Abstract
We utilised a sample of 299 adult females aged between 19 and 86 years, carrying FMR1 alleles with small CCG expansions ranging from 50 to 141 repeats to analyse the relationships between psychological symptoms as assessed by the Symptom Checklist-90-Revised (SCL-90-R) and the size of the CGG repeat in the FMR1 gene. There were highly significant (negative) correlations between the size of the CGG repeat and a great majority of SCL-90-R subscale scores and all the global indices, suggesting that carriers of premutations in the mid-size CGG repeat range may be at greatest risk for development of psychiatric disorder.
Keywords: FMR1 premutation, premutation female carriers, psychological symptoms, CGG repeat size, regression analysis
Introduction
Premutation (PM) is the recognized category of Fragile X Mental Retardation 1 (FMR1) alleles containing CGG expansions between 55 and 200 repeats [1]. In females these common alleles (1 per 113 to 259 individuals) [2–4] are associated with a risk of further expansion into the full mutation range (>200) over one generation, leading to severe developmental abnormality, Fragile X Syndrome (FXS) [5]; on the other hand, the PM carrier status itself is associated with many phenotypic abnormalities in either sex, as reviewed in:[6,7]. Fragile X-associated Primary Ovarian Insufficiency (FXPOI) manifesting with a spectrum of diminished ovarian functions including premature menopause was the first recognized disorder in ~20% of PM carriers [6,8]. Fragile X Associated Tremor-Ataxia Syndrome (FXTAS), highly prevalent (~40%) in male PM carriers, occurs in approximately 16% of female PM carriers, where neurological and cognitive impairments tend to be milder [7,9].
Although psychiatric disorder appears to be common in female PM carriers, the type and severity of these problems varies between different studies, ranging from the absence of significant disorder [10], to high rates (between 41 and ~56%) of lifetime diagnosis of all affective disorders, with anxiety, depression and obsessive compulsive disorders being most prevalent [11–14], with a tendency to cluster in the same individuals (15). Other confounding effects, biases related to pre-selection of the PM carrier or control samples, or low power, may account for conflicting results from comparative studies. Here we adopted a different approach by relating the primary symptom dimensions and global indices of SCL-90-R to the size of the CGG expansion within the PM range.
Materials and Methods
The SCL-90-R [16] is a self-report instrument that covers a broad range of relevant psychological symptom clusters, with good validity (0.77 – 0.90), and reliability (.80 – 0.90). The Wechsler Adult Intelligence Scale (WAIS-III; [17]), including a prorated short form based on the Block-Design and Vocabulary subtests, which has been shown to be comparable with FSIQ (except for extreme scores, which did not occur in our sample) [18], was used to assess cognitive status.
The PCRs and Southern Blot analysis were used to assess the size of CGG expansion (see Legend to Table 1), with all assays fully validated by internal and external quality assessment to provide precision of +/− one repeat
Table 1.
List of variables and summary statistics for the American (US) and Australian (AU) samples, separately and combined. Two-sample t-tests were used to test for the difference between means if the data was normally distributed; the nonparametric Mann-Whitney test was used to determine the difference in the medians (p-values).
| All | US | AU | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | p-value | |
| CGG repeat* | 299 | 88.09 | 17.62 | 182 | 90.72 | 17.14 | 117 | 83.95 | 17.64 | 0.0013 |
| Age | 299 | 44.10 | 12.61 | 182 | 45.12 | 13.18 | 117 | 42.54 | 11.55 | 0.1330 |
| Menopausal age | 110 | 41.91 | 7.130 | 56 | 41.52 | 7.670 | 54 | 42.31 | 6.58 | 0.5605 |
| Prorated IQ‡ | 260 | 105.9 | 13.73 | 144 | 108.9 | 12.85 | 116 | 102.02 | 13.87 | < 0.0001 |
| Anxiety† | 294 | 42.69 | 10.52 | 182 | 40.02 | 8.380 | 112 | 47.02 | 12.13 | < 0.0001 |
| Depression† | 294 | 43.70 | 11.39 | 182 | 39.27 | 9.480 | 112 | 50.89 | 10.56 | < 0.0001 |
| Interpersonal Sensitivity† | 294 | 45.40 | 11.23 | 182 | 41.76 | 9.750 | 112 | 51.33 | 10.98 | < 0.0001 |
| Hostility† | 294 | 45.87 | 9.490 | 182 | 43.81 | 8.200 | 112 | 49.22 | 10.46 | < 0.0001 |
| Phobic Anxiety† | 294 | 45.25 | 8.250 | 182 | 43.98 | 7.060 | 112 | 47.31 | 9.57 | < 0.0001 |
| Paranoid Ideation † | 294 | 44.16 | 9.570 | 182 | 41.79 | 8.440 | 112 | 48.02 | 10.07 | < 0.0001 |
| Obsessive-Compulsive† | 294 | 48.47 | 11.09 | 182 | 45.71 | 9.870 | 112 | 52.95 | 11.53 | < 0.0001 |
| Psychotism† | 294 | 43.59 | 10.98 | 182 | 39.04 | 8.780 | 112 | 50.97 | 10.16 | < 0.0001 |
| Somatization† | 293 | 48.34 | 10.31 | 182 | 47.87 | 10.00 | 111 | 49.10 | 10.79 | 0.3243 |
| GSI†† | 294 | 44.01 | 11.75 | 182 | 40.08 | 10.10 | 112 | 50.40 | 11.48 | < 0.0001 |
| PSDI†† | 294 | 43.18 | 11.36 | 182 | 39.81 | 10.30 | 112 | 48.66 | 10.89 | < 0.0001 |
| PST†† | 294 | 45.12 | 11.78 | 182 | 41.74 | 10.71 | 112 | 50.60 | 11.42 | < 0.0001 |
| VIQ ‡ | 215 | 104.0 | 14.65 | 144 | 107.0 | 13.26 | 71 | 97.86 | 15.48 | < 0.0001 |
.Genomic DNA was isolated from peripheral blood lymphocytes using standard methods (Qiagen). For Southern blot analysis, 5–10 micrograms of isolated DNA was digested with EcoRI and NruI. Hybridization was performed using the FMR1 genomic dig labelled StB12.3 probe. DNA was also amplified by PCR primers c and f [5]. PCRs were performed using the method previously described: in [Tassone F, et al. J Mol Diagn 2008;10: 43–9], for the American sample, and in: [Khaniani MS, et al. Mol Cytogenet 2008;1:5] for the Australian sample.
Nine primary symptoms dimensions are assessed by the 90 items rated on a five-point Likert scale of distress during the past 7 day, ranging from 0 to 4.
Three global indices provide measures of overall psychological distress: the Global Severity Index (GSI), the Positive Symptom Total (PST) and the Positive Symptom Distress Index (PSDI).
Both primary symptoms and global indices are in the form of T scores with the normal range of 40–60.
Pro-rated IQ and the standard composite score VIQ (Verbal Intelligence Quotient); VIQ normal range of 90–110.
In Australia, 117 females aged 18–79 were ascertained through the affected probands or other relatives attending Victorian Clinical Genetic Services (VCGS); none of these females manifested FXTAS. The size of the CGG repeat (in blood) ranged from 50 to 141, including several subjects with the larger intermediate size alleles ranging from 45–55 CGGs [1].
The US sample consisted of 182 females aged 19–86 ascertained through the affected probands seen at the Fragile X Research and Treatment Center, MIND Institute, UC Davis, 29 females were diagnosed with FXTAS spectrum. The size of the CGG repeat ranged from 56 to 138.
Shaprico-Wilk statistics at the 5% significance level was used to test for normality of distributions. Principal components were used to combine the SCL-90-R subscale scores into linear weight combination of the original (inter-correlated) variables. First principal component (PC1) accounted for 70% of the variation for the SCL-90-R measures, weights ranging from 0.3 to 0.36. The second PC (not considered further) accounted for an additional 8% of variation. The relationship between the cognitive and the SCL-90-R scores, and PC1 (outcome variables), and CGG repeat number (predictor), adjusting for age, country and VIQ, was assessed using multiple linear regressions. The least square estimation was used initially to calculate regression coefficients. Robust regression was applied to down-weight the effect of outliers.
All analyses were conducted using the STATA statistical package, version 11.2 (http://www.stata.com).
Results
There were significant differences in means (or medians) between the American and Australian samples for the majority of symptom scores, with all the means being within the normal range (Table 1). Pro-rated IQ was included as a potential predictor of SCL-90-R scores, and VIQ was significantly correlated with the size of the CGG repeat in the US sample.
Initial regression analyses revealed significant interactions between CGG repeat size and site, resulting in differences between the two samples in the correlations between Pro-rated IQ, VIQ, Obsessive-Compulsive subscale and CGG size, but after adjusting for age and/or VIQ, were no longer significant (p>0.05). The results of regression analysis for the two samples combined showed that the majority of SCL-90-R global and partial scores were significantly and inversely correlated with the size of CGG repeat (Table 2). The size effect of CGG expansion was the strongest for the global scores, especially PSDI and GSI, and for Somatization and Obsessive-Compulsive subscales.
Table 2.
Relationship between CGG repeats (predictor) and outcome variables: prorated IQ, VIQ, SCL-90-R global indices and domain scores, and PC1 assessed using linear regression method, adjusted for country, age and VIQ and country (whenever significant).
| N | Coef. | s.e | p-value | Std Coef. | |
|---|---|---|---|---|---|
| Prorated IQ | 171 | 0.008 | 0.034 | 0.807 | 0.011 |
| VIQ | 171 | −0.086 | 0.064 | 0.178 | −0.104 |
| Anxiety | 291 | −0.092 | 0.034 | 0.008 | −0.151 |
| Depression | 210 | −0.111 | 0.039 | 0.005 | −0.176 |
| Interpersonal Sensitivity | 210 | −0.112 | 0.042 | 0.009 | −0.179 |
| Hostility | 210 | −0.063 | 0.040 | 0.115 | −0.128 |
| Phobic Anxiety | 169 | −0.028 | 0.032 | 0.385 | −0.059 |
| Paranoid Ideation | 210 | −0.102 | 0.036 | 0.005 | −0.192 |
| Obsessive Compulsive | 210 | −0.121 | 0.043 | 0.005 | −0.197 |
| Psychotism | 210 | −0.077 | 0.038 | 0.047 | −0.129 |
| Somatization | 168 | −0.150 | 0.045 | 0.001 | −0.255 |
| GSI | 210 | −0.131 | 0.043 | 0.002 | −0.201 |
| PSDI | 291 | −0.136 | 0.036 | < 0.001 | −0.210 |
| PST | 210 | −0.111 | 0.044 | 0.012 | −0.171 |
| PC1 | 209 | −0.010 | 0.004 | 0.006 | −0.188 |
Std Coef. = estimated standardised coefficient representing the size effect of CGG expanded repeat, where outcome variable and predictors were standardised to have mean zero and variance of 1.
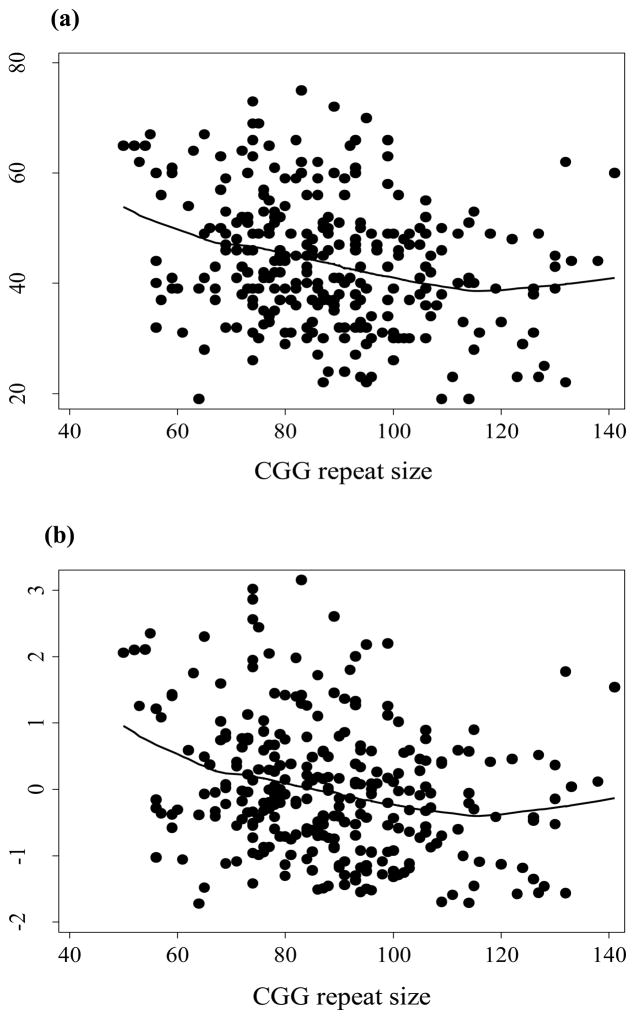
The scatterplots (presented in Figure 1a and 1b for GSI and PC1) showed that the SCL-90-R scores and global indices were highest for alleles within the 60–80 repeat range and the most obvious and consistent downward trend was between 80–100 CGG repeats. We did not have enough data to observe this trend after passing their minimum at approximately 100–120 repeats.
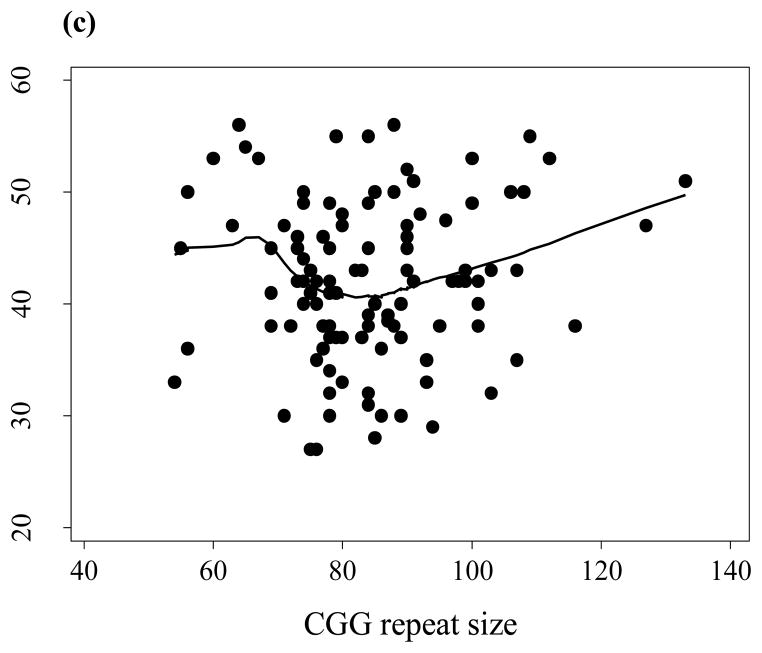
Figure 1.
Scatterplots and regression line representing the relationships between CGG repeat size and the Global Severity Index, GSIT (a), the First Principal Component, PC1, both based on the SCL-9-R self-report instrument (b); and menopausal age (c), using nonparametric locally weighted regression.
We also compared SCL-90-R scores between the two categories of CGG sizes (Table 3). The rationale for using 100 rather than 120 repeats as the cut-off value between those categories was the smallness of our subsample of females carrying larger PM alleles, especially considering that the range with significant effect could only be accurately determined using the non-linear regression models. The means (or medians) were significantly higher in the ≤ 100 category than in >100 category for most of the SCL-90-R subscale and global scores. After relevant adjustments, the differences remained significant for GSI and PDSI, Anxiety, Obsessive-Compulsive, and Somatization scores.
Table 3.
Comparison of the prorated IQ, VIQ, SCL-90-R global indices and domain scores, and PC1 between samples with less than or equal to 100 CGG repeats, and with greater than 100 CGG repeats.
| ≤100 CGG repeats | >100 CGG repeats | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | p-value* | p-value** | |
| Prorated IQ‡ | 199 | 105.9 | 13.39 | 59 | 105.7 | 14.99 | 0.9051 | 0.644 |
| Anxiety† | 229 | 43.48 | 10.87 | 63 | 39.63 | 8.720 | 0.0217 | 0.038 |
| Depression† | 229 | 44.56 | 11.58 | 63 | 40.43 | 10.27 | 0.0153 | 0.089 |
| Interpersonal Sensitivity† | 229 | 46.24 | 11.43 | 63 | 42.00 | 9.820 | 0.0233 | 0.137 |
| Hostility† | 229 | 46.48 | 9.640 | 63 | 43.71 | 8.800 | 0.0737 | 0.177 |
| Phobic Anxiety† | 229 | 45.52 | 8.620 | 63 | 44.22 | 6.840 | 0.4589 | 0.473 |
| Paranoid Ideation † | 229 | 44.61 | 9.880 | 63 | 42.11 | 7.950 | 0.1099 | 0.256 |
| Obsessive-Compulsive† | 229 | 49.16 | 11.38 | 63 | 45.56 | 9.450 | 0.0220 | 0.047 |
| Psychotism† | 229 | 44.14 | 11.50 | 63 | 41.41 | 8.690 | 0.1795 | 0.398 |
| Somatization† | 228 | 49.23 | 10.40 | 63 | 44.84 | 9.280 | 0.0026 | 0.041 |
| GSI† | 229 | 45.12 | 11.94 | 63 | 39.70 | 10.11 | 0.0037 | 0.042 |
| PSDI† | 229 | 44.10 | 11.75 | 63 | 39.79 | 9.320 | 0.0076 | 0.007 |
| PST† | 229 | 46.09 | 11.78 | 63 | 41.17 | 11.04 | 0.0032 | 0.113 |
| VIQ ‡ | 161 | 104.5 | 15.08 | 53 | 102.6 | 13.40 | 0.4119 | 0.307 |
| PC1 | 228 | 0.080 | 1.030 | 63 | −0.300 | 0.830 | 0.0083 | 0.108 |
p-values were computed using either two-sample t-test or nonparametric Mann-Whitney tests.
p-values using analysis of covariance (regression), adjusted for country, age and verbal IQ whenever appropriate.
T score values, with a normal range of 40–60,
Index scores with a normal range of 90–110.
We also explored the relationship of menopausal age with the CGG size in a subsample of 110 post-menopausal females (Figure 1c) which showed the maximum decrease in menopausal age at 60–80 repeats. The (linear) relationship between menopausal age and either CGG repeat size or SCL-90-R scores in a small sample was significant for Depression only (slope=−0.302; p=0.030).
Discussion
In a sample of female PM carriers from Australia and US we have demonstrated significant correlations between CGG repeat size and the SCL-90-R, with two global indices (PSD1 and GSI) and the Somatization and Obsessive-Compulsive subscale scores showing the highest values for effect size. Although the two samples were significantly different in the number of relevant features, the results were consistent in both combined and individual samples, after adjustments for the respective sites (countries) and other confounders in the regression analysis. These adjustments also corrected for the difference in the frequency of FXTAS, which was diagnosed in nearly 15% of the US, compared with nil in the Australian sample. A scarcity of FXTAS in Australian females, and much less severe manifestations of this disorder in the affected males have been noticed previously (19, Loesch unpublished data), the reason for this discrepancy is still unexplained.
Our data have demonstrated a significant effect of PM alleles on psychological status; however, this effect is moderate, with the scores above clinically significant threshold of 63 [16] occurring in less than 10% (23) of all participants. Therefore we hypothesize that those alleles may contribute to shifting the distribution of psychiatric distress scores towards the clinically significant range rather than being the sole cause of psychiatric disorder in the majority of carriers. The earlier finding that the PM females have increased sensitivity to major life changes leading to depression and anxiety [20] strongly supports this view. Considering all the above, as well as the smallness of our subsample with clinically significant SCL-90-R scores, we used the total sample representing a broader range of those scores in correlation with the CGG repeat size.
The important finding was that all subscales or global scores were negatively correlated with CGGs, with the relevant scatterplots suggesting that these relationships may not be linear. However, we could not verify this claim because of limitation in the data which did not cover the full PM range of repeat sizes. Nevertheless, significant differences between the two CGG size categories in both global (PSI and PDS) and several subscale scores strongly support this claim.
Notably, the results of earlier studies [12, 21] in large independent samples of PM female carriers have been indicative of a predominant effect of the midsize CGG repeat range on mood disorders such as anxiety and depression. In the more recent study [20], this effect was found to be linked to the negative life events in mothers of children with FXS, thus reinforcing the concept of the elevated risk of psychiatric symptoms in the carriers of midsize expansion size exposed to the life stress. The reported midsize range may vary according the dependent variable being studied [20], the size and composition of the sample and the statistical model applied.
The phenomenon of the greatest risk of a condition being associated with the midsize CGG range in PM females was first reported for FXPOI using non-linear regression models [22], and later confirmed in several independent samples [23–25]. Our present results, though based on a much smaller sample with truncated distribution of CGG sizes, are clearly consistent with those earlier findings.
A hypothesis attributing the non-linear effect of CGG size within the PM range on severity of FXPOI manifestations to qualitative differences in the FMR1 RNA transcript [23] may also be applicable to psychiatric symptoms. Those differences may result either from a deleterious structure of this transcript in the mid-PM range allowing inappropriate protein binding, or from the alternative transcription initiation sites for FMR1 varying with repeat number as previously shown [26]. The finding of neurotoxic effects of FMR1mRNA over a repeat sizes <100 CGGs [27] supports the concept of maximal mRNA toxicity in the mid-PM range.
Because of already acknowledged limitations of this study, we recommend that the results are confirmed by data covering the whole range of PM expansion sizes, so that the non-linear regression models can be applied to better characterize its relationship with the phenotype, and to accurately determine the range with clinically significant effects. The data from subjects with the intermediate size alleles [6] should also be included in order to be able to identify the minimum size of repeat that may elevate the risk of psychiatric disorder.
Acknowledgments
This study was supported by the National Institutes of Child Health and Human Development Grant HD 36071 HD02274, to Prof R Hagerman and Dr DZ Loesch, and MH078041 to Prof D Hessl; NHMRC project grant No 330400, to Dr DZ Loesch and Prof E Storey We thank Drs Paige Simpson and Sarah Sherwell for conducting neuropsychological testing in a proportion of this study’s participants
Footnotes
Conflict of Interest Statement: Nothing to declare
References
- 1.Maddalena A, Richards CS, McGinniss MJ, et al. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Genet Med. 2001;3 (3):200–5. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toledano-Alhadef H, Basel-Vanagaite L, Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69(2):351–60. doi: 10.1086/321974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hantash FM, Goos DM, Crossley B, et al. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet Med. 2011;13(1):39–45. doi: 10.1097/GIM.0b013e3181fa9fad. [DOI] [PubMed] [Google Scholar]
- 4.Indhumathi N, Singh D, Chong SS, et al. Fragile X CGG repeat variation in Tamil Nadu, South India: a comparison of radioactive and methylation-specific polymerase chain reaction in CGG repeat sizing. Genet Test Mol Biomarkers. 2012;16(2):113–22. doi: 10.1089/gtmb.2011.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67 (6):1047–58. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 6.Loesch DZ, Hagerman RJ. Unstable mutations in the FMR1 gene and the phenotypes. In: Hannah AJ, editor. Tandem Repeat Polymorphisms: Genetic Plasticity, Neural Diversity and Disease. Texas, USA: Landes Bioscience; 2011. [Google Scholar]
- 7.Hagerman RJ, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurology. 2013;12:786–98. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherman SL. Premature ovarian failure in the Fragile X syndrome. Am J Med Genet (Semin Med Genet) 2000;97 :189–94. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 9.Hall DA, O’Keefe JA. Fragile X-associated tremor ataxia syndrome: the expanding clinical picture, pathophysiology, epidemiology and update on treatment. Tremor Other Hyperkinet Mov. 2012;2 doi: 10.7916/D8HD7TDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiss A, Freund L, Abrams MT, Kazazian H. Neurobehavioral effects of the fragile X premutation in adult women: a controlled study. Am J Hum Genet. 1993;52(5):884–94. [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Revenga L, Madrigal I, Algret M, Santos M, Mila M. Evidence of depressive symptoms in fragile-X syndrome premutated females. Psychiatr Genet. 2008;18:153–5. doi: 10.1097/YPG.0b013e3282f97e0b. [DOI] [PubMed] [Google Scholar]
- 12.Roberts J, Bailey D, Jr, Mankowski J, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(1):130–39. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- 13.Bourgeois JA, Coffey SM, Rivera SM, et al. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70:852–62. doi: 10.4088/JCP.08m04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraan CM, Hocking DR, Bradshaw JL, et al. Neurobehavioural evidence for the involvement of the FMR1 gene in female carriers of fragile X syndrome. Neurosci Biobehav Rev. 2013;37:522–47. doi: 10.1016/j.neubiorev.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Bailey D, Raspa M, Olmsted M, Holiday D. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A(16):2060–9. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR. SCL-90-R: Symptom Checklist-90-R. Administration, scoring and procedures manual. National Computer Systems. 3. Minneapolis, Minnesota: 1994. [Google Scholar]
- 17.Wechsler DA. Wechsler Adult Intelligence Scale—III. New York: Psychological Corporation; 1997a. [Google Scholar]
- 18.Engelhart CI, Eisenstein N, Johnson V, Losonczy M. Comparison of linear equating and prorated forms for estimating WAIS-R FSIQ in a neuropsychological population. Clin Neuropsychol. 1999;13:95–9. doi: 10.1076/clin.13.1.95.1971. [DOI] [PubMed] [Google Scholar]
- 19.Loesch DZ, Churchyard A, Brotchie P, et al. Evidence for, and a spectrum of, neurological involvement in fragile X premutation: FXTAS and beyond. Clin Genet. 2005;67:412–17. doi: 10.1111/j.1399-0004.2005.00425.x. [DOI] [PubMed] [Google Scholar]
- 20.Seltzer MM, et al. Differential sensitivity to life stress in FMR1 premutation carriers mothers of children with fragile X syndrome. Health Psychology. 2012;31:612–22. doi: 10.1037/a0026528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter JE, Allen E, Abramowitz A, et al. Investigation of phenotypes associated with mood and anxiety among male and female Fragile X premutation carriers. Behavior Genet. 2008a;38(5):493–502. doi: 10.1007/s10519-008-9214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan A, Marcus M, Epstein M, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–12. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 23.Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006;14:253–5. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen EG, Sullivan A, Marcus M, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22 :2142–52. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- 25.Tejada M, Garcia-Alegria E, Bilbao A, et al. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause. 2008;15(5):945–9. doi: 10.1097/gme.0b013e3181647762. [DOI] [PubMed] [Google Scholar]
- 26.Beilina A, Tassone F, Schwartz PH, Sahota P, Hagerman PJ. Redistribution of transcription start sites within the FMR1 promoter region with expansion of the downstream CGG-repeat element. Hum Mol Genet. 2004;13:543–9. doi: 10.1093/hmg/ddh053. [DOI] [PubMed] [Google Scholar]
- 27.Hoem G, Raske C, Garcia-Arocena D, et al. CGG-repeat length threshold for FMR1 RNA pathogenesis in a cellular model for FXTAS. Hum Mol Genet. 2011;20:2161–70. doi: 10.1093/hmg/ddr101. [DOI] [PMC free article] [PubMed] [Google Scholar]