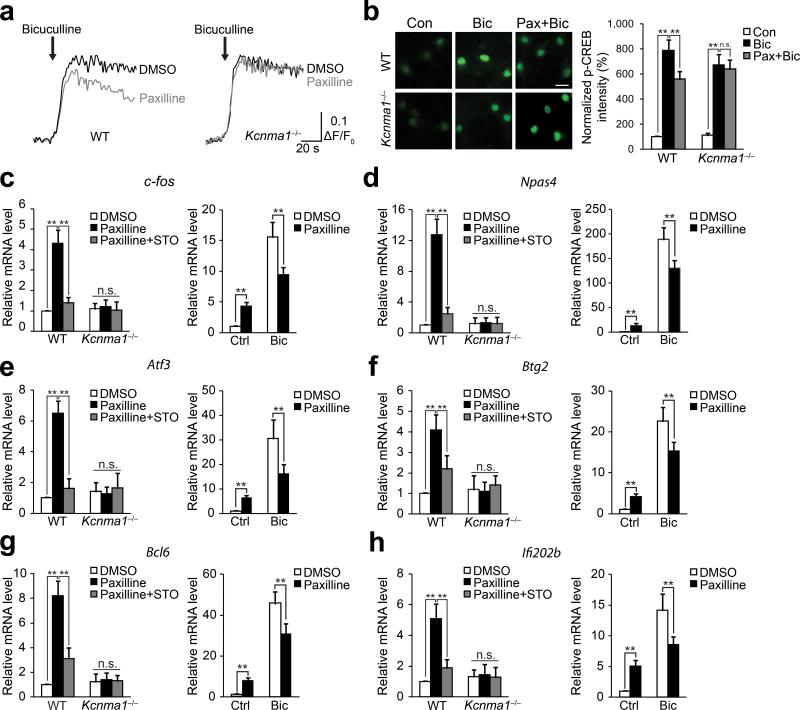
Hippocampal neurons were preincubated with IbTx to exclude the involvement of pmBK channels (Supplementary Fig. 4) and were stimulated with the GABAA receptor antagonist, bicuculline (50 μM). GABAergic interneurons exert a tonic inhibition onto the network. Removal of GABAergic inhibition with bicuculline leads to enhancement of neuronal activity and induces robust [Ca2+]nu elevation and CREB phosphorylation12,31 (Fig. 4a). Bicuculline-induced [Ca2+]nu elevation and CREB phosphorylation are highly dependent on the filling of the perinuclear Ca2+ store, and a reduction of the Ca2+ store will significantly compromise the bicuculline-induced effects31. As shown in Fig. 4a,b, paxilline pretreatment attenuated both bicuculline-induced [Ca2+]nu elevation and CREB phosphorylation in wild-type mice because paxilline induced the reduction of the perinuclear Ca2+ store content in advance (Fig. 4a,b and Fig. 2). In contrast, paxilline failed to compromise bicuculline-induced [Ca2+]nu elevation and CREB phosphorylation in the neurons from Kcnma1–/– mice (Fig. 4a,b).
Figure 4. nBK channels regulate synaptic activity-evoked [Ca2+]nu elevation, CREB phosphorylation and gene expression.
(a) Bicuculline-induced changes of fluorescence intensity in nuclear calcium imaging of intact neurons after DMSO or paxilline pretreatment. (b) Left, bicuculline-induced CREB phophorylation in intact neurons with or without paxilline pretreatment. Scale bar represents 20 μm. Right, summary of the data. One-way ANOVA (N = 5 for each group; P = 4.98 × 10–5, F2,12 = 25.29 for wild-type group, P = 3.15 × 10–5, F2,12 = 27.76 for Kcnma1–/– group) and post hoc test. (c) Left, mRNA level of c-fos after treatments as indicated. One-way ANOVA (P = 0.0015, F2,6 = 23.34 for wild-type group; P = 0.90, F2,6 = 0.11 for Kcnma1–/– group) and post hoc test. Right, bicuculline-induced c-fos expression after DMSO or paxilline pretreatment (unpaired t-test, P = 0.0053, t4 = –5.50 for control group; P = 0.0093, t4 = 4.70 for bicuculline group). (d) Left, mRNA level of Npas4 after treatments as indicated. One-way ANOVA (P = 0.00090, F2,6 = 28.02 for wild-type group; P = 0.99, F2,6 = 0.00999 for Kcnma1–/– group) and post hoc test. Right, bicuculline-induced Npas4 expression after DMSO or paxilline pretreatment (unpaired t-test, P = 0.0035, t4 = –6.16 for control group; P = 0.0082, t4 = 4.87 for bicuculline group). (e) Left, mRNA level of Atf3 after treatments as indicated. One-way ANOVA (P = 0.00048, F2,6 = 35.38 for wild-type group; P = 0.90, F2,6 = 0.11 for Kcnma1–/– group) and post hoc test. Right, bicuculline-induced Atf3 expression after DMSO or paxilline pretreatment (unpaired t-test, P = 4.29 × 10–5, t4 = –19.25 for control group; P = 0.0023, t4 = 6.88 for bicuculline group). (f) Left, mRNA level of Btg2 after treatments as indicated. One-way ANOVA (P = 0.018, F2,6 = 8.36 for wild-type group; P = 0.16, F2,6 = 0.16 for Kcnma1–/– group) and post hoc test. Right, bicuculline-induced Btg2 expression after DMSO or paxilline pretreatment (unpaired t-test, P = 0.0076, t4 = –4.98 for control group; P = 0.0057, t4 = 5.40 for bicuculline group). (g) Left, mRNA level of Bcl6 after treatments as indicated. One-way ANOVA (P = 0.0011, F2,6 = 25.61 for wild-type group; P = 0.93, F2,6 = 0.074 for Kcnma1–/– group) and post hoc test. Right, bicuculline-induced Bcl6 expression after DMSO or paxilline pretreatment (unpaired t-test, P = 0.0034, t4 = –6.21 for control group; P = 0.0040, t4 = 5.96 for bicuculline group). (h) Left, mRNA level of Ifi202b after treatments as indicated. One-way ANOVA (P = 0.011, F2,6 = 10.45 for wild-type group; P = 0.93, F2,6 = 0.075 for Kcnma1–/– group) and post hoc test. Right, bicuculline-induced Ifi202b expression after DMSO or paxilline pretreatment (unpaired t-test, P = 0.0094, t4 = –4.69 for control group; P = 0.0030, t4 = 6.44 for bicuculline group). N = 3 independent cultures from at least 3 litters for each group in c–h. Fisher's least significant difference test was used for post hoc test in one-way ANOVA. Error bars represent the mean ± s.e.m.. **P < 0.01; n.s., no significant difference. WT, wild-type.
To determine whether the effects of nBK channels blockade extended to differences in activity-evoked gene transcription, we performed Real Time quantitative Reverse Transcription PCR (qRT-PCR) to analyze the expression of activity-dependent and nuclear Ca2+-sensitive genes12,33,34 after IbTx preincubation. Indeed, paxilline induced increases in c-fos, Npas4, Atf3, Btg2, Bcl6 and Ifi202b expression (Fig. 4c–h), but not GADD45β and GADD45γ expression (data not shown), during basal neuronal activity. Paxilline-induced genes expression was blocked by STO-609 and was completely abolished in the neurons from Kcnma1–/– mice (Fig. 4c–h). Similar to the effects on bicuculline-induced [Ca2+]nu and CREB phosphorylation, paxilline pretreatment significantly attenuated bicuculline-induced elevation of the genes expression (Fig. 4c–h). Taken together, these results indicate that the nBK channels play an important role in regulating synaptic activity-evoked CREB phosphorylation and gene expression in E-T coupling.
nBK channels regulate dendritic arborization
Dendritic arborization is critically important for neuronal development and function, and the regulation of dendritic geometry is highly dependent on E-T coupling13. Given that the nBK channel is important in controlling nuclear Ca2+ signaling (Fig. 2 and 3) and gene expression (Fig. 4) in E-T coupling, we further explored the roles of nuclear Ca2+ signaling and the nBK channels in the regulation of dendritic arborization.
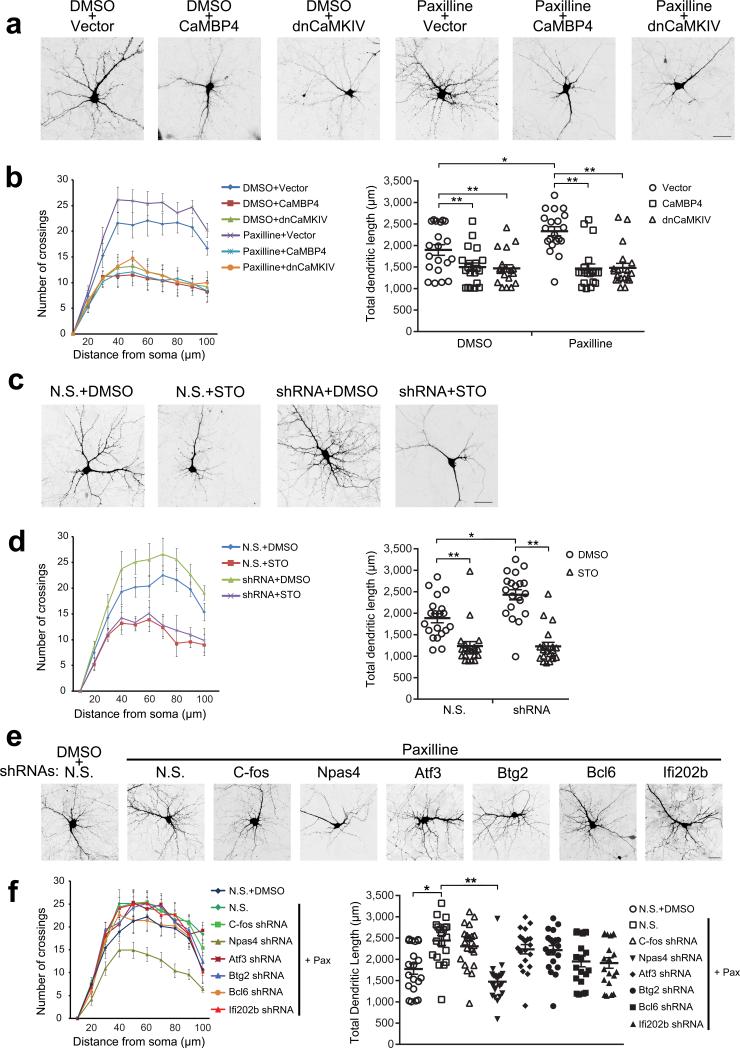
To first determine the role of nuclear Ca2+ signaling in the regulation of dendritic arborization in basal condition, we expressed CaMBP4 or dnCaMKIV in hippocampal neurons. CaMBP4 contains four repeats of the M13 CaM binding peptide and can effectively inactivate the nuclear Ca2+/CaM complex and block genomic responses induced by nuclear Ca2+ signaling12,13,15. Consistent with the previous study13, morphological analyses showed that the expression of CaMBP4 or dnCaMKIV could reduce the dendritic complexity assessed by Sholl analysis and the dendritic length (Fig. 5a,b), suggesting that nuclear Ca2+ signaling is important for the basal dendritic arborization.
Figure 5. nBK channels regulate dendritic arborization via nuclear Ca2+/CaMKIV signaling.
(a) Representative micrographs of hippocampal neurons transfected with an expression vector for GFP or cotransfected with expression vectors for GFP and CaMBP4 or for GFP and CaMKIVK75E (dnCaMKIV), with DMSO or paxilline treatment. (b) Sholl analysis (left) and quantification of total dendritic length (right) in hippocampal neurons treated as indicated. Right, one-way ANOVA (P = 0.0070, F2,57 = 5.42 for DMSO group; P = 6.22 × 10–8, F2,57 = 22.51 for paxilline group) and post hoc test; unpaired t-test (P = 0.010, t38 = –2.70) for comparison between vector groups after DMSO or paxilline treatment. (c) Representative micrographs of hippocampal neurons transfected with GFP-tagged plasmids containing BK channel-specific shRNAs or non-silencing shRNAs, with DMSO or STO-609 treatment. (d) Sholl analysis (left) and quantification of total dendritic length (right) in hippocampal neurons treated as indicated. Right, unpaired t-test (P = 0.00011, t38 = 4.32 for non-silencing shRNAs group; P = 0.0015, t38 = –3.42 for BK channel-specific shRNAs group; P = 1.17 × 10–9, t38 = 7.99 for comparison between DMSO groups after non-silencing shRNAs or BK channel-specific shRNAs transfection). (e) Representative micrographs of hippocampal neurons transfected with GFP-tagged plasmids containing gene-specific shRNAs or non-silencing shRNAs, with DMSO or paxilline treatment. (f) Sholl analysis (left) and quantification of total dendritic length (right) in hippocampal neurons treated as indicated. Right, one-way ANOVA (P = 6.80 × 10–8, F7,152 = 7.64) and post hoc test. For the data in b,d,f, n = 20 cells from 4 independent cultures from at least 4 litters for each group. Fisher's least significant difference test was used for post hoc test in one-way ANOVA. Error bars represent the mean ± s.e.m.. Scale bar = 30 μm. *P < 0.05, **P < 0.01. N.S., non-silencing shRNAs.
To further investigate the role of nBK channels in the regulation of dendritic arborization, hippocampal neurons were pretreated with IbTx to exclude the involvement of pmBK channel (Fig. 5 and Supplementary Fig. 4). Morphometric analyses revealed that neurons treated with paxilline showed a significant increase both in the complexity of the dendrites (Fig. 5a) and in the total dendritic length (Fig. 5b). Importantly, this paxilline-induced increase was reversed by the expression of CaMBP4 or dnCaMKIV (Fig. 5a,b), suggesting that paxilline-induced increase in dendritic complexity and length is also dependent on the nuclear Ca2+ signaling. Similarly, neurons transfected with BK channel-specific shRNAs showed a significant increase both in the dendritic complexity and in the total dendritic length (Fig. 5c,d). STO-609 suppressed basal dendritic arborization and reversed the shRNA-induced increase, once again suggesting that both basal and paxilline-induced dendritic arborizations are dependent on nuclear Ca2+ signaling. To determine the specific genes mediating paxilline-induced morphological changes, we used shRNAs to knock down the expression of nBK channel-target genes (Supplementary Fig. 5). As shown in Fig. 5e,f and Supplementary Fig. 6, only shRNA against Npas4 significantly reduced dendritic arborization, and reversed paxilline-induced morphological changes, suggesting the involvement of Npas4 in both basal and paxilline-induced dendritic arborization. Taken together, these results indicate that nBK channels can regulate dendritic arborization in a nuclear Ca2+/CaMKIV signaling-dependent manner.
DISCUSSION
In this study, we demonstrate for the first time to our knowledge that functional BK channels are expressed on the NE of hippocampal neurons and that the activities of nBK channels regulate synaptic activity-triggered nuclear Ca2+ signal, CREB activation, gene expression, and dendritic arborization. These findings reveal the important role of the nBK channel as a key regulator and molecular linker between neuronal activities, nuclear Ca2+ signaling, and gene expression at the NE level.
The positive expression of BK channels in the nuclear envelope was based on immunofluorescence, immunoelectron microscopy and immunoblot data in both intact cells and isolated nuclei (Fig. 1a–f). The use of Kcnma1–/– mice further confirmed the specificity of the labeling (Fig. 1). Most importantly, excised patches of nuclear membrane confirmed the presence of the nBK channels that exhibit similar voltage-dependence, Ca2+-dependence, single channel conductance and pharmacological properties to the BK channels found in the plasma membrane (Fig. 1g–i). Thus, several independent lines of evidence indicate the presence of functional BK channels in the NE, supporting the notion that the BK channels have multiple sites of function, including the plasma membrane22,35-37, mitochondria17,18,38, and, as shown here, the NE.
Nuclear Ca2+ is a potent signal in regulating neuronal gene expression, and represents a key player in the dialog between synapse and nucleus11,12,31. However, the source of nuclear Ca2+ has been debated for many years6, and the mechanisms by which nuclear Ca2+ is regulated by synaptic activity are incompletely understood. It has been proposed that the nuclear pore complex (NPC) is large enough to provide routes for passive diffusion of ions to cross the NE, which would imply that nuclear Ca2+ signaling is dependent on cytosolic Ca2+ changes6,7. However, numerous evidences suggested that nucleus has an autonomous Ca2+ signaling system, which can generate its own independent Ca2+ transients6–10. (1) NPC size is regulated by the filling state of the NE lumen. Depletion of perinuclear Ca2+ stores has been shown to induce a conformational change of the NPC and reduce the NPC permeability6. (2) The ability to obtain high-resistance gigaohm seals during patch clamping in the nucleus-attached mode, when dozens of NPCs may be encompassed by the patch pipette, suggested that the NPCs can exist in a closed state that restricts ion flow39. (3) The ion movement seems not freely across NPC, because studies show that there are differences in ion concentrations between cytoplasm and nucleoplasm40–43. (4) When HepG2 cells were stimulated with extracellular ATP, nucleoplasmic Ca2+ signal preceded cytosolic Ca2+ signal by ~450 ms, suggesting the independence of nucleoplasmic Ca2+ signal9. (5) In the present study, application of paxilline to intact neurons induces nucleoplasmic Ca2+ elevation without raising cytosolic Ca2+ concentration (Fig. 2b), suggesting that the nucleoplasmic Ca2+ is independent from cytoplasmic Ca2+ and NPC-mediated Ca2+ transport. Nonetheless, the existence of this autonomous nuclear Ca2+ signaling system does not exclude the presence of the NPC-mediated Ca2+ transport.
Partly due to a lack of suitable experimental methods, the ionic gradient for K+ across the NE remains unknown in intact cells. Recent studies in isolated nuclei loaded with K+-sensitive fluorescent indicators showed that the K+ concentration in the perinuclear space is much lower than the cytoplasm and nucleoplasm41. Therefore the changes in the nuclear K+ channel activity would alter the K+ flux across the NE and ultimately lead to the alteration of the ΔΨn. Indeed, our results showed that the nBK channels, with the similar electrophysiological properties to pmBK channels (Fig. 1g–i), regulate the ΔΨn (Fig. 2a), supporting the existence of the K+ gradient across the NE. Nevertheless, all these results were obtained from isolated nuclei, and need further confirmation in intact cells. It is noteworthy that the open probability of RyRs is elevated upon changes of voltage24,44–46. Thus, it is highly probable that RyRs may be affected by paxilline-induced ΔΨn as revealed by the voltage-sensor DiOC6(3) (Fig. 2a). Similar voltage-induced RyRs-mediated Ca2+ release were observed in pancreatic beta cells24. Nonetheless, we do not exclude the existence of other voltage-sensitive pathways that directly or indirectly trigger the release of nuclear calcium24.
Coupling between synaptic excitation and gene expression is critical for a broad range of long-term changes in nervous system functions. Although many intracellular molecules, such as calmodulin, MAPKs and CaMKs, have been shown to participate in synapse-to-nucleus signaling, faster modulators are also required to convey fast information-laden synaptic electrical signals6,26. Via rapid ion channel kinetics, the nBK channel may function as a fast molecular modulator between neuronal activity and nuclear Ca2+ to convey the signaling from synapse to nucleus. The NE is inaccessible to analysis with patch clamp pipettes and voltage-sensitive dyes in intact neurons, which makes it difficult to measure the activity of nBK channels synchronously during neuronal activity. Therefore, the effects of neuronal activity on the activity of the nBK channel require further exploration. It is noteworthy that neuronal activity can modulate the pmBK channels in a bi-directional manner: activity-induced intracellular Ca2+ elevation and CaMKII activation activate pmBK channels47, whereas activity-induced calcineurin activation has the opposite effect48. If the same regulation also applies to nBK channels, the regulation of nBK channel activity by neuronal activity may be bi-directional as well. The development of suitable experimental systems will help to elucidate this question.
The long-term maintenance of activity-induced, functional adaptations requires nuclear Ca2+ signaling-dependent, CREB-mediated transcription to activate gene expression12,15,26–28. Using immunofluorescence, immunoblot and qRT-PCR, we show that blockade of the nBK channel increases CREB phosphorylation and neuronal activity-dependent gene expression during basal neuronal activity, but compromises those during bicuculline-induced action potential bursting (Fig. 3 and 4). Bicuculline-induced CREB phosphorylation and gene expression need sustained, Ca2+ store-derived nuclear Ca2+ elevation, and depletion of the Ca2+ store will significantly compromise these effects31. In our studies, paxilline pretreatment induced the release of nuclear Ca2+ and the reduction of the Ca2+ store content (Fig. 2), and consequently, compromise the bicuculline-induced nuclear Ca2+ elevation needed for CREB phosphorylation and gene expression (Fig. 4). These results are consistent with studies proposing that filling of the nuclear Ca2+ store is required for synaptically evoked nuclear Ca2+ signaling and CREB-mediated gene expression31. nBK channel-modulated CREB phosphorylation and gene expression is dependent on nuclear Ca2+ signaling because specific blockade of nuclear Ca2+ signaling via dnCaMKIV, PV-NLS, and STO-609, but not via the ERK inhibitor U0126, abolished the effects of nBK channel blockade (Fig. 4). In the intact cell, we cannot exclude the possibility that Ca2+ release from other organelles may drive gene expression in our conditions. However, as shown in Fig. 2 and 3, the NE would be responsible for an important part of these intracellular Ca2+ signals.
The dendritic tree determines the neuronal connectivity and has a strong influence on synaptic transmission and integration of synaptic signals. Moreover, dendritic geometry itself is regulated by neuronal activity. Our results show here that nBK channel blockade causes a significant increase both in the total dendritic length and in the complexity of the dendrites (Fig. 5), further confirming the importance of nuclear Ca2+ in the regulation of dendritic arborization13. Knockdown of one of the nBK channel-target gene, Npas4, expression completely blocked paxilline-induced morphological changes, indicating the critical role of Npas4 in this regulation. Importantly, Npas4 also functions as a transcriptional regulator, with a key role in activity-dependent regulation of inhibitory synapse development33, contextual memory formation34, and neuronal protection12,49. Knockdown of Npas4 significantly inhibits brain-derived neurotrophic factor (BDNF) expression11,33, implicating the potential mechanisms by which Npas4 knockdown blocks paxilline-induced morphological changes.
By controlling nuclear Ca2+ signaling, the nBK channel links neuronal activity to the regulation of CREB activation and gene expression, the control of total dendritic length and branching patterns. The blockade of the nBK channels represents a new strategy for the development of effective therapies for the reduction of dendrite complexity in aging, Alzheimer's disease and autism11,33. These findings may also help explain the various phenotypes, such as Purkinje cell dysfunction37, cerebellar ataxia35,37, and abnormal circadian behavioral rhythms36, found in BK channel global knockout mice.
METHODS Reagents and antibodies
Paxilline, IbTx, STO-609, thapsigargin, EGTA, ruthenium red, heparin, digitonin, 2-APB, U0126, bicuculline, DMSO, DAPI and all the other reagents to make solutions were from Sigma-Aldrich. Fluo-4/AM, Fluo-4/dextran, X-Rhod-1 were from Life Technologies. Antibodies specific for BK channel α-subunit (L6/60, Millipore MABN70, immunocytochemisty (ICC) 20 μg/ml, western blot (WB) 10 μg/ml, reference (Ref.) PMID 16566008), BK channel β4-subunit (Alomone Lab APC-061, ICC 1:100, Ref. PMID 21697543), Lamin B (Abcam ab16048, WB 1:1000, ICC 1:500, Ref. PMID 24265311) GAPDH (Cell Signalling 3683S, WB 1:1000, Ref. PMID 22895339), Calnexin (Cell Signalling 2433S, WB 1:1000, Ref. PMID 19815548), COX IV (Cell Signalling 4850S, WB 1:1000, Ref. PMID 19737931), phospho-CREB (Cell Signalling 9198S, WB 1:1000, ICC 1:500, Ref. PMID 17906627), CREB (Cell Signalling 9197S, WB 1:1000, Ref. PMID 20864036), phospho-CaMKIV (Santa Cruz Biotechnology sc-28443-R, WB 1:200, Ref. PMID 18559891), CaMKIV (Cell Signalling 4032S, WB 1:1000, Ref. PMID 21784978), phospho-ERK (Cell Signalling 9101S, WB 1:1000, Ref. PMID 11782377), and ERK (Cell Signalling 9102S, WB 1:1000, Ref. PMID 21669876), actin (Cell Signalling 4967S, WB 1:1000, Ref. PMID 17525163), RyR (Thermo MA3-925, WB 1:1000, Ref. PMID 1645737), Na/K ATPase (Cell Signalling 3010S, WB 1:1000, Ref. PMID 21724843) were used.
Cell culture and transfection and virus infection
Wild-type or Kcnma1–/– mice (C57BL/6J strain) and Sprague-Dawley rats were used for cell culture. All of the experiments were conducted in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (China) and were approved by the Southern Medical University Animal Ethics Committee. Three to four animals were housed per cage on a 12 h light, 12 h dark cycle, similar to those reported in previous publications. Pure primary hippocampal neurons from postnatal day 1-old wild-type or Kcnma1–/– mice (C57BL/6J/129 strain) 36 were cultured in Neurobasal medium with 2% B27 supplement, 0.5 mm glutamine, and 100 U/ml penicillin/streptomycin (Invitrogen, San Diego, CA). Cells were grown at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. The data in Fig. 1d,2b,2c and 3b were done in intact neurons or isolated nuclei of primary cultured hippocampal neurons from postnatal day 1 Sprague-Dawley rat. All experiments were performed on cells cultured for 14 d. DNA transfection was done on DIV 8 by using Lipofectamine 2000 (Invitrogen, San Diego, CA) as described50. BK-GFP plasmid was gift from Dr. Xiangdong Tang51,52. PV-NLS plasmid was gift from Dr. Michael Nathanson32. Kinase-inactive form of CaMKIV (dn CaMKIV, CaMKIVK75E) and CaMBP4 plasmids were gifts from Dr. Hilmar Bading12. For shRNA, three different sequences were obtained from Shanghai GeneChem (shBK 1, shBK 2 and shBK 3), cloned and tested for silencing efficiency. shBK (1) and (2) (GTGATGCCAAAGAAGTTAA and GCACTTACGTACTGGGAATGT) was selected. The targeting sequences for all RyRs or RyR2 are ACATGGAGACCAAGTGCTT and GGAAAGAAGTCGATGGCAT, respectively25. As control, a scramble version of this sequence was used. For the studies on dendrite morphology, neurons were analyzed 4–5 days after transfection. In lentivirus-delivery shRNA, virus was constructed and packed by Shanghai GeneChem Co.,Ltd, and infection was performed following the manual. 107/ml of the virus was used to transfect the cultured hippocampal neurons. The shRNA target sequences of nBK channel-regulated gene are listed below:
Atf3 (1) 5’-TCCTAGCCTGTCAACATAATA-3’,
(2) 5’-TATTATGTTGACAGGCTAGGA-3’.
Npas4 (1) 5’-TCTGTGACTTAACGTCTTCAA-3’,
(2) 5’-GGGCGACAGTATCTACGATAT -3’.
Btg2 (1) 5’- GGACGCACTGACCGATCATTA-3’,
(2) 5’-GCTATCGCTGTATCCGTATCA-3’.
c-fos (1) 5’- GCAGATCTGTCCGTCTCTAGT-3’,
(2) 5’-GCTGAAGGCAGAACCCTTTGA-3’.
Bcl6 (1) 5’-GCACGCTAGTGATGTTCTTCT-3’,
(2) 5’-GGACATCTTGACGGACGTTGT-3’.
Ifi202b(1) 5’- GCTACAGAGAGCCAATATTAC-3’,
(2) 5’-GCTGCTCCTAACCAAATTATT-3’.
Western blot
Western blot were performed as described50. Briefly, protein concentration was measured by BCA Protein Assay Kit (Thermo). Samples were combined with 2 × SDS loading buffer, boiled for 10 min, and loaded onto 10% or 4–20% gradient polyacrylamide-SDS gel. Proteins were then transferred to a PVDF membrane (Millipore, Billerica, MA) for 2 h at 350 mA, and membranes were incubated in Odyssey Blocking Buffer (LI-COR) for 2 h at room temperature. After incubation with primary antibodies for overnight at 4°C, the blots were washed three times in TBS containing 0.1% Tween 20 for 15 min and then incubated with peroxidase- or IRDye-conjugated secondary antibody for 1 h in TBS, 0.1% Tween 20 at room temperature. Immunoreactivity was detected by chemiluminescence with ECL reagent or LiCor imaging system.
Extraction of plasma membrane protein
For surface biotinylation, neurons were cooled on ice, washed twice with ice-cold PBS containing 1mM CaCl2 and 0.5 mM MgCl2, and then incubated with PBS containing 1mM CaCl2, 0.5 mM MgCl2, and 1 mg/ml Sulfo-NHS-SS-Biotin (Pierce) for 30 min at 4°C. Unreacted biotin was quenched by washing cells three times with ice-cold 100mM Glycine (pH7.4). Cultures were harvested in RIPA buffer. Homogenates were centrifuged at 132,000 rpm for 20 min at 4°C. The resulting supernatant volume was measured and 15% of it separated as the total protein. The remaining 85% of the homogenate was rotated overnight at 4°C with Streptavidin beads (Pierce). Precipitates were washed with RIPA buffer and analyzed by immunoblotting with each antibody.
Nuclei isolation
Cultured neurons were collected and resuspended in Buffer A (2.0 mM MgCl2, 125 mM KCl, 10 mM HEPES (pH 7.5), protease inhibitors). After swelling on ice for 10 min, the cell membrane and cytoskeleton were disrupted by 15 brief homogenization. The solution were filtered with 3 layers of gauze, and then centrifuged with 1000g for 10min. The precipitate were resuspended in Buffer N (0.25 M sucrose in buffer A), and covered on the top of Buffer B (1.1 M sucrose in buffer A) in a centrifuge tube and centrifuged with 1000g for 10 min, at 4°C. We only performed experiments in intact and clean nuclei (free of ER contamination). All the data of isolated nuclei were from at least 3 independent cultures from at least 3 litters. The isolated nuclei were treated with Triton X100 buffer (1% Triton X-100, 100 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 0.5 mM CaCl2,1 mM DTT) for 7 min, and centrifuged with 650 g for 5 min. The resultant precipitate was saved as the denuded nuclei fraction and the supernatant as the nuclear envelope fraction for further analyses. Alternatively, nuclei isolation followed the previous report31. Nuclei were prepared by lysing hippocampal neurons at room temperature in PBS (pH 7.2) containing 0.5% (v/v) NP-40, 7.5 mM K+ 2EGTA, 2.5 mM Ca2+EGTA and 2.5 (free Ca2+ concentration is 60 nM). The free Ca2+ concentration was increased to 1 μM by adding 6.2 volumes of 10 mM Ca2+EGTA. After 60 to 90 s, 1.8 volumes of 4× sample buffer was added, nuclei were scraped off the dish, boiled for 7 min, and subjected to SDS-PAGE and immunoblot analyses.
Nuclear protein extraction
Nuclear protein was extracted using the PIERCE Nuclear Protein Extraction Kit (Thermo), following the manual. Briefly, cells were collected by centrifugation, 500g for 3 min at 4°C. Resuspend cells in 200μl precooled CER I by 15 sec of intense vortex, and incubate on ice for 10 min. Add 11μl precooled CER II to the solution, and after 5 sec of intense vortex, incubate it on ice for 1 min. After 5 sec of intense vortex, centrifuge the solution at 16000g for 5 min, at 4°C, discard the supernatant. Add 100μl NER to the precipitate and vibrate it intensively for 15 sec, and incubate it on ice for 10 min. Repeat this step 4 times, and then centrifuge the solution at 16000g for 10 min, at 4°C. The supernatant was purified nuclear protein.
Patch-clamp experiments
For whole-cell recordings, electrodes were with a final resistance of 5–10 MΩ when filled with pipette solution (in mM): 120.0 potassium gluconate, 15.0 HEPES, 2.2 CaCl2, 1.0 MgCl2, 4.0 EGTA, and 4.0 HEDTA, pH7.35, 305mOsm. Currents were recorded using an Axopatch 200B (Axon Instruments, Foster City, CA) amplifier at a sampling rate of 1–5 kHz and leak-subtracted on line. Series resistance never exceeded 25 MΩ and was compensated. Data were acquired and analyzed with pClamp9 (Axon Instruments). Single-channel currents were recorded from nuclei positively labeled with ethidium homodimer-1 by using standard patch-clamp recording procedures24. Currents were recorded in symmetrical 135 mM K using a patch-clamp amplifier (Axopatch 200; Axon Instruments) at a sampling rate of 5 kHz and filtered at 1 kHz filter. Experiments were performed at RT. High resistance seals were formed (5–7 GΩ), indicating minimal contamination by the endoplasmic reticulum (ER) membrane24. Under these circumstances, it has been proposed that nuclear pores would be occluded or nonconducting because of experimental conditions or lack of cytosolic factors6,39,53. PCLAMP software was used to measure the current magnitude and duration.
Ca2+ imaging
Intact neurons were loading with Fluo-4/AM or X-Rhod-1 AM (Life Technology) in transfected neurons, in HEPPS buffer for 30 min, and washed 3 times before imaging. Single isolated nuclei were loaded as reported24. The membrane impermeant Ca2+ probe Fluo-4/dextran (30 μg/ml; 30 min at 4°C; Life Technologies) loaded the nucleoplasm while the membrane permeant Ca2+ probe Fluo-4/AM (20 μM; 60 min at 4°C) loaded the nuclear envelope. After loading, the nuclei were washed twice with the intracellular medium (125 mM KCl/2 mM K2HPO4/40 mM Hepes/0.1 mM MgCl2, pH 7.2/100 nM Ca2+, with 10.2 mM EGTA and 1.65 mM CaCl2) and then were equilibrated in the same medium supplemented with 1 μM of ATP and 300 nM Ca2+ for a few minutes to load nuclei with Ca2+. After that, they were washed twice again with the intracellular buffer (without ATP and Ca2+). Experiments were done at RT. No probe leakage was detected during the experiment. Ca2+ imaging in single isolated nuclei was performed by using an Olympus FV1000 Confocal microscope. Images were collected at 1 s intervals, and fluorescence was measured by using the Olympus FV1000 software package. The difference (ΔF) between the mean fluorescence measured in a given region of interest (ROI) and the corresponding control value for each ROI (F 0) was expressed as fraction of the control (ΔF/F 0) and was plotted as a function of time.
Nuclear Transmembrane Potential (ΔΨn) Measurements
Isolated nuclei were equilibrated in intracellular medium with DiOC6(3) (200 nM, RT, Life Technologies), which is a fluorescent cationic lipophyllic dye whose incorporation into lumen is proportional to ΔΨ24. After 10 min, the nuclear envelope became stained. The nuclear were washed for 3 times before imaging with Olympus FV1000 Confocal microscope. Images were collected at 1 s intervals, and fluorescence was measured by using the Olympus FV1000 software package. The difference (ΔF) between the mean fluorescence measured in a given region of interest (ROI) and the corresponding control value for each ROI (F 0) was expressed as fraction of the control (ΔF/F 0) and was plotted as a function of time.
Immunocytochemistry
Cells were washed with PBS and fixed for 20 min with 4% paraformaldehyde at room temperature. To permeabilize cells, cells were treated with 0.3% Triton X-100 in PBS solutions. Nonspecific binding was blocked for 1 h at room temperature using 5% bovine serum. Primary antibodies were then added and incubated overnight at 4 °C. Cells were then washed and incubated for 1 h with the fluorescent-labeled secondary antibodies at room temperature, and washed for three times to remove the unbound antibodies.
Phospho-CREB Immunofluorescence
CREB phosphorylation was induced in isolated nuclei by increasing Ca2+ in an EGTA-buffered intracellular medium from 60 nM to 200 nM Ca2+ or by addition of paxilline for 3 min. Nuclei were fixed with 4% paraformaldehyde (wt/vol) and permeabilized with 0.1% Triton X-100 for 10 min. Nuclei were preincubated with blocking buffer; then anti-phospho-CREB antibodies were applied for 16 h at 4°C. Phospho-CREB was visualized with TRITC-conjugated secondary antibodies (1 h, RT). High Ca2+ gave the maximum response.
Morphometric Analyses
For three-dimensional Sholl analysis, total dendritic length and spine morphology were calculated by using Image-J freeware software. Briefly, a z-stack acquisition was imported, calibrated in Image-J, and manually traced. Total dendritic length was then computed. For Sholl analysis, the shell interval was set at 10 μm. All analyses were performed blind.
RNA Extraction and cDNA Synthesis
Total RNA was isolated from hippocampal primary neuron cultures with RNeasy Mini Kit (TAKARA) including an optional DNase I treatment at room temperature for 15 min according to manufacturer's instructions (TAKARA). 1.2 μg of extracted RNA was reverse transcribed into first strand cDNA using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA).
Real Time Quantitative PCR
Quantitative reverse transcriptase PCR (qRT-PCR) was done on a Statagene Mx3000P thermal cycler using Universal qRT-PCR master mix for the indicated genes (TAKARA). The primers were designed and synthesized as follow:
c-Fos sense 5'- GCGGGAGTGGTGAAGACCAT-3',
antisense 5'- GCTTGGAGCGTATCTGTCAGCTC-3'.
Npas4 sense 5'- ACATCATGAGTCTTGCCTGCATC-3',
antisense 5'- TCCAGGTAGTGCTGCCACTATGTC-3'.
Atf3 sense 5'- ATGATGCTTCAACACCCAGGC-3'
antisense 5'- TTAGCTCTGCAATGTTCCTTC-3'
Btg2 sense 5'- GAGCGAGCAGAGACTCAAGGTT-3',
antisense 5'- CGATAGCCAGAACCTTTGGATGG-3'.
Bcl6 sense 5'- CAGAGATGTGCCTCCATACTGC-3',
antisense 5'- CTCCTCAGAGAAACGGCAGTCA-3'.
Ifi202b AAGAAAGGCTGGTTGATGGAGAG-3',
antisense 5'- GGTCAGTTGTCCAGATACCACC-3'.
GADD45β sense 5'- GGAGACATTGGGCACAACCGAA-3',
antisense 5'- CTGCTCTCTTCACAGTAACTGGC-3'.
GADD45γ sense 5'- TCTACGAGTCCGCCAAAGTCCT-3',
antisense 5'- CTCACAGCAGAACGCCTGAATC-3'.
GAPDH sense 5'-GGCACAGTCAAGGCTGAGAATG-3',
antisense 5'-ATGGTGGTGAAGACGCCAGTA-3'.
Expression of target genes was normalized against the expression of GAPDH as endogenous control gene. Data were derived from cells from 3 independent cultures from at least 3 litters.
Statistical Analysis
SPSS 13.0 was used for statistical analysis. The data were collected and processed randomly. No statistical methods were used to predetermine sample size, but our sample sizes are similar to those reported in previous publications.50,54–57 Statistical significances were determined by comparing means of different groups using unpaired t-test or one-way ANOVA followed by Fisher's least significant difference (LSD) test. Two-sided test was used in the analysis. Data met the assumptions of the statistical tests used, and equal variances were formally tested. The criterion for excluding data points were established prior to data collection. An outlier was defined as a value outside the mean ± 3 s.d.. All the immunocytochemistry, immunoelectron microscopy imaging, sholl analysis, qRT-PCR, calcium imaging and electrophysiology experiments were carried out blind. The western blot was not blind when loading the samples, but data collection and analyses were performed blind. For the box and whisker plot, the bottom and top of the box are the first and third quartiles, and the band inside the box is the median. The whisker represents the minimum and maximum of all of the data. The data were expressed as mean ± s.e.m. if not otherwise specified.
Supplementary Material
Acknowledgment
We thank Dr. Xiangdong Tang (Nankai University) for BK-GFP plasmid, Dr. Michael Nathanson (Yale University) for PV-NLS plasmid, Dr. Hilmar Bading (University of Heidelberg) for CaMKIVK75E and CaMBP4 plasmids, Dr. Feng-Xia Liang and Microscopy Core of New York University Medical Center for immunoelectron microscopy analysis. This work was supported by the National Natural Science Foundation of China (Grants 81030022; 81329003; U1201225 to TM.G., 30900581 to B.L., 81001129 to L.H.), the Program for Changjiang Scholars and Innovative Research Team (Grant IRT1142), Guangdong Natural Science Foundation (Grant 9351051501000003; CXZD1018 to TM.G., 10451051501004726 to L.H.), Guangzhou Science and Technology Project (Grant 7411802013939), Medical Scientific Research Foundation of Guangdong Province (B2010170 to L.H.), the National Heart, Lung, and Blood Institute (Grant R01-HL102758 to A.L.M.).
Footnotes
The authors declare no competing financial interests.
Author contributions:
B.L. and TM.G. designed the study; B.L., W.J., L.H. conducted biochemistry, Ca imaging, immunocytochemistry experiments; B.L., P.W., and Z.L. conducted electrophysiological experiments, B.L. and S.L., cultured neurons; A.K.F, A.L.M, MH.H, and XH. Z. contributed new reagents/analytic tools; B.L., W.J., P.W., Z.L., and L.H. collected and analyzed data; B.L., L.H., and TM.G. wrote the manuscript.
A supplementary methods checklist is available
References
- 1.Wheeler DG, et al. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–1124. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calcraft PJ, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong XP, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wegierski T, et al. TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. EMBO J. 2009;28:490–499. doi: 10.1038/emboj.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signalling. J.Cell Sci. 2009;122:2337–2350. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- 7.Carafoli E, Nicotera P, Santella L. Calcium signalling in the cell nucleus. Cell Calcium. 1997;22:313–319. doi: 10.1016/s0143-4160(97)90016-6. [DOI] [PubMed] [Google Scholar]
- 8.Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- 9.Leite MF, et al. Nuclear and cytosolic calcium are regulated independently. Proc Natl Acad Sci U S A. 2003;100:2975–2980. doi: 10.1073/pnas.0536590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stehno-Bittel L, Luckhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 11.Bading H. Nuclear calcium signalling in the regulation of brain function. Nat.Rev.Neurosci. 2013;14:593–608. doi: 10.1038/nrn3531. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SJ, et al. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS.Genet. 2009;5:e1000604. doi: 10.1371/journal.pgen.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauceri D, Freitag HE, Oliveira AM, Bengtson CP, Bading H. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron. 2011;71:117–130. doi: 10.1016/j.neuron.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Limback-Stokin K, Korzus E, Nagaoka-Yasuda R, Mayford M. Nuclear calcium/calmodulin regulates memory consolidation. J.Neurosci. 2004;24:10858–10867. doi: 10.1523/JNEUROSCI.1022-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J.Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misonou H, et al. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas RM, et al. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience. 2006;139:1249–1261. doi: 10.1016/j.neuroscience.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 18.Singh H, et al. MitoBK(Ca) is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci U S A. 2013;110:10836–10841. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong LW, Gao TM, Huang H, Zhuang ZY, Tong Z. Transient forebrain ischemia induces persistent hyperactivity of large conductance Ca2+-activated potassium channels via oxidation modulation in rat hippocampal CA1 pyramidal neurons. Eur.J.Neurosci. 2002;15:779–783. doi: 10.1046/j.1460-9568.2002.01908.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu H, et al. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee US, Cui J. BK channel activation: structural and functional insights. Trends Neurosci. 2010;33:415–423. doi: 10.1016/j.tins.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat.Rev.Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 23.Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol. 1997;136:531–544. doi: 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quesada I, et al. Nuclear KATP channels trigger nuclear Ca(2+) transients that modulate nuclear function. Proc Natl Acad Sci U S A. 2002;99:9544–9549. doi: 10.1073/pnas.142039299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu B, Yamaguchi H, Lai FA, Shen J. Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc.Natl.Acad.Sci.U.S.A. 2013;110:15091–15096. doi: 10.1073/pnas.1304171110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr.Opin.Neurobiol. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 27.Han MH, et al. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J.Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 29.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)-and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 30.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 31.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues MA, et al. Nucleoplasmic calcium is required for cell proliferation. J.Biol.Chem. 2007;282:17061–17068. doi: 10.1074/jbc.M700490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Y, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramamoorthi K, et al. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011;334:1669–1675. doi: 10.1126/science.1208049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J.Biol.Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 36.Meredith AL, et al. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat.Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sausbier M, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc.Natl.Acad.Sci.U.S.A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu W, et al. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 39.Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev. 2001;81:1–19. doi: 10.1152/physrev.2001.81.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Dick DA. The distribution of sodium, potassium and chloride in the nucleus and cytoplasm of Bufo bufo oocytes measured by electron microprobe analysis. J Physiol. 1978;284:37–53. doi: 10.1113/jphysiol.1978.sp012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garner MH. Na,K-ATPase in the nuclear envelope regulates Na+: K+ gradients in hepatocyte nuclei. J Membr Biol. 2002;187:97–115. doi: 10.1007/s00232-001-0155-5. [DOI] [PubMed] [Google Scholar]
- 42.Gifford JD, Galla JH, Luke RG, Rick R. Ion concentrations in the rat CCD: differences between cell types and effect of alkalosis. The American journal of physiology. 1990;259:F778–782. doi: 10.1152/ajprenal.1990.259.5.F778. [DOI] [PubMed] [Google Scholar]
- 43.Rick R. pHi determines rate of sodium transport in frog skin: results of a new method to determine pHi. The American journal of physiology. 1994;266:F367–374. doi: 10.1152/ajprenal.1994.266.3.F367. [DOI] [PubMed] [Google Scholar]
- 44.Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev. 2009;89:1153–1176. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- 45.Chen SR, Li X, Ebisawa K, Zhang L. Functional characterization of the recombinant type 3 Ca2+ release channel (ryanodine receptor) expressed in HEK293 cells. J Biol Chem. 1997;272:24234–24246. doi: 10.1074/jbc.272.39.24234. [DOI] [PubMed] [Google Scholar]
- 46.Zahradnikova A, Meszaros LG. Voltage change-induced gating transitions of the rabbit skeletal muscle Ca2+ release channel. J Physiol. 1998;509( Pt 1):29–38. doi: 10.1111/j.1469-7793.1998.029bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van WI, du LS. Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. J.Neurophysiol. 2011;105:1651–1659. doi: 10.1152/jn.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loane DJ, Hicks GA, Perrino BA, Marrion NV. Inhibition of BK channel activity by association with calcineurin in rat brain. Eur.J.Neurosci. 2006;24:433–441. doi: 10.1111/j.1460-9568.2006.04931.x. [DOI] [PubMed] [Google Scholar]
- 49.Shamloo M, et al. Npas4, a novel helix-loop-helix PAS domain protein, is regulated in response to cerebral ischemia. Eur.J.Neurosci. 2006;24:2705–2720. doi: 10.1111/j.1460-9568.2006.05172.x. [DOI] [PubMed] [Google Scholar]
- 50.Huang L, Li B, Li W, Guo H, Zou F. ATP-sensitive potassium channels control glioma cells proliferation by regulating ERK activity. Carcinogenesis. 2009;30:737–744. doi: 10.1093/carcin/bgp034. [DOI] [PubMed] [Google Scholar]
- 51.Avdonin V, Tang XD, Hoshi T. Stimulatory action of internal protons on Slo1 BK channels. Biophys J. 2003;84:2969–2980. doi: 10.1016/S0006-3495(03)70023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang XD, et al. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–535. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- 53.Taylor CW, et al. IP3 receptors: some lessons from DT40 cells. Immunol.Rev. 2009;231:23–44. doi: 10.1111/j.1600-065X.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 54.Cao X, et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nature medicine. 2013;19:773–777. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- 55.Woo RS, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Chen YJ, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li XM, et al. Contribution of downregulation of L-type calcium currents to delayed neuronal death in rat hippocampus after global cerebral ischemia and reperfusion. J Neurosci. 2007;27:5249–5259. doi: 10.1523/JNEUROSCI.0802-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.