Abstract
The ability to respond adaptively to threats in a changing environment is an important emotional function. The amygdala is a critical component of the neural circuit that mediates many emotion- related processes, and thus likely plays an important role in modulating the peripheral emotional response to threat. However, prior research has largely focused on the amygdala’s response to stimuli that signal impending threat, giving less attention to the amygdala’s response to the threat itself. From a functional perspective, however, it is the response to the threat itself that is most biologically relevant. Thus, understanding the factors that influence the amygdala’s response to threat is critical for a complete understanding of adaptive emotional processes. Therefore, we used functional magnetic resonance imaging (fMRI) to investigate factors (i.e. valence and arousal of co-occurring visual stimuli) that influence the amygdala’s response to threat (loud white-noise). We also assessed whether changes in amygdala activity varied with the peripheral expression of emotion (indexed via skin conductance response; SCR). The results showed that threat-elicited amygdala activation varied with the arousal, not valence of emotional images. More specifically, threat-elicited amygdala activation was larger to the threat when presented during high arousal (i.e. negative & positive) vs. low arousal (i.e. neutral) images. Further, the threat-elicited amygdala response was positively correlated with threat-elicited SCR. These findings indicate the amygdala’s response to threat is modified by the nature (e.g. arousal) of other stimuli in the environment. In turn, the amygdala appears to mediate important aspects of the peripheral emotional response to threat.
Keywords: fMRI, emotion, threat, amygdala, skin conductance
An important function of emotion is that it allows one to respond more effectively to threats in the environment. The response to threat is an important aspect of emotional behavior given the direct biological impact it has on survival. More specifically, survival is dependent upon the ability to avoid, escape, or defend against a threat once it is encountered. Further, threat-elicited behavior is influenced by other environmental factors, including the emotional context in which the threat occurs (Cook et al., 1992; Cuthbert et al., 1998; Panayiotou et al., 2011; Reagh & Knight, 2013; vanOyen Witvliet & Vrana, 1995; Vrana et al., 1988). Therefore, understanding the neural mechanisms of threat-related processes is important for a complete understanding of emotional behavior. However, prior neuroimaging research has largely focused on the emotional response to cues that signal or contextualize threat rather than the threat itself. Thus, there is a critical gap in our knowledge of the neural substrates of threat-elicited emotional behavior.
The amygdala is a critical component of the neural circuit that mediates many emotion-related functions. Specifically, the amygdala processes the content of emotional stimuli (Klumpp et al., 2011; LeDoux, 2003; Phan et al., 2002; Phelps & LeDoux, 2005) and mediates important aspects of the peripheral expression of the emotional response (Cheng et al., 2003, 2006; Ciocchi et al., 2010; Helmstetter, 1992; Knight et al., 2005, LeDoux, 2007). Further, prior human neuroimaging research has demonstrated that the amygdala’s response to a threat is modified by the nature of other events in the environment (Dunsmoor et al., 2008; Sarinopoulos et al., 2010; Wood et al., 2012, 2013). Therefore, threat-elicited amygdala activity may be influenced by the emotional content of other environmental stimuli, and may play a key role in modulating the peripheral emotional response to threat.
Prior work indicates that behavioral and autonomic responses to threat (i.e. burst of white-noise) are modulated by the emotional content (i.e. valence and arousal) of other co- occurring events (Cook et al., 1992; Cuthbert et al., 1998; Vrana et al., 1988). For example, the startle eye-blink response is typically enhanced by negative images and attenuated by pleasant images (Bradley et al., 1991; Cuthbert et al., 1998; Lang et al., 1998; Vrana et al., 1988). In contrast, the skin conductance response (SCR) elicited by a startle probe varies with arousal level (vanOyen Witvliet & Vrana, 1995). Thus, the emotional response to a threat appears to be modulated by both the valence and arousal of other events in the environment.
Although a number of human neuroimaging studies have investigated the amygdala’s role in processing emotional content (i.e. negative, neutral, and positive images), limited attention has been given to the effect these images have on the amygdala’s response to the threat (i.e. burst of white noise). This prior work has demonstrated greater amygdala activity to negative compared to neutral images (Breiter et al., 1996; Whalen et al., 2001; Yang et al., 2002). However, the amygdala’s response to positive stimuli is less clear. For example, prior research has demonstrated decreased amygdala activity to positive compared to neutral images (Morris et al., 1996; Whalen et al., 1998), whereas others have observed increased amygdala activity to positive versus neutral pictures (Breiter et al., 1996; Kensinger & Schacter, 2006; Somerville et al., 2004; Yang et al., 2002). Although the response to positive stimuli is somewhat ambiguous, the amygdala does appear capable of processing both positive and negative stimuli. However, it is unclear how these processes influence the amygdala’s response to a separate, yet co-occurring threat.
Several human neuroimaging studies have measured both psychophysiology and amygdala activation while viewing emotional images (Anders et al., 2004, 2008; Eippert et al., 2007; Hamann et al., 2002; Heller et al., 2011; Johnstone et al., 2007; Lee et al., 2012; Pissiota et al., 2003; Urry et al., 2006). However, few have investigated the neural processes that mediate changes in the peripheral emotional response (Anders et al., 2004; Johnstone et al., 2007; Lee et al., 2012; Urry et al., 2006). The few studies that have investigated these brain-behavior relationships, have primarily focused on the cognitive regulation of emotion (Johnstone et al., 2007; Lee et al., 2012; Urry et al., 2006). These studies have shown amygdala activity that varies with corrugator supercilii electromyography (EMG), a valence sensitive measure (Lee et al., 2012), and pupil dilation, an index of autonomic arousal (Johnstone et al., 2007; Urry et al., 2006). However, these neurophysiological relationships were observed during emotional images, and were not elicited by the presentation of a threat (e.g. startle probe) leaving questions regarding the neural mechanisms that modulate the response to threat unanswered.
Prior neuroimaging research investigating the behavioral response to threat, in relation to brain activity, has been limited. Further, the threat-related behavior (i.e. startle eye-blink) assessed in the limited work that has been completed was compared to amygdala activity elicited by other emotional stimuli (i.e. images from the International Affective Picture system; IAPS), not by the threat (i.e. startle probe) itself (Anders et al., 2004). In this prior study, the startle eye- blink response and IAPS image-elicited amygdala activity both varied with the valence of the images presented (Anders et al., 2004). In contrast, the SCR data collected in this prior study varied with arousal (Anders et al., 2004). However, the amygdala activation and SCR were both elicited by the presentation of IAPS stimuli, not the threat itself (Anders et al., 2004). Further, no relationship was observed between amygdala activity and SCR expression (Anders et al., 2004), which is inconsistent with fear conditioning research that has repeatedly demonstrated a relationship between amygdala activity and SCR production (Cheng et al., 2003, 2006, 2007; Dunsmoor et al., 2008; Knight et al., 2005; Wood et al., 2012). Thus, there remains a gap in our understanding of whether the amygdala’s influence over the peripheral response to a threat is modulated by the content of other emotional stimuli (e.g. IAPS images) in the environment.
Individual differences in affect also appear to influence amygdala reactivity and the peripheral response to emotional stimuli (Cook et al., 1992; Grillon et al., 1994; Knight et al., 2011; Sehlmeyer et al., 2011; Somerville et al., 2004). For example, healthy individuals with high state anxiety show greater amygdala activity to neutral facial expressions than those with low anxiety (Somerville et al., 2004). Prior work has also demonstrated an enhanced amygdala response to negative emotional stimuli for patients with anxiety compared to healthy individuals (Shah, et al., 2009). Further, high trait anxiety has been repeatedly linked with an exaggerated peripheral emotional response to aversive stimuli (Butler et al., 1990; Cook et al., 1992; Grillon et al., 1994, 1998, 2002; Knight et al., 2011), as well as increased amygdala reactivity (Brühl et al., 2011; Indovina et al., 2011) in anticipation of aversive events. Therefore, identifying the relationship between trait anxiety and the threat-elicited neurophysiological response may provide a more complete understanding of these emotional processes in general.
The present study investigated the amygdala’s role in the emotional modulation of the peripheral response to a threat. More specifically, this study was designed to determine whether the amygdala’s response to threat is modulated by the valence or arousal of other stimuli in the environment (i.e. IAPS). Although prior work has demonstrated that the emotional content of other environmental stimuli influences the behavioral response elicited by an acoustic startle probe (Bradley et al., 1991; Cook et al., 1991, 1992; Cuthbert et al., 1998; Lang et al., 1998; Vrana et al., 1988), there remains a gap in our understanding of the influence the emotional content (i.e. valence and arousal) of these other stimuli on threat-elicited amygdala activity and subsequent peripheral emotional autonomic response.
Materials and Methods
Participants
Twenty-two healthy right-handed volunteers were recruited for this study based on recommendations from prior work (Simmons et al., 2011). The data for one volunteer was excluded from the analyses due to scanner malfunction leaving a total of twenty-one participants (13 female, 8 male; age = 21.19 ± 0.83 years (mean ± SEM); range = 19-34 years). Three non- responsive (SCR < 0.05 μSiemens) participants were excluded from the SCR data analyses. These three participants were also excluded from the brain-behavior analyses, leaving eighteen participants in the analyses that included SCR (10 female, 8 male; age = 21.33 ± 0.95; range = 19-34). All subjects provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board.
Stimuli
Participants were exposed to an emotion modulation procedure in which IAPS (Lang et al., 1990) images (8 s duration; 2, 4, or 6 s ITI) were presented in combination with a loud white- noise (100 dB sound pressure level; 500 ms duration). The IAPS is a set of color images with content that varies in its emotional valence (negative to positive) and arousal (low to high). Standardized valence and arousal ratings (Lang et al., 1998) were used to select 3 categories of stimuli of 1) low valence and moderate arousal (i.e. negative images; valence = 2.52 ± 0.31, arousal = 5.78 ± 0.53), 2) high valence and moderate arousal (i.e. positive images; valence = 7.45 ± 0.33, arousal = 5.78 ± 0.58), and intermediate valence and low arousal (i.e. neutral images; valence = 5.04 ± 0.36, arousal = 2.74 ± 0.34). A total of 216 distinct images (72 negative, 72 neutral, and 72 positive) were presented over three 874 s duration functional magnetic resonance imaging (fMRI) scans (24 negative, 24 neutral, and 24 positive trials were presented during each scan). In order to disentangle the hemodynamic response to the white-noise (i.e. threat) and IAPS stimuli, the threat was presented 2, 4, or 6 s after image onset through magnetic resonance (MR) compatible pneumatic headphones (IFIS-SA). The threat was presented during 33% of each type of emotional image (i.e. during 8 negative, 8 neutral, and 8 positive stimuli during each of the 3 scans). In total, there were 72 presentations of the threat (during 24 negative, 24 neutral, and 24 positive images). The emotional images were presented in a pseudorandom order such that no more than two of the same trial type were consecutively presented, and presentations of the threat were separated by at least 16 s. Presentation software (Neurobehavioral Systems, Inc.; Albany, CA) was used to present IAPS images on an MR compatible IFIS-SA (Invivo Corp.; Gainesville, FL) LCD video screen located above the participant’s head and viewed through a mirror attached to the RF coil.
Skin conductance response
An MRI compatible physiological monitoring system (Biopac Systems; Goleta, CA) was used to collect SCR data as described in previously published work (Knight & Wood, 2011). SCR was sampled (2,000 Hz) with a pair of disposable radio- translucent electrodes (1 cm diameter, Biopac Systems; Goleta, CA) from the distal phalanx of the middle and ring fingers of the nondominant hand. SCR data were processed using Biopac AcqKnowledge 4.1 software. A 1 Hz low pass digital filter was applied and SCR data were resampled at 200 Hz. Threat- and image-elicited SCR were assessed to determine if emotional modulation (i.e. an effect for valence or arousal) was simply produced by the images alone, or if differences in response amplitude were specific to the threat. Threat-elicted SCRs were calculated as the maximum SCR during the 10 s following the white-noise as compared to baseline (the skin conductance level at response onset). SCRs in response to the IAPS images alone (i.e. image-elicited) were calculated as the maximum SCR during image presentation as compared to baseline. SCRs with amplitude less than 0.05 μSiemens were scored as 0. Data were square root transformed prior to statistical analysis. SCR data were evaluated by repeated measures ANOVA with a factor for image valence (i.e. negative, neutral, and positive) and subsequent t-test comparisons (1-tailed) to evaluate modulation of the peripheral emotional response. MRI compatible electromyography (EMG) equipment was not available in our imaging facility when this study was completed. Therefore, startle EMG data were not collected.
State–Trait Anxiety Inventory
Participants completed the State-Trait Anxiety Inventory (STAI; Form Y) for Adults (Spielberger, 1983) prior to the conditioning session. The STAI consists of self-assessment scales that measure state and trait anxiety in terms of negative affect (Grös et al., 2007). Scores on the state scale reflect current anxiety levels, while trait anxiety scores reflect a relatively long-term predisposition for anxiety (Spielberger, 1983). No other measures were administered.
Functional MRI
Structural and functional imaging was completed on a 3 Tesla Siemens Allegra scanner. High-resolution anatomical images (MPRAGE) were obtained in the sagittal plane using a T1 weighted series (TR = 2300 ms, TE = 3.9 ms, flip angle = 12°, FOV = 25.6 cm, matrix = 256 × 256, slice thickness = 1 mm) to serve as an anatomical reference. Blood oxygen level dependent fMRI of the entire brain was conducted using a gradient-echo echoplanar pulse sequence in an oblique-axial orientation (TR = 2000 ms, TE = 30 ms, flip angle = 70°, FOV = 24 cm, matrix = 64 × 64, slice thickness = 4 mm) during each block of stimulus presentations. Functional image processing was performed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Echo-planar time series data were corrected for slice timing offset, motion corrected, concatenated, reregistered to the fifth volume of the first imaging block, and spatially filtered using a 4 mm full-width-at-half-maximum Gaussian filter.
Functional MRI data were analyzed at the individual subject level using the input from all stimuli in a multiple linear regression analysis using a gamma variate hemodynamic response function. Regressors accounted for brain activity related to negative, neutral, and positive IAPS images, white-noise (threat) presentations, and head motion parameters. Three white-noise threat regressors were included in the analysis to model distinct fMRI signal response patterns. The first threat regressor’s amplitude was modulated on a trial-by-trial basis in relation to IAPS image valence (i.e. negative, neutral, & positive) based on published norms (Lang et al., 1998). The second threat regressor’s amplitude was modulated on a trial-by-trial basis in relation to IAPS image arousal (i.e. high & low), also based on previously published norms (Lang et al., 1998). The third was an unmodulated threat regressor. The valence and arousal amplitude modulated reference waveforms were the regressors of interest for the primary analysis. Regressors for behavioral data collected during the study were not included in the analysis given that STAI ratings do not vary on a trial-by-trial basis and because an SCR regressor would be expected, based on prior work (vanOyen Witvliet & Vrana, 1995; Vrana, 1995), to explain similar variance to the IAPS image arousal regressor described above.
For the primary group analyses, functional maps reflecting beta coefficients from valence and arousal modulated threat regressors were converted to the Talairach and Tournoux (1988) stereotaxic coordinate system using a nonlinear transformation to the TT_N27 template. Based on our a priori hypotheses about the amygdala’s role in modulation of the emotional response, group level analyses were restricted to this brain area using an anatomical mask (see Supplemental Figure 1) to reduce the number of voxel-wise comparisons. Monte Carlo simulations were conducted in AFNI using 3dClustSim to determine threshold criteria to correct for multiple comparisons. The simulations indicated that an uncorrected p < 0.005 and cluster size of 62 mm3 (1.1 voxels of 3.75 × 3.75 × 4.00 mm dimension) resulted in FWE corrected significance threshold of p < 0.05. Functional maps representing valence and arousal modulated activity were included in separate t-test (1-tailed) comparisons vs. no effect to determine if threat-elicited amygdala activity varied with IAPS image valence, arousal, or both. In addition, a whole brain analysis was also conducted to determine the specificity of results obtained for the amygdala (see Supplemental Table 1). Monte Carlo simulations indicated that an uncorrected p < 0.005 and cluster size of 788 mm3 (14 voxels of 3.75 × 3.75 × 4.00 mm dimension) resulted in FWE corrected significance threshold of p < 0.05 for the whole brain analyses.
Additional analyses of the amygdala data were completed to obtain descriptive statistics and to investigate individual differences in brain-behavior relationships. At the individual subject level, the three original threat regressors from the primary analysis (i.e. valence modulated, arousal modulated, and unmodulated regressors) were replaced with three new regressors that represented threat presentations on negative, neutral, and positive trials separately. Consistent with the primary analysis, regressors also accounted for image presentation for negative, neutral, and positive trials. Functional ROI representing the volume of amygdala activity identified in the primary analysis were then used to extract the threat-elicited percent signal change data from the bilateral amygdala. The percent signal change data were also extracted for image-elicited amygdala activation to evaluate the amygdala’s response to IAPS images alone. Although the study was designed to disentangle the hemodynamic response to the threat and IAPS images, we analyzed both threat- and image-elicited fMRI signal responses as a manipulation check to ensure the observed effects were specific to the threat. The amygdala’s response on negative, neutral, and positive trials was then combined to obtain averaged threat-elicited and image- elicited responses for each subject. These data were then correlated (1-tailed) with the combined (i.e. the average of negative, neutral, and positive trials) SCR expression across subjects to investigate the amygdala’s role in the peripheral expression of the emotional response. In addition, trait anxiety scores were correlated with the fMRI and SCR data to investigate whether individual differences in trait anxiety varied with amygdala activity or the peripheral expression of emotion (i.e. SCR).
Results
Skin conductance response
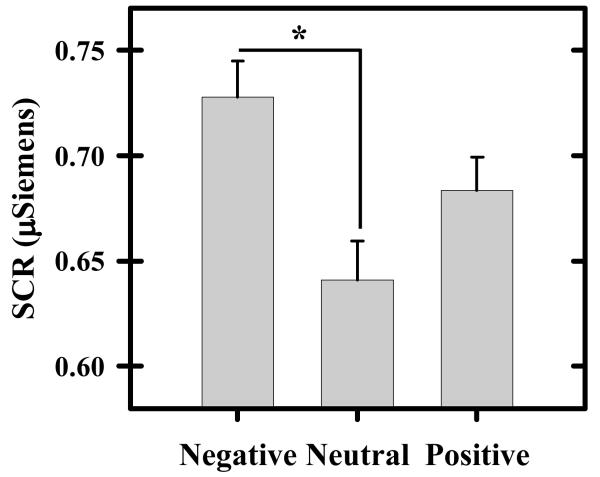
Significant differences in threat-elicited SCR expression were observed in the present study. Repeated measures ANOVA revealed a significant quadratic relationship in the SCR elicited by the threat during negative, neutral, and positive images (F [1, 17] = 5.46; p < 0.05, η 2p = .24). Subsequent paired t-test comparisons revealed a larger SCR to the threat during negative images (mean ± SEM [adjusted for between subject variance (Loftus & Masson, 1994)]: 0.73 ± 0.02) compared to neutral images (0.64 ± 0.02; t[17] = 2.74, p < 0.05, d = 0.65), while threat-elicited SCR fell at an intermediate level during positive images (0.68 ± 0.02) and did not differ from the threat response produced during negative (t = 1.65) or neutral stimuli (t = 1.43) (Figure 1). Repeated measures ANOVA revealed no differences in SCR elicited by the IAPS images alone for negative, neutral, or positive stimuli (F [1, 17] < 1.00).
Figure 1.
Threat-elicited skin conductance response (SCR). Larger SCRs were produced by the threat presented during negative compared to neutral valence IAPS images. Threat-elicited SCR during images of positive valence fell at an intermediate level and did not differ from negative or neutral stimuli. Asterisk reflects significant difference (p < 0.05). Error bars reflect SEM after adjusting for between-subject variance (Loftus & Masson, 1994).
Functional MRI
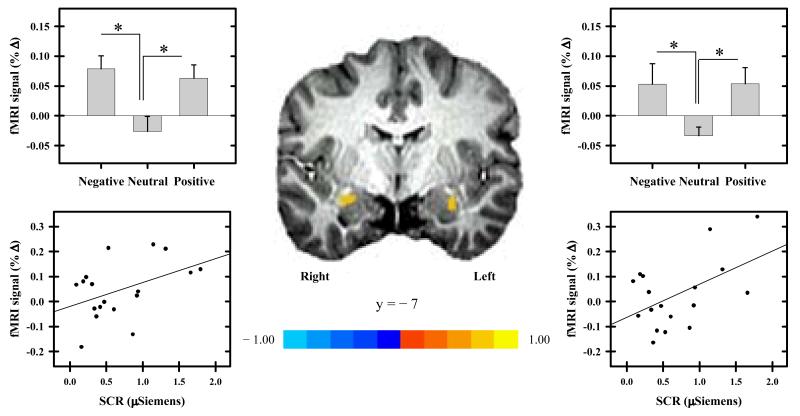
The primary fMRI data analysis indicated that the threat-elicited fMRI signal response within the bilateral amygdala varied with the arousal (Table 1), but not the valence of IAPS images. Threat-related amygdala activation was larger during high arousal versus low arousal IAPS images. More specifically, threat-related amygdala activation was greater in response to both negative and positive images compared to neutral images (Figure 2, Table 1). There were no differences in threat-elicited amygdala activity for negative vs. positive stimuli. We also evaluated amygdala activation in response to the IAPS images alone (i.e. image-elicited activity). Image-elicited activation from the functional amygdala ROI only revealed greater activation to negative vs. neutral images (t[20] = 2.17, p < 0.05, d = 0.47) within the right amygdala. No other differences in image-elicited activation were observed within the right amygdala (t = 1.00), and no differences in image-elicited amygdala activity were observed within the left amygdala (t ≤ 1.00).
Table 1.
Threat-elicited amygdala activation.
| Talairach coordinates | Negative v. Neutral |
Negative v. Positive |
Neutral v. Positive |
SCR | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Amygdala | Vol (mm3) |
x | y | z | t, d | t, d | t, d | r |
| Right | 196 | 25.4 | −7.2 | −10.7 | 2.55, 0.56 | n.s. | −2.13, 0.47 | .44 |
| Left | 141 | −27.4 | −6.0 | −13.4 | 1.91, 0.42 | n.s. | −3.22, 0.71 | .51 |
Note: Location, volumes, and coordinates from Talairach and Tournoux (1988) for the center of mass for areas of activation. Significance criteria: Arousal modulated t-test (N = 21); t[20] > 2.84, p < 0.05 (corrected), d = 0.62. Pearson correlations (N = 18 due to 3 SCR non-responders) between amygdala activity and SCR were conducted across subjects on the combined (i.e. average of negative, neutral, and positive) threat response.
Figure 2.
Threat-elicited amygdala activity. The threat-elicited fMRI signal response observed within the amygdala was enhanced by images of high arousal content (i.e. both negative and positive images; top graphs). Error bars reflect SEM after adjusting for between-subject variance (Loftus & Masson, 1994), and the asterisk indicates significant difference. The threat-elicited response within the bilateral amygdala varied with the amplitude of the threat-elicited SCR such that as threat-elicited amygdala activity increased, the magnitude of the threat-elicited SCR increased (bottom graphs). Pearson correlations between amygdala activity and SCR were conducted across subjects on the combined (i.e. average of negative, neutral, and positive) threat response.
Additional analyses demonstrated a correlation between combined threat-elicited (i.e. the average threat response on negative, neutral, and positive trials) amygdala activity and SCR. Significant positive correlations were observed between bilateral amygdala activity and SCR elicited by the threat (Figure 2, Table 1). This relationship was not observed between combined image-elicited (i.e. the average response to negative, neutral, and positive images) amygdala activation and image-elicited SCR. We also investigated the relationship between trait anxiety level (mean ± SEM 31 ± 1.1; range 24 - 40) and the fMRI and SCR data (i.e. threat and image- elicited). Trait anxiety level was not correlated with amygdala activation or with SCR expression.
Discussion
Understanding the neural substrates of the emotional response to threat is important given the direct impact a threat has on an organism’s survival. However, prior neuroimaging work has largely focused on stimuli that predict or contextualize threat, leaving questions about the neural mechanisms of threat specific processes unanswered. Therefore, the present study used fMRI to investigate whether threat-related activity within the amygdala is influenced by the valence or arousal of other stimuli (i.e. IAPS images) in the environment. Results from the current study, indicate that threat-elicited amygdala activity varies with the arousal, but not the valence of IAPS images. The present findings also revealed brain-behavior relationships specific to threat-elicited emotional behavior. More specifically, as threat-elicited amygdala activity increased the amplitude of threat-elicited SCR also increased.
Converging lines of research have demonstrated that the amygdala processes the emotional content of sensory input (Davis, 1992; Davis & Whalen, 2001; Fanselow, 1994; LeDoux, 2007; Rogan et al., 1998). For example, increased amygdala activity has been previously observed in response to emotionally arousing stimuli (Cahill et al., 1996; Whalen et al., 2001; Yang et al., 2002), regardless of whether the valence was negative or positive (Breiter et al., 1996; Garavan et al., 2001; Hamann et al., 1999, 2002; Kensinger & Schacter, 2006; Somerville et al., 2004; Yang et al., 2002). However, these prior studies did not evaluate emotional modulation of amygdala activity to a co-occurring threat. A better understanding of threat-related amygdala function is important given the biological significance of an appropriate threat response for survival. Therefore, the present study assessed threat-specific amygdala activity. Findings from the current study demonstrate that the amplitude of the threat-elicited amygdala response is larger during images of high arousal (i.e. negative & positive) vs. low arousal (i.e. neutral) content. This finding demonstrates that amygdala activity elicited by a threat is modulated by the emotional arousal associated with other events in the environment (e.g. IAPS images), and indicates that emotional processes modulate the amygdala’s response to a separate, yet co-occurring threat.
In addition, we observed threat-elicited amygdala activity that varied with the amplitude of threat-elicited SCR. This neurophysiological relationship cannot be explained by the emotional content of the images alone given that the amygdala activity and SCR elicited by the images were not correlated. Therefore, the present findings suggest the amygdala plays a critical role in modulating the peripheral emotional response to a threat. Prior human neuroimaging research investigating fear conditioning has also demonstrated a consistent relationship between amygdala activity and SCR (Cheng et al., 2003, 2006, 2007; Dunsmoor et al., 2008; Knight et al., 2005; Wood et al., 2012). However, the vast majority of this prior work only investigated this neurophysiological relationship during the anticipation of a threat (Cheng et al., 2003, 2006, 2007; Knight et al., 2005; Tabbert et al., 2006). Limited prior work has focused on the amygdala’s role in the production of the emotional response to the threat itself (Dunsmoor et al., 2008; Wood et al., 2012) and none of these studies have demonstrated that arousal level modulates threat-elicited amygdala activation. The present study extends this prior work by demonstrating that arousal level impacts the threat-elicited amygdala activity that varies with the peripheral emotional response.
Although the present study observed a significant relationship between threat-elicited amygdala activity and SCR, threat-elicited EMG was not assessed. MR compatible EMG equipment was not available at the time this study was completed. Therefore, EMG data could not be collected. Typically, the startle EMG response varies with the valence of emotional images (Bradley et al., 1991; Cuthbert et al., 1998; Lang et al., 1998; Vrana et al., 1988), and prior research has demonstrated a relationship between amygdala function and the startle response during fear conditioning (Klumpers et al., 2010; van Well et al., 2012). Therefore, future studies would benefit from monitoring both EMG and SCR to further examine the relationship between threat-elicited amygdala activity and behavioral measures that are sensitive to distinct aspects of emotion (i.e. valence and arousal). Future studies may also benefit by assessing subjective valence and arousal ratings from study participants. Subjective ratings of valence and arousal were not collected from the participants in the current study. Instead, we used previously published normative ratings (Lang et al., 1998). Although the use of normative ratings is a common practice, subjective ratings from individual participants in a study are likely to vary around norms and could be used to identify individual differences in brain activation that subserves this aspect of the emotional experience.
In conclusion, emotional modulation of the threat-elicited neurophysiological response was observed in the present study. Although prior research has shown modulation of the emotional response to other events within the environment (e.g. IAPS images alone), we have demonstrated modulation of the emotional response to the threat itself. The current findings indicate threat-elicited amygdala activity is modulated by the emotional arousal, but not the valence of other events in the environment. Further, the threat-elicited fMRI signal response within the amygdala varied with the peripheral expression of emotion (i.e. SCR). The ability to respond adaptively to threats, as circumstances change within one’s environment, is critical for healthy emotional function, and although cues that signal impending threat are important, it is the response to the threat itself that is most biologically relevant from a functional perspective. Therefore, understanding the neural mechanisms of threat-related emotional behavior may provide important new insights into adaptive and maladaptive processes that mediate resilience and susceptibility to affective disorders.
Supplementary Material
Acknowledgements
This research was supported by the University of Alabama at Birmingham Faculty Development Grant Program and NIH grant MH098348 to D.C.K.
References
- Anders S, Eippert F, Weiskopf N, Veit R. The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: an fMRI study. Social Cognitive and Affective Neuroscience. 2008;3(3):233–43. doi: 10.1093/scan/nsn017. doi:10.1093/scan/nsn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Human Brain Mapping. 2004;23(4):200–9. doi: 10.1002/hbm.20048. doi:10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Startle and emotion: lateral acoustic probes and the bilateral blink. Psychophysiology. 1991;28(3):285–95. doi: 10.1111/j.1469-8986.1991.tb02196.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1946894. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–87. doi: 10.1016/s0896-6273(00)80219-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8938120. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Rufer M, Delsignore A, Kaffenberger T, Jäncke L, Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Research. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. doi:10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. The American Journal of Psychiatry. 1990;147(10):1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:15, 8016–21. doi: 10.1073/pnas.93.15.8016. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=38867&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behavioral Neuroscience. 2006;120(6):1187–95. doi: 10.1037/0735-7044.120.5.1187. doi:10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: Stimulus processing versus response expression. Behavioral Neuroscience. 2003;117(1):3–10. doi: 10.1037//0735-7044.117.1.3. doi:10.1037/0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory. 2007;14(7):485–490. doi: 10.1101/lm.632007. doi:10.1101/lm.632007.sion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I, Lüthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–82. doi: 10.1038/nature09559. doi:10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Cook EW, 3rd, Davis TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29(6):633–45. doi: 10.1111/j.1469-8986.1992.tb02038.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1461954. [DOI] [PubMed] [Google Scholar]
- Cook EW, 3rd, Hawk LW, Davis TL, Stevenson VE. Affective individual differences and startle reflex modulation. Journal of Abnormal Psychology. 1991;100(1):5–13. doi: 10.1037//0021-843x.100.1.5. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16406216. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8812068. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley M, McManis M, Lang PJ. Probing affective pictures: attended startle and tone probes. Psychophysiology. 1998;35(3):344–7. doi: 10.1017/s0048577298970536. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9564755. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. doi:10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11244481. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage. 2008;40(2):811–7. doi: 10.1016/j.neuroimage.2007.11.042. doi:10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28(5):409–23. doi: 10.1002/hbm.20291. doi:10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1(4):429–438. doi: 10.3758/BF03210947. doi:10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12(12):2779–83. doi: 10.1097/00001756-200108280-00036. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11522965. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Goddard A, Woods SW, Davis M. Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry. 1994;35:431–439. doi: 10.1016/0006-3223(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry. 1998;44(10):1027–36. doi: 10.1016/s0006-3223(98)00034-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9821567. [DOI] [PubMed] [Google Scholar]
- Grös DF, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): Comparison to the State-Trait Anxiety Inventory (STAI) Psychological Assessment. 2007;19(4):369–81. doi: 10.1037/1040-3590.19.4.369. doi:10.1037/1040-3590.19.4.369. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2(3):289–93. doi: 10.1038/6404. doi:10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman J, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13(2):135–41. doi: 10.1111/1467-9280.00425. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11933997. [DOI] [PubMed] [Google Scholar]
- Heller AS, Greischar LL, Honor A, Anderle MJ, Davidson RJ. Simultaneous acquisition of corrugator electromyography and functional magnetic resonance imaging: a new method for objectively measuring affect and neural activity concurrently. NeuroImage. 2011;58(3):930–4. doi: 10.1016/j.neuroimage.2011.06.057. doi:10.1016/j.neuroimage.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. The amygdala is essential for the expression of conditional hypoalgesia. Behavioral Neuroscience. 1992;106(3):518–28. doi: 10.1037//0735-7044.106.3.518. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1319714. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Núñez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69(3):563–71. doi: 10.1016/j.neuron.2010.12.034. doi:10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(33):8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. doi:10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger E, Schacter D. Processing emotional pictures and words: Effects of valence and arousal. Cognitive, Affective & Behavioral Neuroscience. 2006;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Raemaekers MAHL, Ruigrok ANV, Hermans EJ, Kenemans JL, Baas JMP. Prefrontal mechanisms of fear reduction after threat offset. Biological Psychiatry. 2010;68(11):1031–1038. doi: 10.1016/j.biopsych.2010.09.006. doi:10.1016/j.biopsych.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberzon I. Trait anxiety modulates anterior cingulate activation to threat interference. Depression and Anxiety. 2011;28(3):194–201. doi: 10.1002/da.20802. doi:10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Lewis EP, Wood KH. Conditioned diminution of the unconditioned skin conductance response. Behavioral Neuroscience. 2011;125(4):626–31. doi: 10.1037/a0024324. doi:10.1037/a0024324. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. NeuroImage. 2005;26(4):1193–200. doi: 10.1016/j.neuroimage.2005.03.020. doi:10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight DC, Wood KH. Investigating the neural mechanisms of aware and unaware fear memory with FMRI. Journal of Visualized Experiments: JoVE. 2011;(56):1–6. doi: 10.3791/3083. doi:10.3791/3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97(3):377–95. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2274614. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44(12):1248–63. doi: 10.1016/s0006-3223(98)00275-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9861468. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23(4-5):727–38. doi: 10.1023/A:1025048802629. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14514027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Current Biology: CB. 2007;17(20):868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala- prefrontal coupling underlies individual differences in emotion regulation. NeuroImage. 2012;62(3):1575–81. doi: 10.1016/j.neuroimage.2012.05.044. doi:10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1(4):476–490. doi: 10.3758/BF03210951. doi:10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(31):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Panayiotou G, vanOyen Witvliet CV,, Robinson JD, Vrana SR. A startling absence of emotion effects: Active attention to the startle probe as a motor task cue appears to eliminate modulation of the startle reflex by valence and arousal. Biological Psychology. 2011;87(2):226–233. doi: 10.1016/j.biopsycho.2011.03.001. doi:10.1016/j.biopsycho.2011.03.001.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. doi:10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–87. doi: 10.1016/j.neuron.2005.09.025. doi:10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, Fredrikson M. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. European Journal of Neuroscience. 2003;18(5):1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. doi:10.1046/j.1460-9568.2003.02855.x. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Knight DC. Negative, but not positive emotional images modulate the startle esponse independent of conscious awareness. Emotion. 2013;13(4):782–791. doi: 10.1037/a0032286. doi:10.1037/a0032286. [DOI] [PubMed] [Google Scholar]
- Rogan M, Stäubli U, Ledoux J. Fear conditioning induces associative long-term potentiation of the amygdala. Nature. 1998;391(February):818. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex. 2010;20(4):929–40. doi: 10.1093/cercor/bhp155. doi:10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schöning S, Kugel H, Pyka M, Pfleiderer B, Konrad C. Neural correlates of trait anxiety in fear extinction. Psychological Medicine. 2011;41(4):789–98. doi: 10.1017/S0033291710001248. doi:10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. Journal of Psychiatry Neuroscience. 2009;34(4):296–302. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2702447&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science. 2011;22(11):1359–66. doi: 10.1177/0956797611417632. doi:10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological Psychiatry. 2004;55(9):897–903. doi: 10.1016/j.biopsych.2004.01.007. doi:10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Spielberger C. State-Trait Anxiety Inventory for Adults. Mind Garden; Redwood City, CA: 1983. [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. NeuroImage. 2006;32(2):761–770. doi: 10.1016/j.neuroimage.2006.03.038. doi:10.1016/j.neuroimage.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26(16):4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. doi:10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Well S, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: Evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cognitive, Affective & Behavioral Neuroscience. 2012 doi: 10.3758/s13415-012-0089-7. doi:10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanOyen Witvliet CV,, Vrana SR. Psychophysiological responses as indices of affective dimensions. Psychophysiology. 1995;32(5):436–43. doi: 10.1111/j.1469-8986.1995.tb02094.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7568637. [DOI] [PubMed] [Google Scholar]
- Vrana SR. Emotional modulation of skin conductance and eyeblink responses to startle probe. Psychophysiology. 1995;32(4):351–7. doi: 10.1111/j.1469-8986.1995.tb01217.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7652111. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97(4):487–91. doi: 10.1037//0021-843x.97.4.487. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3204235. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9412517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12894812. [DOI] [PubMed] [Google Scholar]
- Wood KH, Kuykendall D, Ver Hoef LW, Knight DC. Neural substrates underlying learning-related changes of the unconditioned fear response. The Open Neuroimaging Journal. 2013;7:41–52. doi: 10.2174/1874440001307010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC. Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. NeuroImage. 2012;60(1):787–99. doi: 10.1016/j.neuroimage.2011.12.048. doi:10.1016/j.neuroimage.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, Reiss AL. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13(14):1737–41. doi: 10.1097/00001756-200210070-00009. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12395114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.