Abstract
Currently, there are no FDA approved medications for treatment of cocaine addiction underscoring the dire need to develop such a product. There is an accumulating body of evidence that L-tetrahydropalmatine (L-THP), a non-selective dopamine antagonist, can be used for the treatment of cocaine addiction. Indeed, the FDA recently approved its usage in a Phase I study in cocaine abusers and it was indispensable to develop a simple and sensitive method for the simultaneous determination of L-THP and cocaine in human plasma. We developed a UPLC-FLD method for quantitation of these molecules using an ACQUITY BEH C18 column (2.1 × 50mm, 1.7um) and a mobile phase that consisted of 5 mM ammonium phosphate (PH=4.75), methanol, and acetonitrile (v:v:v, 78:16:6). Venlafaxine was used as the internal standard while hexane was used for the liquid-liquid extraction. The flow rate was 0.4ml/min with fluorescence detection using an excitation wavelength of 230nm and emission detection wavelength of 315nm. This method was selective, linear and sensitive with a lower limit of quantification of 2.5 ng/mL for both cocaine and L-THP. The intra-day precision of cocaine and L-THP was <9.50% while the accuracy was <4.29%. The inter-day precision of cocaine and L-THP was <9.14%, and the accuracy was <12.49%. The recovery for cocaine and L-THP ranged from (43.95 - 50.02%) and (54.65 - 58.31%), respectively. In comparison to forty reported cocaine quantitation methods this method is simple, sensitive and cost-effective and can be used for simultaneous quantitation of L-THP and cocaine. This method meets the FDA guidelines and can be used in current and future clinical studies.
Keywords: cocaine, L-THP, UPLC, analysis, human plasma, fluorescence
1. INTRODUCTION
L-tetrahydropalmatine (L-THP), a non-selective dopamine antagonist, has recently emerged as a promising agent for cocaine addiction [1]. It is an alkaloid, isolated from a Chinese herbal medicine called Corydalis yanhusuo, and has been used in China for 40 year as an analgesic. Primarily a modest dopamine receptor antagonist, L-THP also interacts with several other neurotransmitter receptors, such as the serotonin receptor, 5-hydroxytryptamine (5-HT) [2], the Gamma Amino Butyric Acid (GABA) receptors [3] and the alpha-1 adrenergic receptor [4–6]. The effectiveness of L-THP as a non-opioid analgesic and sedative/anxiolytic agent resulted in the approval of purified L-THP for this indication by the Chinese State Food and Drug Adminisration (SFDA) and has led to extensive investigation of the pharmacological properties of L-THP. The finding that the analgesic (and other) effects of L-THP were likely attributable to dopamine (DA) receptor antagonism [7,8]. The interactions of L-THP at the D3 dopamine receptor is of particular interest because that receptor has been associated with drug seeking behavior. Furthermore, a recent study conducted in China demonstrated that 21 patients receiving L-THP 30 mg twice daily increased abstinence from heroin as compared to only 9 patients recieving placebo over a 13 mont period [9]. It has been hypothesized that L-THP's unique dopaminergic interactions combined with its interactions with other nondopamine receptors contribute to its effectiveness in attenuating cocaine seeking behavior and reinstatement [10,11], therefore it could be a viable clinical treatment option for coaine addiction.
Currently L-THP in under investigation in a Phase I clinical trial to determine the safety and pharmacokinetics of L-THP alone, and in combination with low dose (40 mg) intranasal cocaine. Therefore an analytic method for determining the concentrations of both L-THP and coaine in human plasma was needed. Previous clinical pharmacokinetic studies involving low dose cocaine (20 to 42 mg) by intranasal administration routes have reported maximum plasma concentrations in the range of approximately (40 to 120 ng/mL) [12–14].To acurately assess the pharmacokinetic profile of cocaine to support this clinical trial a lower limit of quantitation in the range of 1 to 3 ng/mL was required.
Although individual analytic methods for the determination of cocaine and L-THP have been published [15–23], the simutanously mesurement of both agents have not as yet been reported. The advantage for being able to measure both agents within the same assay is obvious for the current and future clinical studies of LTHP for cocaine addiction to provide a simple and cost effective method. Of published methods for cocaine detection, several used more complex and/or intensive chromatographic methods, while others were more applicable to toxicological procedures. Some of the currently published assays, including HPLC and LC/MS/MS for the determination of cocaine, were appliacable in nature, to those commonly employed for the determination of drug concentrations in clinical trials. The previously published analytical methods for the determination of cocaine reported lower limits of detection in plasma ranging from 2.5 to 100 ng/mL whlie using 0.1 to 2 mL of plasma for extraction [21,23–27]. For simplicity, and cost savings, assays using UV or flourescence detection were deemed more applicable to the current study, and flourescence detection was chosen for improve sensitivity.
The degredation of cocaine by plasma esterases has been problematic for assay validation since first described by Bouis and colleagues [28,29]. Additives such as sodium or potassium flouride with or without the addition of acid have been used to improve the stability of cocaine in stored plasma samples [30,31]. Therefore, the assessment of the stability of cocaine in human plasma samples as well as banked donor plasma required additional investigation in the development of an assay for the simultaneous determination of both cocaine and L-THP by the method described herein. An important issue was the potential of stabilizing agents to adversly effect the chromatography and/or stability of L-THP.
Herein, we report an analytical method that is able to measure both L-THP and coaine simultaneously with similar or greater sensitivity for cocaine in comparison with the previously reported methods. The application of this method to support the first pharmacokinetic clinical study of the combination of cocaine in combination with L-THP is presented.
2. MATERIALS AND METHODS
2.1 Chemicals and Regents
L-tetrahydropalmatine (L-THP) was purchased from WuxiGorunjie Technology Co., LTD. (No.97 Wuqiao West Road Wuxi Jiangsu,214044 CN); Cocaine HCl was supplied by NIDA/NIH; Venlafaxine (internal standard), methanol (HPLC grade), acetonitrile (HPLC grade), and hexanes (HPLC grade) were purchased from Sigma (ST. Louis, MO). Water was deionized and purified on a Millipore water purification system (Milford, MA), and grey top tubes (5mL) containing sodium fluoride and potassium oxalate were purchased from (BD, Franklin lakes, NJ, USA).
2.2 Instruments and experimental conditions
Plasma samples were analysed using a UPLC system with a fluorescence detector (Waters, MA). Separation was carried out on an ACQUITY UPLC BEH C18 column (2.1 × 50mm, 1.7um, Waters, MA) maintained at 25 • •. The mobile phase consisted of 5 mM ammonium phosphate (PH=4.75) (solvent A), methanol (solvent B), and acetonitrile (solvent C) (v:v:v, 78:16:6).The flow rate was 0.4ml/min with fluorescence detection at λ of excitation 230nm and emission 315nm.
2.3 Sample preparation and extraction
Human blood samples were collected into grey top tubes containing sodium fluoride and potassium oxalate to maintain cocaine stability as previously described [31], and centrifuged at 5,000 g for 10 minutes, and the resulting plasma samples were stored at −80 • •until analysis. Standard stock solutions of cocaine, L-THP and venlafaxine were prepared in methanol. Standards of cocaine and L-THP in plasma were prepared by spiking blank human plasma with both cocaine and L-THP to achieve final concentrations of 2.5, 6.25, 12.5, 25 62.5, 125, 250 ng/mL for each. Six replicates of each of the three quality control (Q.C): low Q.C. (7.5 ng/mL), medium Q.C. (37.5 ng/mL), and high Q.C. (187.5 ng/mL) samples were prepared in a similar manner. 20 μL of the internal standard (venlafaxine) was added to 200 μL samples to get a final concentration of 1.25 μg/mL. After shaking for 10 s, 1000 μL of hexane was added to each tube, shake-mixed for 20 min, and then centrifuged for 10 min at 10000 rpm (Eppendorf Ltd., Germany) at 4 °C. The clear supernatant, 950 μL, was then transferred and evaporated. The residue was reconstituted in 50 μL of mobile phase, shake-mixed for 10 s and a 15 μL aliquot was used for injection.
2.4 Assay Validation
The assay method was validated to determine selectivity, lower limit of quantitation (LLOQ), linearity, accuracy, precision, recovery and stability according to the FDA guidance for bio-analytical method validation [32]. Additionally, selectivity was determined in plasma obtained from six different sources.
Assessment of precision and accuracy of the assay was performed by linear regression of the peak area ratio of each analyte using a calibration curve over the concentrations of 2.5, 6.25, 12.5, 25, 62.5, 125, and 250 ng/mL. Quality control (QC) samples were prepared as three sets of six replicate samples using concentrations of 7.5, 37.5, and 187.5 ng/mL to assess the intra- and inter-day precision and accuracy of the assay. Additionally, six samples of cocaine and LTHP, 2.5 ng/mL each in plasma were prepared for evaluation of LLOQ; 2.5 ng/mL as the lower limit of quantitation for used for construction of the calibration curve.
The liquid-liquid extraction recovery was evaluated by comparing the analytical results from extracted samples at three different concentrations (n=6 replicates/conc.) in reference to un-extracted standards that represent 100% recovery. Each sample recovery was calculated by comparing the analytical result of extracted sample to the corresponding injection of un-extracted standards. Absolute recovery of the extraction procedure was determined as described by equation 1 below. The recovery of cocaine and L-THP was evaluated at using the three assay quality control concentrations (7.5, 37.5, and 187.5 ng/mL).
| Equation 1 |
To test the stability of cocaine and L-THP in human plasma, six replicates of each QC were thawed at room temperature over 4 hours to test the short-term stability. Three replicates of QCs were thawed at room temperature and refrozen at −80 • •for three cycles and then analyzed. The stock solutions of three QCs were diluted in mobile phase, and then stored at room temperature over 6 hr to test the short term stock solution stability.
Processed samples stability was carried out by preparing 5 replicates of each QC samples and injecting them into the UPLC. The prepared QC samples were kept in the auto sampler for 24 hours and the samples were reinjected. No appreciable change in concentration was observed and the samples proved to be stable for at least 24 hours.
The specificity of the method was studied by challenging it with potential interferences from caffeine and common OTC drugs including: acetaminophen and ibuprofen. None of the studied interferents showed analytical signal(s) at the retention times of Cocainte, L-THP or internal standard peaks respectively.
2.5 Pharmacokinetics Study in Human
This assay method was developed to support a Phase I safety and pharmacokinetic study of cocaine administered with L-THP. Subjects enrolled in this study received L-THP 30 mg orally twice daily or placebo twice daily for four days with co-administration of cocaine 40 mg once intranasal on day four with the morning dose of L-THP. Blood samples were collected for pharmacokinetic analysis into grey top tubes containing sodium fluoride and potassium oxalate, centrifuged at 5,000 g for 10 minutes, and the resulting plasma was stored at −80 • •until analysis. The blood samples were collected from patients at 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120 min, 2.5, 3, 4, 5, 6, 7, 10, 22 and 28.5 hr after cocaine administration. The pharmacokinetic analysis was performed using Phoenix WinNonlin 6.3 (Pharsight, CA) by non-compartmental analysis of the concentration-time data.
3. RESULTS AND DISCUSSION
The current assay was developed for the purpose of supporting clinical trials designed to determine the safety, pharmacokinetics, and pharmacodynamics of cocaine co-administered with L-THP. Herein we report the development and validation of a simple chromatographic assay method for the quantitation of L-THP and cocaine along as well as application of this assay to support a Phase I study of L-THP and cocaine in human subjects with a history of cocaine use.
Since this study is the first human exposure of L-THP co-administered with cocaine, a low intranasal dose of cocaine was chosen to avoid potential adverse events that might result in a clinically significant interaction between these two drugs. Thus, these circumstances also necessitated the development of a simple, sensitive, and cost effective assay for the simultaneous determination cocaine and L-THP in human plasma.
Due to stability issues with cocaine as well as the chromatographic pH sensitivity of L-THP, we concluded that pursuing the development of an HPLC assay with fluorescence detection using liquid-liquid extraction following plasma treatment with sodium fluoride would achieve our goal. Therefore, we have developed and validated a simple, stable, and cost effective method for the determination of both agents in human plasma to support upcoming clinical trials. The validation was conducted under the FDA guidance for bioanalytical analysis with respect to the criteria of specificity, selectivity, linearity, accuracy, precision, recovery, and stability.
3.1 Assay Validation
3.1.1 Specificity and Selectivity
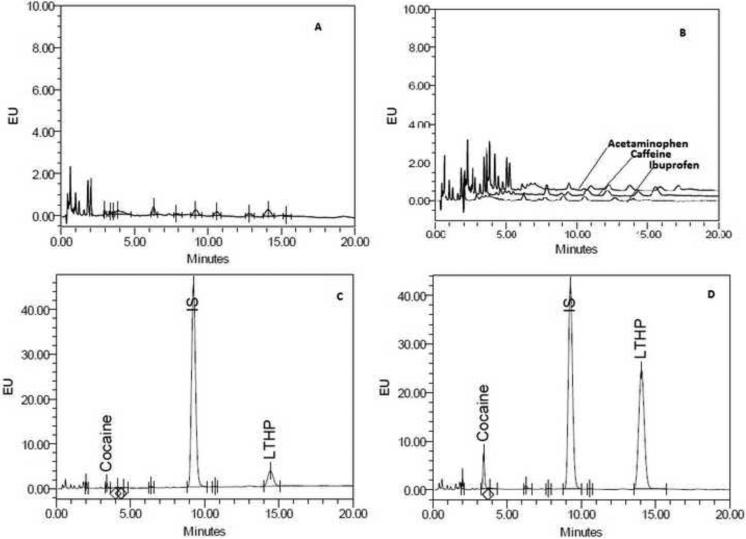
The chromatograms of blank plasma, cocaine, L-THP, and internal standard are shown in Figure 2. The chromatographic run time was 16 minutes with cocaine eluting at 3.6 min, L-THP eluting at 13.5 min and the internal standard eluting at 10.8 min. (figure 2). There were no significant interfering peaks at the retention times of cocaine, L-THP, or the internal standard. The specificity of the method was further studied by challenge with potential interfering agents such as caffeine as well as common over the counter (OTC) drugs including: acetaminophen and ibuprofen. None of the studied agents produced a fluorescence signal at the retention times of Cocaine, L-THP or the internal standard
Figure 2.
Chromatograms of blank plasma (A), Specificity study showing chromatograms of samples spiked by Caffeine, Acetaminphen and Ibuprofen (B), chromatograms of Cocaine and L-THP at LOQ (2.5 ng/ml), and IS at 500 ng/mL (C), a representative chromatogram of a subject's sample at 0.5 hr who received both cocaine and L-THP (D),
3.1.2 Linearity and Lower Limits of Quantification
The calculated peak area ratios of cocaine and L-THP to that of the internal standard versus the nominal concentrations displayed a linear relationship in the tested ranged of 2.5-250 ng/mL. The mean correlation coefficient was 0.9989 ± 0.0008 (range, 0.9964-0.9993). The lower limit of quantification of cocaine and LTHP at 2.5 ng/mL was measured at 2.68 ± 0.12, and 2.62 ± 0.18 (mean ± SD), respectively, as shown in table 1.
Table 1.
Intra-day precision and accuracy of cocaine in human plasma (n=6)
| Nominal concentration (ng/mL) | Measured (Mean ± S.D.) | Precision R.S.D. % | Accuracy Deviation % | |
|---|---|---|---|---|
| Cocaine | 2.5 | 2.68 ± 0.12 | 4.48 | 7.09 |
| 7.5 | 6.74 ± 0.28 | 4.09 | −10.13 | |
| 37.5 | 34.06 ± 1.89 | 5.54 | −9.18 | |
| 187.5 | 185.29 ± 4.85 | 2.62 | −1.18 | |
| L-THP | 2.5 | 2.62 ± 0.18 | 6.87 | 4.64 |
| 7.5 | 6.60 ± 0.26 | 3.92 | −12.03 | |
| 37.5 | 32.82 ± 1.19 | 3.62 | −12.49 | |
| 187.5 | 177.91 ± 10.77 | 6.06 | −5.11 |
3.1.3 Accuracy and Precision
Tables 1 and 2 provide summary data for intra-, and inter-day precision and accuracy of cocaine and L-THP in human plasma. The intra-day precision, as relative standard deviation (R.S.D) of cocaine and L-THP were both less than 9.50%, and the accuracy for each was between −4.29% and 0.85%. For both cocaine and L-THP the inter-day precision was less than 9.14%, and the accuracy was within −12.49% to 9.59%. The results demonstrate that the assay method has accuracy and precision that are in line with the FDA guidance for bioanalytical method validation and should be suitable for GLP and GCP pharmacokinetic studies.
Table 2.
Inter-day precision and accuracy of cocaine and L-THP in human plasma (n=6)
| Nominal concentration (ng/mL) | Measured (Mean ± S.D.) | Precision R.S.D. % | Accuracy Deviation % | |
|---|---|---|---|---|
| Cocaine | 7.5 | 7.26 ± 0.48 | 6.69 | −3.16 |
| 37.5 | 35.89 ± 1.61 | 4.49 | −4.29 | |
| 187.5 | 189.10 ± 7.92 | 4.19 | 0.85 | |
| L-THP | 7.5 | 7.33 ± 0.63 | 8.63 | −2.26 |
| 37.5 | 35.95 ± 2.74 | 7.64 | −4.12 | |
| 187.5 | 182.30 ± 5.65 | 3.10 | −2.77 |
3.1.4 Recovery and Stability
The absolute recovery of the analytes ranged from 43.95% to 50.02% for cocaine and from 54.65% to 58.31% for L-THP (Table 3). The observed changes of cocaine and L-THP concentration determined during short-term stability testing (Table 4) were acceptable and within the limits specified by the FDA [32]. Plasma samples were stable for three freeze and thaw cycles (Table 4) and these results revealed no significant degradation of cocaine or L-THP in human plasma under these conditions. Processed sample stability testing indicated absence of any appreciable change in concentration and the samples were proven to be stable for at least 24 hours as presented in table 4 and the stock solutions were found to be stable over 6 hr at room temperature.
Table 3.
Recovery of cocaine and L-THP in human plasma (n=6)
| Nominal concentration (ng/mL) | Recovery % (Mean ± S.D.) | |
|---|---|---|
| Cocaine | 7.5 | 43.95 ± 1.70 |
| 37.5 | 44.41 ± 2.57 | |
| 187.5 | 50.02 ± 0.48 | |
| L-THP | 7.5 | 54.65 ± 3.33 |
| 37.5 | 55.03 ± 3.02 | |
| 187.5 | 58.31 ± 3.91 | |
| IS | 125 | 40.33 ± 2.03 |
Table 4.
Stability of cocaine and L-THP in human plasma
| Sample condition | Nominal concentration (ng/mL) | Measured (mean ± S.D.) | Deviation % | |
|---|---|---|---|---|
| Cocaine | Short-term (4 hr) n=6 | 7.5 | 6.23 ± 0.21 | −16.85* |
| 37.5 | 33.42 ± 0.79 | −10.88 | ||
| 187.5 | 166.52 ± 7.70 | −11.19 | ||
| Freeze/thaw cycle n=3 | 7.5 | 7.44 ± 0.38 | −0.82 | |
| 37.5 | 35.15 ± 1.37 | −6.25 | ||
| 187.5 | 176.48 ± 11.15 | −5.87 | ||
| Processed sample stability n=5 | 7.5 | 8.90 ± 0.58 | 16.36* | |
| 37.5 | 37.84 ± 0.45 | 0.91 | ||
| 187.5 | 183.36 ± 3.63 | 1.04 | ||
| L-THP | Short-term (4 hr) n=6 | 7.5 | 6.98 ± 0.25 | −6.98 |
| 37.5 | 34.64 ± 0.83 | −7.62 | ||
| 187.5 | 177.54 ± 6.13 | −5.31 | ||
| Freeze/thaw cycle n=3 | 7.5 | 7.51 ± 0.63 | 0.08 | |
| 37.5 | 34.63 ± 1.55 | −7.66 | ||
| 187.5 | 167.72 ± 13.77 | −10.55 | ||
| Processed sample stability n=5 | 7.5 | 7.24 ± 0.047 | 0.78 | |
| 37.5 | 35.24 ± 0.60 | 0.36 | ||
| 187.5 | 179.54 ± 6.45 | 0.26 |
These variations are below 20% deviation, which meet the criteria set forth by FDA for low concentration [32].
3.2 Results of Pharmacokinetics Study
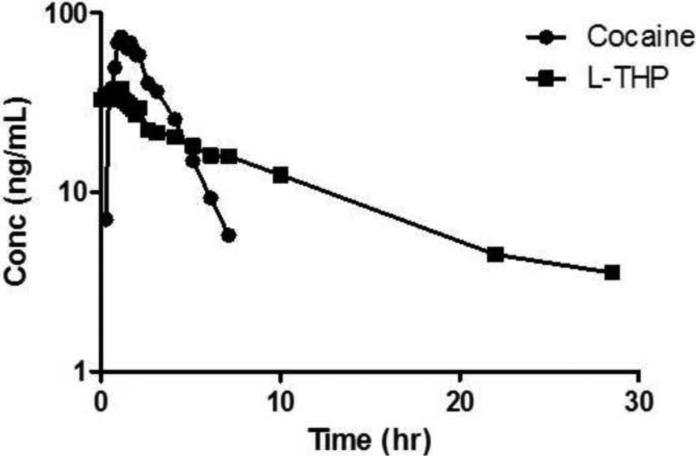
The assay method described was successfully applied to human plasma samples obtained from subjects receiving L-THP 30 mg orally twice daily followed by a single intranasal dose of 40 mg cocaine. The L-THP and cocaine concentration verse time profiles on Study Day 4 from a representative healthy volunteer are shown in Figure 3. The maximum observed concentration (Cmax) of cocaine and L-THP were 73.79 ng/mL and 38.03 ng/mL, respectively, and the minimum observed concentrations (Cmin) were 5.77 ng/mL and 3.55 ng/mL, respectively. The present LLOQ of 2.5ng/mL for cocaine and L-THP proved to be sufficient for this as well as future clinical pharmacokinetic studies using these drugs in combination. The clearance (CL/F) of cocaine and L-THP were 172.76 L/hr and 111.90 L/hr, respectively and the AUCs were 228.65 hr*μg/L and 163.95 hr*μg/L respectively.
Figure 3.
The plasma concentration-time profile for a subject who administered cocaine (40mg, intranasal administration) and L-THP (30mg, P.O. administration).
4. CONCLUSION
To support the previously described Phase I study as well as and forthcoming clinical trials involving combinations of cocaine and L-THP we set out to develop and validate a simple, sensitive, and cost effective UPLC assay to simultaneously determine their concentrations in human plasma. We have also demonstrated the ability to maintain the stability of cocaine in plasma samples and standards as well as in processed solutions. A search of previously published chromatographic methods revealed many methods involving various techniques from differing matrices. Some of the methods reviewed proved relevant to the clinical trials described. Although a number of these methods provided a low limit of quantitation, a vast majority involved mass spectrometric detection or the use of large quantities of plasma. To support clinical trials with multiple blood draws the use of high volumes (1 mL or greater) for extraction was not practical. Additionally, for the purpose of cost effectiveness, methods employing UV or fluorescence detection were attempted to provide more cost efficiency. Through the development process we determined that fluorescence detection allowed for a greater sensitivity in conjunction with the simultaneous determination of cocaine and L-THP in human plasma. Due to the degradation of cocaine in human plasma by plasma esterases, the development of a simple, inexpensive processing method resulting in stable plasma cocaine samples was a priority. We were successful in this endeavor and demonstrated that cocaine was stable for up to 3 days at room temperature, and 2 months under refrigeration when samples were collected into grey top tubes.
The development of this method allowed for the accurate quantitation of cocaine and L-THP in human plasma following low dose nasal inhalation administration of cocaine with a sufficiently LLOQ as to determine cocaine's elimination half-life of while sampling for a minimum of three half-lives after the observed maximum concentration (Cmax) (Figure 3). This method allows for the simultaneous determination of cocaine and L-THP in human plasma and is adequate for clinical pharmacokinetic studies.
Highlights.
The new method allows for the simultaneous determination of cocaine and LTHP in human plasma.
The LLOQ of method is adequate for pharmacokinetic study.
The method was first applied in clinical study.
Figure 1.
Chemical structures of Cocaine, L-THP, and Venlafaxine
ACKNOWLEDGEMENT
The study was funded by a grant from NIDA/NIH (DP1DA031401) to JB Wang. M. Yu was supported by Chinese Scholarship Council, Beijing, China.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang JB, Mantsch JR. L-Tetrahydropalamatine: a Potential New Medication for the Treatment of Cocaine Addiction. Future Med. Chem. 2012;4:177–86. doi: 10.4155/fmc.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi Z-X, Yang Z, Li S-J, Li X, Dillon C, Peng X-Q, et al. Levo-tetrahydropalmatine inhibits cocaine's rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropharmacology. 2007;53:771–82. doi: 10.1016/j.neuropharm.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halbsguth C, Meissner O, Häberlein H. Positive cooperation of protoberberine type 2 alkaloids from Corydalis cava on the GABA(A) binding site. Planta Med. 2003;69:305–9. doi: 10.1055/s-2003-38869. [DOI] [PubMed] [Google Scholar]
- 4.Jin GZ, Xu J, Zhang FT, Yu LP, Li JH, Wang XL. Relevance of the sedative-tranquilizing effect of l-tetrahydropalmatine to brain monoaminergic neurotransmitters. Acta Pharm. Sin. 1983;4:4–10. [PubMed] [Google Scholar]
- 5.Jin GZ, Wang XL, Shi WX. Tetrahydroprotoberberine--a new chemical type of antagonist of dopamine receptors. Sci. Sin. Ser. B. 1986;29:527–34. [PubMed] [Google Scholar]
- 6.Lu ZZ, Wei X, Jin GZ, Han QD. Antagonistic effect of tetrahydroproberberine homologues on alpha 1-adrenoceptor. Acta Pharm. Sin. 1996;31:652–6. [PubMed] [Google Scholar]
- 7.Wu G, Jiang JW, Wu GC, Cao XD. Effects of four dopamine agonists on l-tetrahydropalmatine-induced analgesia and electroacupuncture analgesia in rabbits. Acta Pharmacol. Sin. 1990;11:196–200. [PubMed] [Google Scholar]
- 8.Hu JY, Jin GZ. Supraspinal D2 receptor involved in antinociception induced by l-tetrahydropalmatine. Acta Pharmacol. Sin. 1999;20:715–9. [PubMed] [Google Scholar]
- 9.Yang Z, Shao Y, Li S, Qi J, Zhang M, Hao W, et al. Medication of l-tetrahydropalmatine significantly ameliorates opiate craving and increases the abstinence rate in heroin users: a pilot study. Acta Pharmacol. Sin. 2008;29:781–8. doi: 10.1111/j.1745-7254.2008.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueroa-Guzman Y, Mueller C, Vranjkovic O, Wisniewski S, Yang Z, Li S-J, et al. Oral administration of levo-tetrahydropalmatine attenuates reinstatement of extinguished cocaine seeking by cocaine, stress or drug-associated cues in rats. Drug Alcohol Depend. 2011;116:72–9. doi: 10.1016/j.drugalcdep.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantsch JR, Wisniewski S, Vranjkovic O, Peters C, Becker A, Valentine A, et al. Levo-tetrahydropalmatine attenuates cocaine self-administration under a progressive-ratio schedule and cocaine discrimination in rats. Pharmacol. Biochem. Behav. 2010;97:310–6. doi: 10.1016/j.pbb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cone EJ, Oyler J, Darwin WD. Cocaine disposition in saliva following intravenous, intranasal, and smoked administration. J. Anal. Toxicol. 1997;21:465–75. doi: 10.1093/jat/21.6.465. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson P, Van Dyke C, Jatlow P, Barash P, Byck R. Intranasal and oral cocaine kinetics. Clin. Pharmacol. Ther. 1980;27:386–94. doi: 10.1038/clpt.1980.52. [DOI] [PubMed] [Google Scholar]
- 14.Cone EJ. Pharmacokinetics and Pharmacodynamics of Cocaine. J. Anal. Toxicol. 1995;19:459–478. doi: 10.1093/jat/19.6.459. [DOI] [PubMed] [Google Scholar]
- 15.Jamdar SC, Pantuck CB, Diaz J, Mets B. A rapid, sensitive assay for cocaine and its metabolites in biological fluids using solid-phase extraction and high-performance liquid chromatography. J. Anal. Toxicol. 2000;24:438–41. doi: 10.1093/jat/24.6.438. [DOI] [PubMed] [Google Scholar]
- 16.Lin SN, Moody DE, Bigelow GE, Foltz RL. A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. J. Anal. Toxicol. 2001;25:497–503. doi: 10.1093/jat/25.7.497. [DOI] [PubMed] [Google Scholar]
- 17.Crouch DJ, Alburges ME, Spanbauer a C., Rollins DE, Moody DE. Analysis of cocaine and its metabolites from biological specimens using solid-phase extraction and positive ion chemical ionization mass spectrometry. J. Anal. Toxicol. 1995;19:352–8. doi: 10.1093/jat/19.6.352. [DOI] [PubMed] [Google Scholar]
- 18.Jatlow P, Nadim H. Determination Chromatography of Cocaine Concentrations in Plasma by High-Performance Liquid. Clin. Chem. 1990;36:1436–1439. [PubMed] [Google Scholar]
- 19.Chao-Wu L, Shuo Z, Hai-Qing G, Xiu-Mei Z. Determination of L-tetrahydropalmatine in human plasma by HPLC and pharmacokinetics of its disintegrating tablets in healthy Chinese. Eur. J. Drug Metab. Pharmacokinet. 2011;36:257–62. doi: 10.1007/s13318-011-0045-x. [DOI] [PubMed] [Google Scholar]
- 20.Khan M, Gupta PK, Cristie R, Nangia a, Winter H, Lam FC, et al. Determination of pharmacokinetics of cocaine in sheep by liquid chromatography. J. Pharm. Sci. 1987;76:39–43. doi: 10.1002/jps.2600760112. [DOI] [PubMed] [Google Scholar]
- 21.Brunetto R, Gutiérrez L, Delgado Y, Gallignani M, Burguera JL, Burguera M. High-performance liquid chromatographic determination of cocaine and benzoylecgonine by direct injection of human blood plasma sample into an alkyl-diol-silica (ADS) precolumn. Anal. Bioanal. Chem. 2003;375:534–8. doi: 10.1007/s00216-002-1710-3. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Ye M, Bi K, Guo D. Liquid chromatography-tandem mass spectrometry for the identification of L-tetrahydropalmatine metabolites in Penicillium janthinellum and rats. Biomed. Chromatogr. 2006;20:95–100. doi: 10.1002/bmc.534. [DOI] [PubMed] [Google Scholar]
- 23.Bystrowska B, Adamczyk P, Moniczewski A, Zaniewska M, Fuxe K, Filip M. LC/MS/MS evaluation of cocaine and its metabolites in different brain areas, peripheral organs and plasma in cocaine self-administering rats. Pharmacol. Rep. 2012;64:1337–49. doi: 10.1016/s1734-1140(12)70931-3. [DOI] [PubMed] [Google Scholar]
- 24.Fernández MDMR, Wille SMR, Kummer N, Di Fazio V, Ruyssinckx E, Samyn N. Quantitative analysis of 26 opioids, cocaine, and their metabolites in human blood by ultra performance liquid chromatography-tandem mass spectrometry. Ther. Drug Monit. 2013;35:510–21. doi: 10.1097/FTD.0b013e31828e7e6b. [DOI] [PubMed] [Google Scholar]
- 25.a Kolbrich E, Barnes AJ, a Gorelick D, Boyd SJ, Cone EJ, Huestis M. a. Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration. J. Anal. Toxicol. 2006;30:501–10. doi: 10.1093/jat/30.8.501. [DOI] [PubMed] [Google Scholar]
- 26.Rook EJ, Hillebrand MJX, Rosing H, van Ree JM, Beijnen JH. The quantitative analysis of heroin, methadone and their metabolites and the simultaneous detection of cocaine, acetylcodeine and their metabolites in human plasma by high-performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2005;824:213–21. doi: 10.1016/j.jchromb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 27.Rofael H, Abdel-Rahman M. Development and validation of a high-performance liquid chromatography method for the determination of cocaine, its metabolites and ketamine. J. Appl. Toxicol. 2002;128:123–128. doi: 10.1002/jat.837. [DOI] [PubMed] [Google Scholar]
- 28.Stewart D, Inaba T. Cocaine metabolism: cocaine and norcocaine hydrolysis by liver and serum esterases. Clin. Pharmacol. Ther. 1979;25:464–468. doi: 10.1002/cpt1979254464. [DOI] [PubMed] [Google Scholar]
- 29.Stewart DJ, Inaba T, Tang BK, Kalow W. Hydrolysis of cocaine in human plasma by cholinesterase. Life Sci. 1977;20:1557–63. doi: 10.1016/0024-3205(77)90448-9. [DOI] [PubMed] [Google Scholar]
- 30.Brogan WC, Kemp PM, Bost RO, Glamann DB, a Lange R, Hillis LD. Collection and Handling of Clinical Blood Samples to Assure the Accurate Measurement of Cocaine Concentration. J. Anal. Toxicol. 1992;16:152–154. doi: 10.1093/jat/16.3.152. [DOI] [PubMed] [Google Scholar]
- 31.Baselt RC, Yoshikawa D, Chang J, Li J. Improved long-term stability of blood cocaine in evacuated collection tubes. J. Forensic Sci. 1993;38:935–7. [PubMed] [Google Scholar]
- 32.FDA Guidance for Industry Bioanalytical Method Validation. 2001.