Summary
Objective
Redox status and inflammation are important in the pathophysiology of numerous chronic diseases. Epidemiological studies have linked vitamin D status to a number of chronic diseases. We aimed to examine the relationships between serum 25-hydroxyvitamin D (25(OH)D) and circulating thiol/disulfide redox status and biomarkers of inflammation.
Design
This was a cross-sectional study of N=693 adults (449 females, 244 males) in an apparently healthy, working cohort in Atlanta, GA. Plasma glutathione (GSH), cysteine (Cys), and their associated disulfides were determined with high performance liquid chromatography, and their redox potentials (Eh GSSG and Eh CySS) were calculated using the Nernst equation. Serum inflammatory markers included interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α, assayed on a multiplex platform; and C-reactive protein (CRP), assayed commercially. Relationships were assessed with multiple linear regression analyses.
Results
Serum 25(OH)D was positively associated with plasma GSH (β ± SE: 0.002 ± 0.0004) and negatively associated with plasma Eh GSSG (β ± SE: −0.06 ± 0.01) and Cys (β ± SE: −0.01 ± 0.003) (P<0.001 for all); statistical significance remained after adjusting for age, gender, and race, percent body fat, and traditional cardiovascular risk factors (P=0.01-0.02). The inverse relationship between serum 25(OH)D and CRP was confounded by percent body fat, and full adjustment for covariates attenuated serum 25(OH)D relationships with other inflammatory markers to non-statistical significance.
Conclusions
Serum 25(OH)D concentrations were independently associated with major plasma thiol/disulfide redox systems, suggesting that vitamin D status may be involved in redox-mediated pathophysiology.
Keywords: vitamin D, redox, oxidative stress, inflammation, cytokines, glutathione, cysteine
Introduction
Oxidative stress and inflammation are known contributors to the development of modifiable chronic diseases, such as cardiovascular disease, type 2 diabetes mellitus, and chronic kidney disease 1, 2. Studies have shown that vitamin D deficiency, as indicated by low circulating serum 25-hydoxyvitamin D (25(OH)D) concentrations, is also a risk factor for these chronic metabolic diseases 3-5. Although mechanism(s) linking vitamin D status to these chronic metabolic diseases has not been elucidated, it is possible that vitamin D status, by impacting oxidative stress and/or inflammation, may contribute to disease risk.
Data to date suggest that vitamin D in its biologically active form (1,25-dihydroxyvitamin D or calcitriol) has anti-oxidant and anti-inflammatory effects in vitro 6-8. Some 6, 9, 10, but not all 11-13, interventional and cross-sectional studies have suggested an impact of vitamin D on circulating oxidative and inflammatory markers. As adiposity is associated with both lower serum 25(OH)D and greater oxidation and inflammation 14, 15, confounding effects of adiposity may be a contributing factor to the equivocal results.
The purpose of this study was to investigate the specific association between vitamin D status, as indicated by serum 25(OH)D concentrations, with circulating thiol/disulfide redox status and biomarkers of inflammation in an ambulatory cohort of adult university-affiliated employees living in the Atlanta metropolitan area. We measured the plasma aminothiols glutathione (GSH, a major intracellular antioxidant) and cysteine (Cys, a major extracellular antioxidant) and their associated disulfides, glutathione disulfide (GSSG) and cystine (CySS), respectively, as indexes of oxidative stress in this population. A more oxidized state of the GSH/GSSG and Cys/CySS redox couples is associated with chronic metabolic disease risk, including arterial stiffness, endothelial dysfunction, carotid intima-media thickness, and the Framingham Risk Score 16-19. Although the link between vitamin D and GSH has been shown in specific populations (e.g., end stage renal disease20, the relationship between vitamin D status and the plasma glutathione and cysteine thiol/disulfide redox systems has not been previously investigated in a broad population.
The Emory/Georgia Tech Predictive Health Initiative cohort was established for the purpose of identifying biomarkers and other measurable variables that predict health in an actively working population followed over several years21. Our aim was to determine if vitamin D status specifically was related to thiol/disulfide redox and inflammation in a cross-sectional study of generally healthy adults. The data presented could potentially provide a rationale for subsequent longitudinal follow-up intervention studies of supplemental vitamin D.
Subjects and methods
Participants
Participants were generally healthy adults (ages 18 and older) recruited between January 2008 and February 2013 to participate in the Emory/Georgia Tech Predictive Health Initiative cohort established within the Center for Health Discovery and Well Being (http://predictivehealth.emory.edu) 21. Recruitment to join the study was based on a random list of all Emory employees as well as leaders from the Emory and Georgia Tech communities. Spouses, family, and friends of Emory Employees were also welcome to join the study for a membership fee. All subjects applied online and underwent an initial screening process. Exclusion criteria were: hospitalization for acute or chronic disease within the previous year; history of severe psychosocial disorder within the previous year; addition of new prescription medications to treat a chronic condition within the previous year (with the exception of changes in anti-hypertensive or anti-diabetic agents); history of substance/drug abuse or alcoholism within the previous year; current active malignant neoplasm; history of malignancy other than localized basal cell cancer of skin during the previous 5 years; uncontrolled or poorly controlled autoimmune, cardiovascular, endocrine, gastrointestinal, hematologic, infectious, inflammatory, musculoskeletal, neurologic, psychiatric or respiratory disease; any acute illness (such as viral infection) in the previous 12 weeks before baseline visits.
Protocol
All testing and data collection was performed over two baseline visits within 3 weeks of each other. The first visit occurred after an overnight fast and included blood draws and questionnaires regarding demographic information, health history and current status, tobacco use, and medication and supplement use. Body composition testing was performed on either the 1st or 2nd visit. Physical activity was assessed using a survey developed from the Cross-Cultural Activity Participation Study 22 and coded as whether or not participants met the 2007 American College of Sports Medicine and American Heart Association recommendations for moderate and vigorous physical activity 23. Percent body fat was determined with dual energy X-ray densitometry (GE Lunar Densitometry iDXA, GE Healthcare, Waukesha, WI). Blood pressure was measured with an automated machine (Omron, Kyoto, Japan). For this study, diabetes status was defined by self-report or fasting glucose ≥ 7 mmol/L; hypertension was defined by self-report, use of blood pressure-lowering medication, or blood pressure ≥ 140/90 mmHG; hyperlipidemia was defined by self-report or use of lipid–lowering medications. The study was approved by the Emory University Institutional Review Board, and all participants provided informed consent. Only participants with available serum 25(OH)D measurements were included in these analyses (N = 693).
Laboratory Measurements
Serum 25(OH)D concentrations were measured commercially with liquid chromatography/tandem mass spectrometry (Quest Diagnostics Nichols Valencia, Valencia, CA). This laboratory participates in the Vitamin D External Quality Assessment Scheme. Plasma GSH, GSSG, Cys, CySS, and the CySS-GSH mixed disulfide (CySSG) were measured by high performance liquid chromatography, as described 24. In brief, blood was centrifuged after addition of a preservative solution containing iodoacetic acid. Supernatants were stored at –80°C in 10% perchloric acid and 0.2M boric acid until derivatization with dansyl chloride and quantification of GSH, GSSG, Cys, CySS, and CySSG concentrations using HPLC with fluorescence detection. The Nernst equation was used to calculate the redox potential [Eh in millivolts (mV)] of each redox couple (Eh GSSG and Eh CySS, respectively). Higher (less negative) Eh values are indicative of greater oxidative stress. Plasma redox-related measurements were available for 673 participants. Serum high-sensitivity CRP was measured commercially using nephelometry (Quest Diagnostics Nichols Valencia, Valencia, CA). Serum CRP values <1.0 mg/L were assigned a value of 0.90 mg/L for statistical analyses. Serum CRP was available for 692 participants. Serum interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), and interferon-gamma (IFNγ) concentrations were measured using a Fluorokine® MultiAnalyte Profiling multiplex kit (R&D Systems, Minneapolis, MN) with a Bioplex analyzer (Bio-Rad, Hercules, CA); values were available for 675 participants. Serum matrix metalloproteinase 9 /neutrophil gelatinase-associated lipocalin complex (MMP-9/NGAL) concentrations were measured in duplicate using a standard ELISA kit (R&D Systems, Minneapolis, MN). Serum MMP-9/NGAL was available for 672 participants. The manufacturer’s reported inter- and intra-assay CV ranges are 5.1-7.6% and 2.3-4.1%, respectively. Fasting glucose, insulin, and lipids were assayed commercially (Quest Diagnostics Nichols Valencia, Valencia, CA).
Statistical Analyses
Quantitative variables were summarized using means and standard deviations. Continuous variables were log-transformed for statistical analyses if their distributions deviated from normality, including GSH, GSSG, CySSG, all inflammatory markers, insulin, glucose, and triglycerides. For statistical analyses, a constant of 1 was added to all variables that required log-transformation and included values of 0 (IL-6, TNF-α, and IFNγ). Two-group t-tests were performed to investigate differences between groups dichotomized by sex, African American vs Caucasian ethnicity, supplemental vitamin D intake, serum 25(OH)D less than 75 nmol/L or above, and serum 25(OH)D less than 50 nmol/L or above. ANCOVA was used to investigate seasonal differences. Winter was defined as a study visit in December, January, or February; Spring as March, April, or May; Summer as June, July, or August; and Fall as September, October, or November. Pearson correlations and/or Spearman rank correlations were performed to examine bivariate associations between variables.
Multiple linear regression analyses were performed to investigate the relationship between serum 25(OH)D concentrations (independent variable) and markers of inflammation and oxidative stress (dependent variables) with age, gender, race (Caucasian/African American/Other), and percent body fat as a priori covariates. We considered interactions between 25(OH)D concentrations and race, gender, vitamin D supplement use, diabetic status, or vitamin D deficiency status for any of the circulating redox and inflammatory markers. In the absence of effect modification, interaction terms were dropped in subsequent models. Post-hoc multiple linear regression model building was performed using statistically significant redox and inflammatory determinants of serum 25(OH)D identified in a priori models. We also incorporated the inflammatory biomarker (IL6) found to be consistently associated with redox variables in bivariate analyses. Post-hoc models for the GSH/GSSG redox variables also included plasma CySS concentrations, and models for the Cys/CySS redox variables also included plasma GSH concentrations. GSH and CySS concentrations were each included in post-hoc models for the pro-inflammatory variables. Additional covariates in post-hoc models were season of blood draw (summer/fall/winter/spring) and traditional risk factors for cardiovascular disease identified in preliminary analyses as having a statistically significant bivariate relationship with serum 25(OH)D (waist-to-hip ratio; use of blood pressure, glucose, or lipid-lowering medications (yes/no); meeting recommendations for vigorous physical activity (yes/no); tobacco use (yes/no); systolic blood pressure; diastolic blood pressure; and fasting glucose, insulin, high-density lipoprotein cholesterol, and total cholesterol concentrations). Assessment of variance inflation factors did not indicate high co-linearity between predictor variables. All analyses were performed using SAS (version 9.3, SAS Institute, Cary NC) and a significance level of 0.05.
Results
Demographic and clinical characteristics of the 693 subjects with available serum 25(OH)D (among 719 participants enrolled in the parent cohort) are described in Table 1. A majority were Caucasian, and the mean BMI was in the overweight range. Less than one-third of participants had hypertension and less than one-third had hyperlipidemia. Less than 10% had diabetes. Sixty-five participants (9.4%) had impaired fasting glucose concentrations (5.6-6.9 mmol/L). Mean serum 25(OH)D concentrations of the entire cohort was in the normal range (Table 1). Approximately half the cohort (49.2%) had serum 25(OH)D concentrations <75 nmol/L; 17.9% had serum 25(OH)D concentrations <50 nmol/L. Serum 25(OH)D concentrations were positively associated with age (Pearson r = 0.16, P<0.001) and negatively associated with percent body fat (r = –0.24, P<0.001), as well as several additional clinical risk biomarkers (Supplementary Table 1).
Table 1.
Demographic and clinical characteristics of participants in the Emory Predictive Health Initiative cohort with available serum 25(OH)D levels (N = 693)
| Characteristic | Mean ± SD or N (%) |
|---|---|
| Age (yr) | 48.2 ± 11.0 |
| Female Gender | 449 (65%) |
| Race | |
| Caucasian | 497 (71.7%) |
| African American | 156 (22.5%) |
| American Indian/Alaskan Native | 3 (0.43%) |
| Asian | 33 (4.8%) |
| Other | 1 (0.14%) |
| Mixed | 3 (0.43%) |
| Hispanic/Latino Ethnicity | 9 (1.3%) |
| BMI (kg/m2) | 28.0 ± 6.41 |
| Percent body fat (%) | 35.9 ± 8.92 |
| Vitamin D supplement use4 | 281 (50.7%)3 |
| Fasting glucose (mmol/L) | 5.0 ± 1.0 |
| Fasting insulin (pmol/L) | 41.7 ± 52.1 |
| Systolic blood pressure (mmHG) | 121 ± 16 |
| Diastolic blood pressure (mmHG) | 76 ± 11 |
| Diabetes (%)5 | 41 (6.1%) |
| Hypertension (%)6 | 164 (30.4%) |
| Hyperlipidemia (%)7 | 158 (29.3%) |
| 25(OH)D (nmol/L) | 76.9 ± 30.7 |
n=690;
n=673;
n=554
Self-report of use of any supplements containing vitamin D
Self-reported diagnosis or fasting glucose ≥ 7 mmol/L
Self-reported diagnosis, use of blood pressure-lowering medication, or blood pressure ≥ 140/90 mmHG
Self-reported diagnosis or use of lipid–lowering medications
Half of the participants reported intake of any oral supplements containing vitamin D. Participants reporting intake of any supplement containing vitamin D had a lower BMI (P = 0.03), lower fasting insulin (P = 0.03), lower prevalence of hypertension (P = 0.003), and higher serum 25(OH)D (76.1 ± 30.5 vs 101.1 ± 32.2 nmol/L, P<0.001). Additional detailed demographic information in participants dichotomized by vitamin D supplement intake is provided in Supplementary Table 2.
African Americans had significantly lower serum 25(OH)D concentrations (57.7 ± 23.7 nmol/L) compared to Caucasians (83.1 ± 30.0 nmol/L, P<0.001; t-test) and other ethnic groups (73.9 ± 31.2 nmol/L; P=0.004). Detailed comparisons of the demographic and clinical characteristics between African Americans and Caucasians are provided in Supplementary Table 3. Serum 25(OH)D concentrations did not differ between the genders (males: 79.1 ± 28.5 vs. females: 75.6 ± 31.7 nmol/L, P=0.15; t-test). Serum 25(OH)D did not significantly vary by season.
Redox status and inflammation
Mean blood concentrations of biomarkers of redox status and inflammation are described in Table 2. Participants with serum 25(OH)D concentrations <75 nmol/L had lower plasma GSH, CySSG and Eh CySS (P<0.01 for all) and higher Cys, CySS, Eh GSSG, IL-6, and CRP (P<0.001). Results were similar if participants were dichotomized by serum 25(OH)D <50 nmol/L, although Eh CySS was no longer different between groups (P=0.22, Supplementary Table 4). Mean concentrations of measured biomarkers within tertiles of serum 25(OH)D levels are presented in Table 3.
Table 2.
Circulating markers of redox and inflammation of participants in the Emory Predictive Health Initiative cohort with available serum 25(OH)D levels1
| Characteristic | All | 25(OH)D < 75 nmol/L n = 341 |
25(OH)D ≥ 75 nmol/L n = 352 |
P |
|---|---|---|---|---|
| GSH (μM)2 | 1.73 ± 0.68 (1.60) | 1.64 ± 0.68 (1.51) | 1.83 ± 0.66 (1.73) | <0.001 |
| GSSG (μM)2 | 0.06 ± 0.05 (0.05) | 0.06 ± 0.05 (0.05) | 0.06 ± 0.04 (0.05) | 0.19 |
| Eh GSSG (mV)2 | −135.8 ± 10.1 | −134.5 ± 11.2 | −137.1 ± 8.7 | <0.001 |
| Cys (μM)2 | 9.3 ± 2.2 | 9.72 ± 2.30 | 8.95 ± 1.99 | <0.001 |
| CySS (μM)2 | 84.8 ± 18.3 | 87.34 ±18.54 | 82.21 ±17.71 | <0.001 |
| Eh CySS (mV)2 | −70.0 ± 5.8 | −70.6 ± 6.0 | −69.3 ± 5.5 | 0.005 |
| CySSG (μM)2 | 2.59 ± 0.93 (2.47) | 2.47 ± 0.99 (2.34) | 2.71 ± 0.85 (2.59) | <0.001 |
| CRP (mg/L)3 | 3.1 ± 4.3 (1.5) | 0.37 ± 0.46 (0.18) | 0.25 ± 0.39 (0.12) | <0.001 |
| IL-6 (pg/mL)4 | 1.59 ± 2.89 (0.97) | 2.04 ± 3.76 (1.21) | 1.15 ± 1.48 (0.80) | <0.001 |
| IL-8 (pg/mL) | 9.6 ± 10.1 (8.2) | 10.4 ± 13.5 (8.2) | 8.8 ± 4.5 (8.1) | 0.19 |
| TNF-α (pg/mL)5 | 4.14 ± 8.23 (3.67) | 4.01 ± 2.51 (3.95) | 4.26 ± 11.37 (3.47) | 0.26 |
| IFNγ (pg/mL)5 | 0.25 ± 0.51 (0.18) | 0.26 ± 0.67 (0.15) | 0.24 ± 0.28 (0.20) | 0.55 |
| MMP-9/NGAL (ng/mL)6 |
32.1 ± 22.2 (26.7) | 32.1 ± 22.6 (25.8) | 32.1 ± 21.9 (27.5) | 0.68 |
Reported as mean ± SD with median is listed in parentheses for variables that were not normally distributed. Differences between groups were determined with two-group t-tests. Abbreviations: 25(OH)D, 25-hydroxyvitamin D; GSH, glutathione; GSSG, glutathione disulfide; Eh, redox potential; Cys, cysteine; CySS, cystine; CySSG, cystine-glutathione mixed disulfide; CRP, C-reactive protein; IL-6, interleukin-6; IL-8, interleukin-8; TNF-α, tumor necrosis factor- α; IFNα, interferon-gamma; MMP-9/NGAL, matrix metalloproteinase 9 /neutrophil gelatinase-associated lipocalin complex.
n = 336 and 337 in vitamin D insufficient (25(OH)D < 75 nmol/L) vs sufficient groups (25(OH)D ≥ 75 nmol/L), respectively;
n = 340 and 352 in vitamin D insufficient and sufficient groups, respectively;
n = 337 in both vitamin D insufficient and sufficient groups;
n = 337 and 338 in vitamin D insufficient and sufficient groups, respectively;
n = 335 and 333 in vitamin D insufficient and sufficient groups, respectively.
Table 3.
Circulating markers of redox and inflammation of participants in the Emory Predictive Health Initiative cohort by 25(OH)D tertile1
| Characteristic | < 62.4 nmol/L n = 222 |
62.4–84.9 nmol/L n = 228 |
>84.9 nmol/L n = 243 |
P |
|---|---|---|---|---|
| 25(OH)D (nmol/L) |
45.4 ± 11.3a | 72.9 ± 6.5b | 109.5 ± 23.3c | <0.001 |
| GSH (μM)2 | 1.63 ± 0.70 (1.50)a | 1.67 ± 0.58 (1.60)a | 1.89 ± 0.71 (1.78)b | <0.001 |
| GSSG (μM)2 | 0.06 ± 0.06 (0.05) | 0.06 ± 0.04 (0.05) | 0.06 ± 0.04 (0.05) | 0.53 |
| Eh GSSG (mV)2 | −133.6 ± 11.2a | −135.7 ± 9.8a | −138.1 ± 8.6b | <0.001 |
| Cys (μM)2 | 9.80 ± 2.33a | 9.26 ± 2.23b | 8.97 ± 1.91b | <0.001 |
| CySS (μM)2 | 88.66 ± 19.51a | 84.13 ± 17.74b | 81.68 ± 17.00b | <0.001 |
| Eh CySS (mV)2 | −70.6 ± 6.2 | −69.8 ± 5.8 | −69.5 ± 5.3 | 0.08 |
| CySSG (μM)2 | 2.51 ± 1.06 (2.36)a | 2.46 ± 0.78 (2.43)a | 2.79 ± 0.90 (2.65)b | <0.001 |
| CRP (mg/L)3 | 0.40 ± 0.45 (0.24)a | 0.31 ± 0.53 (0.13)b | 0.23 ± 0.27 (0.12)b | <0.001 |
| IL-6 (pg/mL)4 | 2.12 ± 3.95 (1.30)a | 1.55 ± 2.69 (0.97)b | 1.13 ± 1.45 (0.80)b | <0.001 |
| IL-8 (pg/mL)5 | 10.9 ± 16.2 (8.5) | 9.5 ± 5.5 (8.2) | 8.5 ± 4.3 (8.0) | 0.11 |
| TNF-α (pg/mL)5 | 4.02 ± 2.13 (4.00) | 3.74 ± 2.54 (3.57) | 4.64 ± 13.70 (3.50) | 0.29 |
| IFNγ (pg/mL)5 | 0.24 ± 0.39 (0.11) | 0.25 ± 0.74 (0.16) | 0.27 ± 0.31 (0.22) | 0.13 |
| MMP-9/NGAL (ng/mL)6 |
32.7 ± 23.7 (25.7) | 32.2 ± 21.0 (27.0) | 31.5 ± 22.0 (26.5) | 0.72 |
Reported as mean ± SD with median is listed in parentheses for variables that were not normally distributed. Differences were determined with one-way ANOVA and Tukey’s post hoc comparisons. Tertiles not connected by the same letter are significantly different. Abbreviations: 25(OH)D, 25-hydroxyvitamin D; GSH, glutathione; GSSG, glutathione disulfide; Eh, redox potential; Cys, cysteine; CySS, cystine; CySSG, cystine-glutathione mixed disulfide; CRP, C-reactive protein; IL-6, interleukin-6; IL-8, interleukin-8; TNF-α, tumor necrosis factor- α; IFNγ, interferon-gamma; MMP-9/NGAL, matrix metalloproteinase 9 /neutrophil gelatinase-associated lipocalin complex.
n = 220, 223, 230, respectively;
n = 221 in lowest tertile;
n = 220, 224, 230, respectively;
n = 220, 224, 231, respectively;
n = 219, 221, 228, respectively.
Participants reporting intake of any supplement containing vitamin D had lower serum CRP (P=0.02; t-test; Supplementary Table 5) with no differences in any other marker of redox or inflammation. The difference in serum CRP was no longer statistically significant after adjustment for percent body fat (P = 0.16) or BMI (P = 0.21). Relative to Caucasians, African Americans had significantly lower plasma GSH and CySSG, higher plasma Eh GSSG and CySS, lower serum MMP-9/NGAL, and higher serum IL-6 and CRP (P <0.05 for all; t-test, Supplementary Table 6). Males had significantly lower plasma Cys and serum IL-6 and CRP (P<0.01 for all; t-test) and higher Eh GSSG (P=0.02) and Eh CySS (P=0.004) compared to females. There was significant seasonal variation in plasma GSH and GSSG (P = 0.02 and <0.001, respectively), with peak levels in the summer and the lowest levels in the winter and fall. Eh GSSG significantly differed by season (P = 0.03), with peak levels in the summer and the most reduced levels in the fall. Plasma CySS and Eh CySS also varied by season (P = 0.01 and 0.02, respectively), with peak levels in the winter and lowest levels in the spring and summer. Serum markers of inflammation did not significantly differ by season. Pearson correlations of redox and pro-inflammatory biomarkers with clinical measures of metabolic risk are provided in Supplementary Table 1.
Relationship between serum 25(OH)D and plasma redox biomarkers
The associations of serum 25(OH)D concentrations with plasma redox biomarkers are shown in Table 4. Serum 25(OH)D was positively associated with GSH (β ± SE: 0.002 ± 0.0004, P < 0.001; Figure 1) and CySSG (β ± SE: 0.001 ± 0.0004, P < 0.001), and negatively associated with plasma Eh GSSG (β ± SE: −0.06 ± 0.01, P < 0.001; Figure 2), Cys (β ± SE: −0.01 ± 0.003, P < 0.001; Figure 3), and CySS (β ± SE: −0.09 ± 0.007, P<0.001). However, the relationship of serum 25(OH)D with plasma CySSG, was no longer significant after adjusting for % fat, and the relationship of serum 25(OH)D with CySS was attenuated to non-significance after full adjustment for all covariates. Plasma GSH, Eh GSSG, and Cys remained associated with serum 25(OH)D after full adjustment. Results were similar if BMI was used in place of % body fat or if participants with diabetes (n = 41) were excluded from the analyses. Interactions between 25(OH)D concentrations and race, gender, vitamin D supplement use, diabetic status, or vitamin D deficiency status were not statistically significant for any of the plasma redox biomarkers.
Table 4.
Relationship of serum 25(OH)D (nmol/L) with circulating redox and inflammatory markers1
| Outcome variable | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| β ± SE (R2) | β ± SE (R2) | β ± SE (R2) | β ± SE (R2) | |
| GSH (μM)2 | 0.002 ± 0.0004 (0.04) 3 | 0.002 ± 0.0005 (0.09) 3 | 0.002 ± 0.0005 (0.12) 3 | 0.001 ± 0.0005 (0.20)5,6 |
| GSSG (μM)2 | −0.0002 ± 0.0008 (0.0001) | 0.0001 ± 0.0009 (0.03) | ---------------- | ---------------- |
| Eh GSSG (mV) | − 0.06 ± 0.01 (0.04) 3 | − 0.06 ± 0.01 (0.05) 3 | − 0.05 ± 0.01 (0.07) 3 | −0.04 ± 0.02 (0.15)4,6 |
| Cys (μM) | − 0.01 ± 0.003 (0.02) 3 | − 0.01 ± 0.003 (0.04) 3 | − 0.008 ± 0.003 (0.04) 4 | −0.008 ± 0.004 (0.11)5,7 |
| CySS (μM) | − 0.09 ± 0.007 (0.03) 3 | − 0.11 ± 0.021 (0.19) 3 | − 0.06 ± 0.02 (0.27) 4 | −0.04 ± 0.03 (0.34)7 |
| Eh CySS (mV) | 0.01 ± 0.02 (0.004) | 0.009 ± 0.008 (0.04) | ---------------- | ---------------- |
| CySSG (μM)2 | 0.001 ± 0.0004 (0.02) 3 | 0.001 ± 0.0005 (0.02) 5 | 0.0009 ± 0.0005 (0.03) | ---------------- |
| CRP (mg/L)2 | − 0.005 ± 0.001 (0.04) 3 | − 0.003 ± 0.001 (0.13) 4 | 0.000008 ± 0.001 (0.30) | ---------------- |
| IL-6 (pg/mL)2 | − 0.004 ± 0.0007 (0.04) 3 | − 0.003 ± 0.0008 (0.09) 3 | − 0.002 ± 0.0008 (0.16) 5 | −0.001 ± 0.0009 (0.18)8 |
| IL-8 (pg/mL)2 | − 0.002 ± 0.0008 (0.006) 5 | − 0.002 ± 0.0008 (0.04) 4 | − 0.002 ± 0.0008 (0.04) 5 | −0.002 ± 0.0009 (0.10)8 |
| TNF-α (pg/mL)2 | −0.0009 ± 0.0006 (0.003) | − 0.002 ± 0.0007 (0.02) 5 | −0.001 ± 0.0007 (0.03) | ---------------- |
| IFNγ (pg/mL)2 | 0.0004± 0.0003 (0.003) | 0.0004 ± 0.0003 (0.006) | ---------------- | ---------------- |
| MMP-9/NGAL (ng/mL)2 |
0.001± 0.0009 (0.003) | −0.0001± 0.001 (0.06) | ---------------- | ---------------- |
Boldface indicates statistical significance. Data were analyzed using multiple linear regression. The model R2 is reported in parenthesis.
Model 1: Unadjusted. Model 2: Adjusted for age, race, gender. Model 3: Previous model + percent body fat. Model 4: Previous model + season; waist-to-hip ratio; use of blood pressure, glucose, or lipid-lowering medications; physical activity; tobacco use; systolic blood pressure; diastolic blood pressure; and fasting glucose, insulin, high-density lipoprotein cholesterol, and total cholesterol.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; GSH, glutathione; GSSG, glutathione disulfide; Eh, redox potential; Cys, cysteine; CySS, cystine; CySSG, cystine-glutathione mixed disulfide; CRP, C-reactive protein; IL-6, interleukin-6; IL-8, interleukin-8; TNF-α, tumor necrosis factor- α; IFNγ, interferon-gamma; MMP-9/NGAL, matrix metalloproteinase 9 /neutrophil gelatinase-associated lipocalin.
Values are log-transformed.
P<0.001,
P<0.01,
P<0.05
Model also includes logIL-6 and CySS.
Model also includes logIL-6 and logGSH.
Model also includes logGSH and CySS
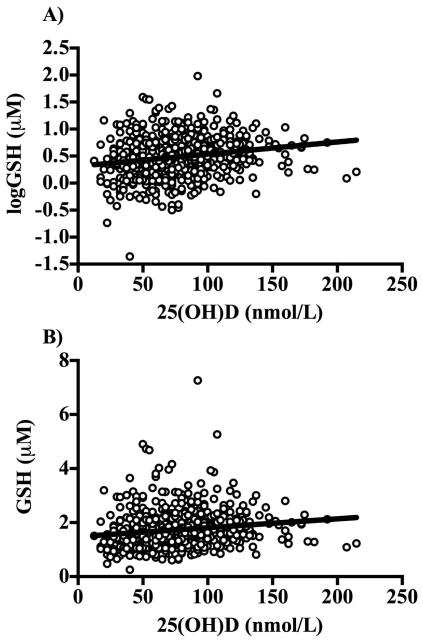
Figure 1.
A) Relationship between serum 25(OH)D concentrations and plasma logGSH (β ± SE: 0.002 ± 0.0004, P < 0.001). The relationships remained statistically significant after adjusting for age, race, gender, percent body fat, season, waist-to-hip ratio, use of blood pressure, glucose, or lipid-lowering medications, physical activity, tobacco use, systolic blood pressure, diastolic blood pressure, and fasting glucose, insulin, high-density lipoprotein cholesterol, total cholesterol, CySS, and IL-6 (β ± SE: 0.001 ± 0.0005, P=0.02). Data were analyzed using multiple linear regression. GSH was log-transformed because it was not normally distributed. B) The scatter plot of serum 25(OH)D versus GSH on the original scale is shown for comparison.
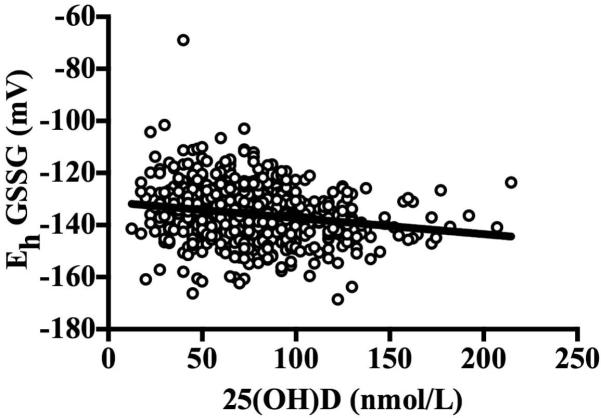
Figure 2.
Relationship between serum 25(OH)D concentrations and plasma Eh GSSG (β ± SE: −0.06 ± 0.01, P < 0.001). The relationships remained statistically significant after adjusting for age, race, gender, percent body fat, season, waist-to-hip ratio, use of blood pressure, glucose, or lipid-lowering medications, physical activity, tobacco use, systolic blood pressure, diastolic blood pressure, and fasting glucose, insulin, high-density lipoprotein cholesterol, total cholesterol, CySS, and IL-6 (β ± SE: −0.04 ± 0.01, P = 0.006). Data were analyzed using multiple linear regression.
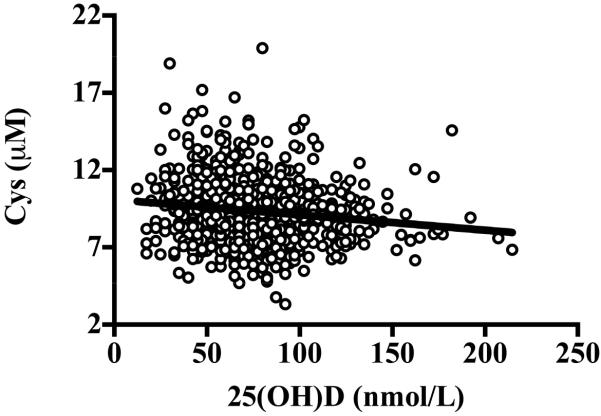
Figure 3.
Relationship between concentration of serum 25(OH)D and plasma Cys (β ± SE: −0.01 ± 0.003, P < 0.001). The inverse relationship remained statistically significant after adjusting for age, race, gender, percent body fat, season, waist-to-hip ratio, use of blood pressure, glucose, or lipid-lowering medications, physical activity, tobacco use, systolic blood pressure, diastolic blood pressure, and fasting glucose, insulin, high-density lipoprotein cholesterol, total cholesterol, GSH, and IL-6 (β ± SE: −0.008 ± 0.004, P = 0.02). Data were analyzed using multiple linear regression.
Relationship between serum 25(OH)D and serum pro-inflammatory cytokines
Although serum 25(OH)D was negatively associated with serum CRP, IL-6, IL-8, and TNF-α in bivariate analyses, the relationships with CRP and TNF-α were no longer significant after adjusting for % body fat (Table 4). Full adjustment for other covariates attenuated the relationships of serum 25(OH)D with serum IL-6 and IL-8 to non- significance (P = 0.11 and 0.08, respectively). Results were similar if BMI was used in place of % body fat, and if participants with diabetes were excluded from the analyses. There were no statistically significant interactions between 25(OH)D concentrations and race, gender, vitamin D supplement use, diabetic status, or vitamin D deficiency status for any of the serum inflammatory cytokines.
Discussion
In this study, we present novel data showing that serum 25(OH)D concentrations are independently associated with the plasma glutathione and cysteine thiol/disulfide redox systems in a large, working cohort of ambulatory adults. Furthermore, our data suggest that adiposity is a potential confounder influencing the relationship between serum 25(OH)D and inflammation, depending on the pro-inflammatory biomarker assayed.
Our study population was comparable to other generally healthy cohorts. The mean serum 25(OH)D concentration (76.9 ± 30.7 nmol/L) of our subjects are in the range of an optimal serum 25(OH)D defined by the Endocrine Society 25 and above the mean serum 25(OH)D concentration reported for the 2000-2006 United States National Health and Nutrition Examination Survey 26. Plasma aminothiol redox and inflammatory biomarkers were within the range we have previously described in other studies in healthy adults 10, 16, 18, 19. The skewed distributions of plasma GSH and GSSG concentrations are consistent with those previously reported in a similar, generally healthy cohort27.
GSH is the major low-molecular weight intracellular antioxidant. Decreased plasma concentrations of GSH or an oxidized Eh GSSG redox potential are associated with type 2 diabetes mellitus 28, and cardiovascular risk factors including carotid intima-media thickness 29. In this study, serum 25(OH)D concentrations were independently associated with higher GSH concentrations and a more reducing Eh GSSG in plasma, suggesting lower oxidative stress as determined by this index. This finding is consistent with a recent study showing a significant increase in serum GSH concentrations with a concomitant decrease in the oxidative stress biomarker malondialdehyde following correction of vitamin D deficiency in children with nutritional rickets 30. In patients on hemodialysis, paricalcitol, a vitamin D receptor agonist, significantly increased blood concentrations of GSH and other antioxidant biomarkers (thioredoxin and catalase) and decreased malondialdehyde and protein carbonyl group concentrations, biomarkers of oxidative stress 20. Potential mechanisms of these apparently redox effects of vitamin D status may include upregulation of genes involved in aminothiol redox states. In a large-scale microarray study by Wang et al 31, a vitamin D response element was identified in the glutamate cysteine ligase (GCL) and thioredoxin reductase genes. Jain et al 7 have recently shown 1,25(OH)2D to upregulate GCL and glutathione reductase expression in vitro. Additional mechanistic studies are needed to validate the link between vitamin D and GSH.
In our cohort, serum 25(OH)D concentrations were also independently and negatively associated with plasma Cys, a relationship that has not been previously investigated. Cys is both a precursor for GSH and a product of GSH metabolism, the latter of which is likely more reflected in extracellular pools32. It has been established previously that the GSH/GSSG and Cys/CySS redox pools are not in equilibrium in the plasma32, and they exhibit differential diurnal plasma variations33. In plasma, Cys is readily oxidized to its disulfide form, CySS, or it is reversibly cleared from circulation by various transporter proteins to maintain a balanced concentration of Cys in cells17. The Cys transporter protein, solute carrier 7 (y+), was identified as a vitamin D responsive gene by Wang et al 31. Thus, the inverse relationship between blood concentrations of 25(OH)D and Cys may reflect vitamin D-mediated Cys turnover via solute carrier 7 (y+) or GCL. Confirmation will require assessments of Cys transporter and direct interconversions using labeled Cys.
We assessed the relationship between serum 25(OH)D and a variety of circulating pro-inflammatory biomarkers and found weak relationships with serum IL-6 and IL-8 after adjusting for adiposity. In vitro, calcitriol and other vitamin D analogs are known to reduce cellular production of inflammatory cytokines through interference with the action of p38 mitogen-activated protein kinase phosphatase-1 8 and of the transcription factor nuclear factor kappa B activity 34. In addition, the IL-8 gene was identified as a vitamin D responsive gene 31. In vivo studies 11, 13, however, including the current study, have not consistently reflected an anti-inflammatory effect of vitamin D. However, it is possible that anti-inflammatory actions of vitamin D are confined to the cellular level and not detected by systemic concentrations of these cytokines or are more readily observed in populations with more severe vitamin D deficiency 9 or chronically activated immune systems, such as cystic fibrosis 35. Our data further indicated that the relationship between serum 25(OH)D and CRP was largely confounded by adiposity, which accounted for a large portion of the variance in CRP. This suggests CRP may not be an appropriate inflammatory biomarker for vitamin D studies.
Strengths of this study included use of a large cohort that was well-phenotyped, collection of blood during the fasted state which controlled for the diurnal variations in GSH/GGSH and Cys/CySS redox states, use of DXA-derived percent body fat (a robust measure of adiposity compared to BMI), and multivariate analysis. As this is a cross-sectional study, a limitation is that we cannot infer causation. Our participants were recruited among university employees and their families; our findings cannot be extrapolated to the general population. Furthermore, it is possible that serum 25(OH)D is a surrogate marker of health, which would encompass reduced oxidative stress and inflammation.
In conclusion, vitamin D status was independently associated with major thiol/disulfide redox systems in a large, ambulatory cohort of university employees. Although further study is needed, these relationships suggest that interactions with redox state/oxidative stress is a potential pathway linking vitamin D status with disorders in which oxidative stress plays a pathophysiologic role. Randomized, placebo-controlled trials are needed to determine if vitamin D supplementation influences the major aminothiol/disulfide redox pools.
Supplementary Material
Acknowledgments
Information upon which this work is based is from the Emory/Georgia Tech Predictive Health Participant Database, and is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000454). Other sources of support for this study include grants from the National Institutes of Health (K23 AR054334 (VT), T32 DK007298-32S1 (JAA), and K24 RR023356 (TRZ)). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the anonymous reviewers for their insightful contributions to improving the manuscript.
Footnotes
Disclosure The authors have nothing to disclose.
References
- 1.Hajjar DP, Gotto AM., Jr Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. The American Journal of Pathology. 2013;182:1474–81. doi: 10.1016/j.ajpath.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovascular Therapeutics. 2012;30:49–59. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–8. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Boer IH, Katz R, Chonchol M, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clinical Journal of the American Society of Nephrology. 2011;6:2141–9. doi: 10.2215/CJN.02640311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol SI, Tsang P, Aggarwal V, et al. Vitamin D status and risk of cardiovascular events: lessons learned via systematic review and meta-analysis. Cardiology in Review. 2011;19:192–201. doi: 10.1097/CRD.0b013e31821da9a5. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez JA, Zughaier SM, Law J, et al. Effects of high-dose cholecalciferol on serum markers of inflammation and immunity in patients with early chronic kidney disease. European Journal of Clinical Nutrition. 2013;67:264–9. doi: 10.1038/ejcn.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain SKM, D Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochemical Biophysical Research Communications. 2013 doi: 10.1016/j.bbrc.2013.06.004. http://dx.doi.org/10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. Journal of Immunology. 2012;188:2127–35. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amer M, Qayyum R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous National Health and Nutrition Examination Survey 2001 to 2006) The American Journal of Cardiology. 2012;109:226–30. doi: 10.1016/j.amjcard.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Ashraf AP, Fisher G, Alvarez J, et al. Associations of C-reactive protein to indices of vascular health and the influence of serum 25(OH)D status in healthy adults. Journal of Nutrition and Metabolism. 2012;2012:475975. doi: 10.1155/2012/475975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorde R, Sneve M, Torjesen PA, et al. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010;50:175–80. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Yiu YF, Yiu KH, Siu CW, et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013;227:140–146. doi: 10.1016/j.atherosclerosis.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Shea MK, Booth SL, Massaro JM, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. American Journal of Epidemiology. 2008;167:313–20. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snijder MB, van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. The Journal of Clinical Endocrinology and Metabolism. 2005;90:4119–23. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 15.Piva S, Tatsch E, Carvalho J, et al. Assessment of inflammatory and oxidative biomarkers in obesity and their associations with body mass index. Inflammation. 2013;36:226–231. doi: 10.1007/s10753-012-9538-2. [DOI] [PubMed] [Google Scholar]
- 16.Ashfaq S, Abramson JL, Jones DP, et al. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. Journal of the American College of Cardiology. 2006;47:1005–11. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 17.Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radical Biology & Medicine. 2011;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel RS, Al Mheid I, Morris AA, et al. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218:90–5. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashfaq S, Abramson JL, Jones DP, et al. Endothelial function and aminothiol biomarkers of oxidative stress in healthy adults. Hypertension. 2008;52:80–5. doi: 10.1161/HYPERTENSIONAHA.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izquierdo MJ, Cavia M, Muniz P, et al. Paricalcitol reduces oxidative stress and inflammation in hemodialysis patients. BMC Nephrology. 2012;13:159. doi: 10.1186/1471-2369-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brigham KL. Predictive health: the imminent revolution in health care. Journal of the American Geriatrics Society. 2010;58(Suppl 2):S298–302. doi: 10.1111/j.1532-5415.2010.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Irwin ML, Addy CL, et al. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. Journal of Women’s Health & Gender-Based Medicine. 1999;8:805–13. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- 23.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 24.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radical Biology & Medicine. 2009;47:1329–38. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff Ferrari H. A., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinololgy and Metabolism. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. The Journal of Nutrition. 2012;142:498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 27.Jones DP, Mody VC, Jr, Carlson JL, et al. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radical Biology and Medicine. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 28.Samiec PS, Drews Botsch C., Flagg EW, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radical Biology & Medicine. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 29.Ashfaq S, Abramson JL, Jones DP, et al. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. Journal of the American College of Cardiology. 2006;47:1005–11. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 30.Dogan M, Cesur Y, Zehra Dogan S., et al. Oxidant/antioxidant system markers and trace element levels in children with nutritional rickets. Journal of Pediatric Endocrinology & Metabolism : JPEM. 2012;25:1129–39. doi: 10.1515/jpem-2012-0153. [DOI] [PubMed] [Google Scholar]
- 31.Wang TT, Tavera Mendoza L. E., Laperriere D, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Molecular Endocrinology. 2005;19:2685–95. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 32.Jones DP, Carlson JL, Mody VC, et al. Redox state of glutathione in human plasma. Free Radical Biology & Medicine. 2000;28:625–35. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 33.Blanco RA, Ziegler TR, Carlson BA, et al. Diurnal variation in glutathione and cysteine redox states in human plasma. The American Journal of Clinical Nutrition. 2007;86:1016–23. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 34.Cohen Lahav M., Shany S, Tobvin D, et al. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrology, Dialysis, Transplantation. 2006;21:889–97. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 35.Grossmann RE, Zughaier SM, Liu S, et al. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. European Journal of Clinical Nutrition. 2012;66:1072–4. doi: 10.1038/ejcn.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.