Summary
Bacillus thuringiensis produces insecticidal Cry and Cyt proteins that are toxic to different insect orders. In addition, Cyt toxins also display haemolytic activity. Both toxins are pore-forming proteins that form oligomeric structures that insert into the target membrane to lyse cells. Cyt toxins play an important role in mosquitocidal activity since they synergize Cry toxins and are able to overcome resistance to Cry toxins. Cry and Cyt toxins interact by specific epitopes, and this interaction is important to induce the synergistic activity observed. It was proposed that Cyt toxins do not interact with protein receptors but directly interacting with the specific midgut cell lipids. Here, we analysed if oligomerization and membrane insertion of Cyt1Aa are necessary steps to synergize Cry11Aa toxicity. We characterized Cyt1Aa helix α-C mutants that were affected in oligomerization, in membrane insertion and also in haemolytic and insecticidal activities. However, these mutants were still able to synergize Cry11Aa toxicity indicating these steps are independent events of Cyt1Aa synergistic activity. Furthermore, the data indicate that formation of stable Cyt1Aa-oligomeric structure is a key step for membrane insertion, haemolysis and insecticidal activity.
Introduction
Bacillus thuringiensis produces insecticidal proteins during sporulation. These proteins, known as Cry and Cyt toxins show toxicity against different insect orders (Bravo et al., 2011). The three-domain Cry proteins (3d-Cry), which have a similar three-dimensional fold (Bravo et al., 2011), have different specificities and are able to kill many different insect orders as well as nematodes (Crickmore et al., 2013).
The Cyt toxins in contrast are composed of a single α–β domain with seven to eight β-strands wrapped by α-helices (Soberón et al., 2012). The Cyt toxins primarily kill dipteran insects. Interestingly, Cyt toxins generally are produced by Bacillus thuringiensis (Bt) strains that also produce 3d-Cry toxins with insecticidal activity against different mosquito and black fly species (Soberón et al., 2012). In addition, it was shown that in vitro Cyt proteins have haemolytic activity (Soberón et al., 2012).
Both the 3d-Cry and Cyt toxins are pore-forming proteins whose crystal inclusions are solubilized after ingestion by larvae and then processed by midgut proteases. The activated toxins undergo structural changes to insert into the membrane and form oligomeric pores that induce osmotic lysis of the midgut cells, leading to the disruption of midgut tissue and larval death. In contrast to Cry proteins, no protein receptors for Cyt proteins have been identified to date, and it was suggested that insect specificity of Cyt toxins resides in their direct interaction with the lipids present in cellular membrane of insect midgut cells (Thomas and Ellar, 1983).
Bacillus thuringiensis subs. israelensis (Bti) produces four 3d-Cry proteins, Cry4Aa, Cry4Ba, Cry10Aa and Cry11Aa, along with two Cyt proteins, Cyt1Aa and Cyt2Ba. Bti has been used worldwide for the control of different mosquito and black fly species that are vectors of important human diseases (Bravo et al., 2011). Interestingly, despite the widespread use of Bti, no resistance to the Bti crystal inclusions has been detected in the field. However, moderate resistance in the field of Aedes spp to single Cry4 toxins has been documented, and it was proposed that this resistance was due to differential decay of Cyt and Cry proteins of Bti crystal under field conditions (Paris et al., 2011). Under laboratory conditions, Culex quinquefasciatus strains with high levels of resistance to Cry4Ba, Cry4Aa or Cry11Aa have been selected (Wirth et al., 1997; 2005). In contrast, resistance to Cyt1Aa could not to be selected even under similar laboratory conditions. Finally, Cyt1Aa synergizes the insecticidal activity of Cry4Aa, Cry4Ba and Cry11Aa and is able to overcome resistance to the three Cry proteins in C. quinquefasciatus resistant populations (Chang et al., 1993; Crickmore et al., 1995; Wirth et al., 1997; 2005).
The mode of action of 3d-Cry toxins has been shown to involve the formation of an oligomeric structure after receptor binding that is proficient in membrane insertion and pore formation (Bravo et al., 2011). Cyt toxins also insert into the membrane forming oligomeric structures that form lytic pores (Soberón et al., 2012). It has been proposed that Cyt1Aa functions as a receptor of Cry toxins since Cyt1Aa synergizes Cry11Aa and Cry4Ba insecticidal activities by specific binding to domain II loop regions of Cry toxins. In the case of Cry11Aa, this interaction facilitates a pre-pore oligomer formation enhancing Cry11Aa activity (Pérez et al., 2005; 2007). Nevertheless, it is not clear if oligomerization and membrane insertion of Cyt1Aa are necessary steps to synergize Cry toxicity.
In the case of the closely related Cyt2Aa and volvatoxin 2 (VVA2) proteins mutations in certain amino acids located in helix α-C affected oligomerization and toxicity of these proteins (Weng et al., 2004; Promdonkoy et al., 2008). But the role of these mutations on Cyt2Aa synergism with other Cry toxins was not analysed.
In this work, we isolated Cyt1Aa helix α-C mutants and show that some of these mutants were affected in oligomer formation, membrane insertion and toxicity. We also show that these mutant proteins were still able to synergize Cry11Aa toxicity, indicating that oligomerization, membrane insertion and toxicity of Cyt1Aa are independent events from the synergistic activity of Cyt1Aa with Cry11Aa.
Results
Construction of Cyt1Aa mutant toxins
Based on information of previously mutagenized Cyt2Aa and VVA2 proteins (Weng et al., 2004; Promdonkoy et al., 2008), we selected helix α-C for analysis. Figure S1 shows the alignment of Cyt1Aa in this region with the amino acid sequences of Cyt2Aa and VVA2 using ClustalW2 multiple sequence alignment (http://www.ebi.ac.uk/Tools/msa/clustalw2/), and the selected residues that were mutagenized in Cyt1Aa are indicated by underlined bold letters. We introduced charged residues in the selected hydrophobic residues to produce six mutants (I116E, V119E, L120K, V122E, L123K and V126E). Two mutants (I116E, V119E) produced very small crystal inclusions that were highly susceptible to degradation after trypsin proteolysis, suggesting that these mutant constructions were not stable, probably compromising the structure of the protein. These proteins were not analysed further. The rest of the mutants (L120K, V122E, L123K and V126E) produce crystal inclusions similar to the wild-type toxin. After trypsin digestion, these mutants gave a 24-kDa fragment similar to the wild-type Cyt1Aa toxin (Fig. S2).
Oligomerization of Cyt1Aa mutant toxins
To determine if oligomerization was affected in these Cyt1Aa mutants, we first optimized the oligomerization assay for the wild-type Cyt1Aa protein by incubating solubilized protoxin with proteinase K in the presence of small unilamellar vesicles (SUV) liposomes, as described in experimental procedures. Samples were then heated at different temperatures for 3 min, separated in SDS-PAGE gels and transferred to polyvinylidene fluoride (PVDF) membranes to be analysed by western blot using a polyclonal anti-Cyt1Aa antibody. Figure 1A shows that Cyt1Aa was able to form large oligomers that migrated as a high-molecular size band, higher than the 250-kDa-marker, in SDS-PAGE after interaction with liposomes. The stability of Cyt1Aa aggregates was analysed at different temperatures showing that they are partially disassembled when incubated at high temperatures. These aggregates are stable under denaturant conditions of SDS-PAGE gels when the samples were heated at 40°C or 60°C. However, when samples were heated at 80°C or 100°C, less oligomers are obtained suggesting that they are sensitive to disassembly at such temperatures (Fig. 1A).
Fig. 1.
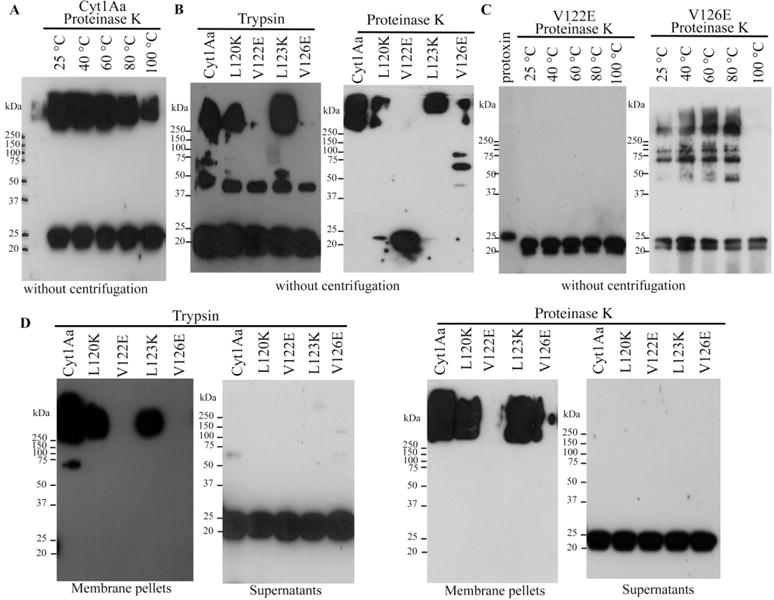
Analysis of oligomerization of Cyt1Aa wild-type and mutant toxins in the presence of SUV.
A. Oligomerization of the wild-type Cyt1Aa protein after incubating the solubilized protoxin 1 h at 37°C with proteinase K the presence of SUV liposomes. Samples were heated 3 min at different temperatures before loading into SDS-PAGE.
B. Oligomerization of different Cyt1Aa mutants after activation with trypsin or proteinase K in the presence of SUV liposomes. Samples were heated 3 min at 65°C before loaded into SDS-PAGE.
C. Analysis of oligomeric structure stability of V122E and V126E mutants after heating at different temperatures. Protoxins were activated with proteinase K in the presence of SUV liposomes, and final samples were heated 3 min at different temperatures before loading into SDS-PAGE.
D. Partition of oligomeric structures into the SUV membranes. Oligomerization of the different Cyt1Aa mutants after activation with trypsin or proteinase K in the presence of SUV liposomes. Samples were centrifuged 30 min at 117 000 g. The pellets containing membrane samples and supernatants were separated, heated 3 min at 65°C before SDS-PAGE. All SDS-PAGE gels were transferred to PVDF and analysed by Western blot using polyclonal anti-Cyt1Aa antibody. Molecular weight markers were Precision Plus Protein Standards All Blue (Bio-Rad), and molecular masses are indicated in kDa.
We analysed the oligomerization of the Cyt1Aa mutants after activation by trypsin or proteinase K in the presence of SUV liposomes. In these experiments, the samples were heated at 65°C before SDS-PAGE. Figure 1B shows that oligomerization is affected in mutants V122E and V126E. Some additional oligomeric bands at intermediate molecular weights were observed. It is possible that these bands may represent different aggregation forms or could also be degradation products of the oligomer due to heat denaturation. It was reported that activation of Cyt1Aa with trypsin induced a cleavage at N-terminal end at residue Arg25, and proteinase K induced cleavages at Arg30 and Val31. The extent of cleavage by proteinase K is greater than with trypsin, since proteinase K is less specific, although aromatic or hydrophobic amino acids are preferred, while trypsin is more specific for positive charged amino acids such as Lys and Arg (Bond, 1990). These differences in the processing could be related to oligomerization, affecting toxin action, since trypsin activated toxin was less efficient in haemolytic activity than proteinase K activated toxin (Al-yahyaee and Ellar, 1995). The oligomerization of mutants V122E and V126E using proteinase K in the assay was further analysed by heating the samples at different temperatures for 3 min before separation in SDS-PAGE. Figure 1C show that the oligomeric structures of mutants V122E and V126E are more sensitive to heat denaturation than the wild-type toxin under similar conditions (compare with Fig. 1A), suggesting that the forces that stabilize the oligomer in these mutants have been weakened. The mutant V122E is more severely affected in oligomerization than V126E, which is able to oligomerize although the oligomer is more sensitive to heat denaturation than the wild-type Cyt1Aa.
Toxin insertion into the membrane was analysed by separating the membrane pellet from the supernatant after centrifugation (and heating the samples 3 min at 65°C before loading the SDS-PAGE). Figure 1D shows that oligomeric structures insert into the membrane and that monomeric structures remain in the supernatants. Mutants V122E and V126E remain in the supernatant as monomeric toxins (Fig. 1D).
To analyse if Cyt1Aa could oligomerize in solution or only in the presence of membranes, we activated Cyt1Aa protoxin with proteinase K in solution and heated the samples at different temperatures before loading them into the SDS-PAGE (Fig. S3). These data show that in absence of lipids, this protein was unable to form oligomeric structures resistant to SDS and moderate heat treatment.
Finally, to analyse if mutants V122E and V126E were able to insert into natural insect membranes, brush border membrane vesicles (BBMV) were isolated from midgut tissue of the A. aegypti larvae, and we analysed toxin oligomerization in the presence of BBMV. Figure 2 shows that Cyt1Aa toxin was able to form oligomers and was recovered in the membrane pellet suggesting that under these conditions the monomeric Cyt1Aa toxin does not insert into the BBMV. In contrast, V122E and V126E mutants did not form oligomers, and monomeric forms of these mutants were recovered only in the supernatant indicating that these mutants were affected in BBMV insertion and oligomerization (Fig. 2B).
Fig. 2.
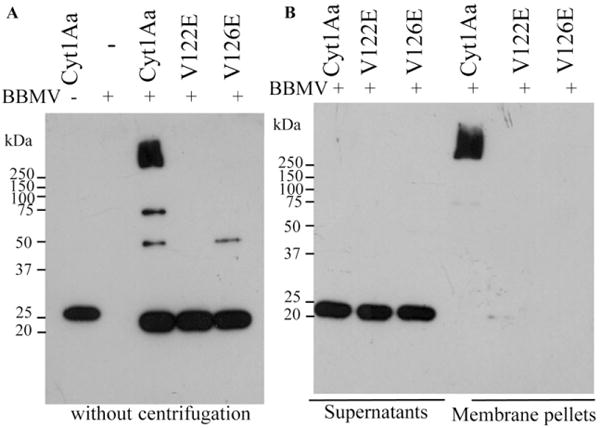
Analysis of oligomerization of Cyt1Aa wild-type and mutant toxins in the presence of BBMV.
A. Oligomerization of the wild-type Cyt1Aa protein and mutants V122E and V126E after incubating the solubilized protoxins 2 h at 37°C with trypsin in the presence of BBMV. Samples were heated 3 min at 65°C before loading them into SDS-PAGE. A control of Cyt1Aa protoxin without BBMV was included as well as control of BBMV without Cyt1Aa incubation.
B. Partition of oligomeric Cyt1Aa in BBMV. Membrane pellet of BBMV was separated by centrifugation 30 min at 117 000 g. Both samples containing membrane pellets or supernatants were heated 3 min at 65°C, before separation in SDS-PAGE. All SDS-PAGE gels were transferred to PVDF and analysed by Western blot using polyclonal anti-Cyt1Aa antibody. Molecular weight markers are as in Fig. 1.
Haemolytic and insecticidal activities of Cyt1Aa mutants
The in vitro haemolytic activity of activated Cyt1Aa against rabbit red blood cells was analysed showing a medium effective concentration (EC50) value of 125 ng ml−1 (Fig. 3). The haemolytic activity of the different Cyt1Aa mutants was analysed, showing that mutants L120K and L123K, which were able to form oligomeric structures, display a haemolytic activity similar to the wild-type toxin. In contrast, mutants V122E and V126E, which were affected in oligomerization, were severely affected in haemolytic activity. V122E showed no haemolytic activity even at 1000 ng ml−1, while V126E showed 30% of haemolysis at this toxin concentration (Fig. 3).
Fig. 3.
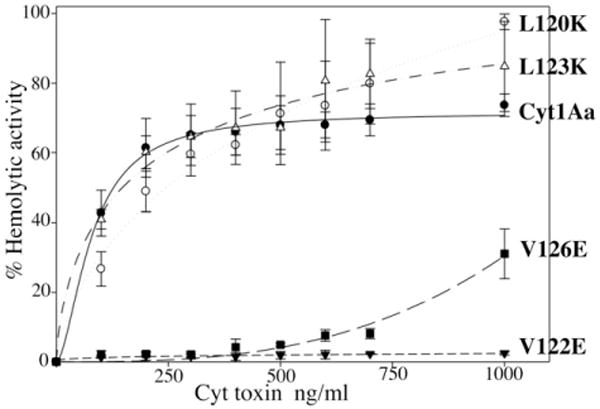
Haemolytic activity of Cyt1Aa and mutant toxins, analysed with rabbit red blood cells. Symbols are as follow: Cyt1Aa toxin, black circles; L120K, white circles; L123K, white triangles; V122E, black triangles; V126E, black squares. These assays were performed three times and standard deviations are shown.
The in vivo insecticidal activity of Cyt1Aa mutants was analysed in bioassays by feeding fourth instar A. aegypti larvae with spore/crystal suspensions. The medium lethal concentration (LC50) of the wild-type Cyt1Aa showed a value of 1100 (880–1480 confidential limits). Mutants L120K and L123K showed better insecticidal activities, since LC50 values were significantly lower than the wild-type toxin (Table 1). In contrast, mutants V122E and V126E were severely affected in their insecticidal activity, since even at 10 000 ng ml−1, which was the highest concentration analysed, no mortality was observed (Table 1). Overall, these data indicated that oligomerization and membrane insertion of Cyt1Aa are important for haemolytic and insecticidal activity.
Table 1.
Insecticidal activity of Cyt1Aa and mutant toxins against fourth instar Aedes aegypti larvae.
| Toxin | Insecticidal activity LC50 value in ng ml−1 |
|---|---|
| Cyt1Aa | 1100 (880–1,480)a |
| L120K | 507 (462–561) |
| L123K | 376 (262–516) |
| V122E | > 10 000 |
| V126E | > 10 000 |
95% confidential limits calculated by Probit statistical analysis.
Synergism of Cyt1Aa proteins with Cry11Aa
We evaluated synergism between Cyt1Aa wild-type or mutant toxins with Cry11Aa. We assumed that the proportion of larvae surviving exposure to a mixture of toxins is the product of the proportions of larvae that survive exposure to each of the toxins separately as explained in experimental procedures (Fernandez-Luna et al., 2010).
The results show that synergism occurred in all mixtures of Cyt1Aa toxins with Cry11Aa that were tested (Table 2). Mortality was 20% with Cry11Aa alone (200 ng Cry11Aa per ml) and 0% with Cyt1Aa wild-type alone (75 ng Cyt1Aa per ml). At the same concentration of 75 ng ml−1, all Cyt1Aa mutants showed 0% mortality. The observed mortality of all mixtures of Cyt1Aa proteins with Cry11Aa (Table 2) was higher than their expected mortality, indicating that all Cyt1Aa mutant proteins were able to induce synergism. However, the synergism induced by mutants V122E and V126E was lower than the wild-type toxin. These data suggest that synergism also occurred with these Cyt1Aa mutants which have lost their insecticidal activity against mosquito larvae, were not haemolytic and were affected in oligomerization and membrane insertion.
Table 2.
Analysis of synergism of Cyt1Aa or mutant toxins with Cry11Aa toxin.
| Toxin | S(toxin)OBSa = (Rep1 + Rep2 + Rep3)/n |
S(Cyt1Aa, Cry11Aa)EXPb = S(Cyt1Aa)OBS × S(Cry11Aa)OBS |
Expected mortalityc = (1 − S(Cyt1Aa, Cry11Aa)EXP) × 100% |
Observed mortalitydCyt1Aa + Cry11Aa |
|---|---|---|---|---|
| Cyt1Aa | 1.00 | 0.80 | 20% | 90 ± 10% |
| L120K | 1.00 | 0.80 | 20% | 93 ± 6% |
| V122E | 1.00 | 0.80 | 20% | 57 ± 15 % |
| L123K | 1.00 | 0.80 | 20% | 90 ± 10% |
| V126E | 1.00 | 0.80 | 20% | 77 ± 15% |
| Cry11Aa | 0.80 |
Observed survival of individual toxin S(toxin)OBS corresponds to the observed proportion of larvae that survived to the exposure to Cyt1Aa or mutant toxins. Observed mortality was 20% with Cry11Aa at 200 ng ml−1 and 0% with Cyt1Aa or mutant toxins at 75 ng per ml. n = 30 larvae for each toxin tested.
Theoretical proportion of larvae that survive to the toxin-mixture, S(Cyt1Aa, Cry1Aa)EXP = S(Cyt1Aa)OBS × S(Cry11Aa)OBS corresponds to the proportion of larvae expected to survive to the exposure of a mixture of toxins.
Theoretical expected mortality was calculated with (1−S(Cyt1Aa, Cry11Aa)EXP) × 100%.
Experimentally observed mortality with the mixture of toxins using Cry11Aa at 200 ng ml−1 of plus each Cyt1Aa toxin at 75 ng ml−1. These assays were performed three times. Fisher’s exact test showed values of P < 0.001 for each comparison.
Discussion
Few studies have focused on analyzing the role of the α-helices of Cyt toxin on its mechanism of action and synergism with Cry toxins. These α-helices are too short to span the membrane bilayer (Li et al., 1996; Cohen et al., 2011). However, studies performed with synthetic peptides corresponding to helices α-A and α-C showed that they may be involved in the interaction of the toxin with the membrane and in toxin oligomerization (Gazit et al., 1997). The oligomers of Cyt1Aa have a molecular weight of 400 kDa and are formed only after activation in the presence of lipid membranes or insect cells (Chow et al., 1989).
It was reported that a mutation in helix α-C (S108C) of the highly related Cyt2Aa toxin, resulted in an inactive toxin affected in oligomerization (Promdonkoy et al., 2008). However, the effect of this mutation on the capacity of Cyt2Aa to synergize the insecticidal activity of other Cry toxins was not analysed.
We mutagenized helix α-C of Cyt1Aa and analysed the effect on toxin oligomerization, membrane insertion, as well as haemolytic, insecticidal and synergistic activities. We generated six-point mutations in Cyt1Aa, but only four of them produced stable proteins after solubilization and treatment with proteases. Two of these mutants (L120K and L123K) showed increased activity against A aegypti larvae when compared with the wild-type toxin and retain their haemolytic activity. The reason for the increased insecticidal activity of L120K and L123K mutants remains to be analysed. The other two mutants (V122E and V126E) were affected in their insecticidal as well as in their haemolytic activities. Our data indicate that residues V122 and V126 are important for oligomerization since mutations in these residues affected the stability of oligomeric structures, resulting in oligomeric structures highly sensitive to high temperatures and SDS (Figs 1 and 2). It is important to note that V126E still retained a marginal capacity to form oligomers that insert into the membrane after activation with proteinase K (Fig. 1D) that correlated with its low haemolytic activity (Fig. 3).
The most accepted model of Cyt1Aa mode action suggests that Cyt1Aa monomers insert into the membrane and oligomerization of the toxin follows within the membrane plane (see Soberón et al., 2012 for review). The data presented here rebuts this hypothesis, since Cyt1Aa does not insert as a monomer into the membrane and only oligomeric structures insert into the membrane (Figs 1D and 2B) suggesting that toxin oligomerization is an essential step for membrane insertion. A previous report showed that Cyt1Aa and Cyt2Ba monomers remain associated with membrane pellets of multilamellar vesicles of synthetic lipids (Du et al., 1999). However, it is important to mention that these samples were boiled before SDS-PAGE electrophoresis (Du et al., 1999), a condition that destabilizes the oligomer structure to the monomeric form. In another report, the interaction of Cyt2Aa with red blood cell membranes was analysed at 4°C, showing that monomers remained associated with the membrane pellet at this temperature, while only oligomers were observed at other temperatures such as 15°C to 37°C, suggesting that monomers insert into the membrane before toxin oligomerization (Promdonkoy and Ellar, 2003). It remains to be analysed if Cyt monomers are just associated with membranes in a binding interaction or are inserted into the membrane. Also, it is still possible that interaction of Cyt with red blood cell or with insect BBMV have some differences. In any case, our data show that in the absence of lipids, Cyt1Aa was unable to form oligomers resistant to SDS and moderate heat (Fig. S3), suggesting that the presence of membrane is absolutely necessary to induce oligomerization of Cyt1Aa (Figs 1 and 2).
The synergistic activity was analysed showing that all Cyt1Aa mutants were able to synergize Cry11Aa toxicity. Interestingly, V122E and V126E mutants that were affected in oligomer formation, in haemolytic activity and membrane insertion, still synergized Cry11Aa activity. These data indicate that Cyt1Aa insecticidal activity is not important to synergize Cry11Aa toxin and are in agreement with a previous publication where it was shown that in another mosquito species, Anopheles albimanus, Cyt1Aa was not toxic by itself but was able to synergize the toxicity of Cry4Ba and Cry11Aa proteins (Fernandez-Luna et al., 2010). Our data also indicate that haemolytic activity of Cyt1Aa toxin is not necessary to induce synergism of Cry11Aa toxin as observed with the V122E and V126E mutants (Fig. 3).
The binding interaction of Cyt1Aa to Cry11Aa or Cry4Ba was important to potentiate the insecticidal activity of these Cry proteins, and the epitopes involved in this interaction were mapped (Pérez et al., 2005; Canton et al., 2011). The identified regions in Cry11Aa and Cry4Ba toxins correspond to domain II loop α8 and loop 2 respectively, and in the case of Cyt1Aa toxin, the binding region corresponds to the residues located between strand β6 and helix α–E. Site directed mutagenesis of these regions reduced binding affinity among these proteins and correlated with reduced synergism (Pérez et al., 2005; Canton et al., 2011). Recently, it was shown that synergism between Cyt2Aa and Cry4Ba also depends on their interaction through two exposed loop regions of Cry4Ba domain II (Lailak et al., 2013). The binding interaction between Cyt1Aa and Cry11Aa could occur in solution or after insertion into the membrane as shown by co-immunoprecipitation studies and Enzyme-Linked ImmunoSorbent Assay (ELISA) binding assays (Pérez et al., 2005). Furthermore, it was shown that binding of Cry11Aa to Cyt1Aa facilitates formation of Cry11Aa oligomers (Pérez et al., 2007). Based on these data, it was proposed that Cyt1Aa toxin might function as a receptor of Cry11Aa explaining a possible mechanism of synergism (Pérez et al., 2005; 2007). The data presented here support that synergism between these proteins is linked to their binding interaction which could occur in solution or in the membrane plane promoting oligomerization of Cry11Aa. Similarly, it was previously shown that different fragments of the Manduca sexta cadherin protein, which is a receptor of Cry1A toxins in this insect, are able to bind to Cry1Ab or Cry1Ac toxins inducing oligomerization of these Cry toxins, and resulting also in an important increase of the toxicity of these toxins even though that some of these cadherin fragments are not linked to the membrane (Chen et al., 2007; Pacheco et al., 2009). However, V122E and V126E are less efficient at synergizing Cry11Aa, suggesting that insertion of Cyt1Aa into the membrane could be an optimum condition for inducing synergism, since the Cry11Aa oligomer that is formed after interaction of these proteins may have a higher probability to insert in the bilayer.
The data presented here show that oligomerization, membrane insertion, haemolysis and insecticidal activity of Cyt1Aa are independent events of its synergistic activity of Cry11Aa toxicity.
Experimental procedures
Bacterial strains
Bacillus thuringiensis 407 acrystalliferous strain transformed with plasmid pWF45 (Wu et al., 1994) or plasmid pCG6 (Chang et al., 1993) were used to produce Cyt1Aa or Cry11Aa protoxins respectively. Escherichia coli X-L1 blue strain was used as recipient of the mutated cyt1Aa genes constructed in this work. After sequence confirmation of these mutations, these mutant-constructions were transformed into the B. thuringiensis 407 acrystalliferous strain.
Production of Cyt1Aa and Cry11Aa proteins
Bacillus thuringiensis bacterial strains expressing wild-type Cyt1Aa or Cyt1Aa-mutant proteins were grown 4 days at 30°C in solid nutrient broth sporulation medium supplemented with 10 μg/ml erythromycin; Bt strain expressing Cry11Aa was grown in same medium supplemented with 25 μg/ml erythromycin, (Lereclus et al., 1995). Spores and crystal inclusions produced by the Bt strains were observed under an optical microscope, and then harvested and washed three times with 0.3 M NaCl, 0.01 M EDTA, pH 8.0 by centrifugation for 10 min at 8600 g at 4°C. The pellet was suspended in water containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and stored at −20°C. These proteins were solubilized for 1 h at 37°C in 50 mM Na2CO3, 10 mM DTT, pH 10.5, agitation at 350 r.p.m. and centrifuged for 10 min at 8600 g 4°C. The soluble protoxins were recovered in the supernatant. Protein concentrations were determined using the Bradford assay. Finally, Cyt1Aa protoxin was activated by incubation with 1:20 trypsin (Sigma-Aldrich Co., St Louis, MO) w/w for 2 h at 30°C. The optimal pH of trypsin activity is pH 8.5, but it is still active at pH 10.5. The quality of Cry11Aa and Cyt1Aa protoxins as well as the trypsin-activated toxins was analysed in SDS-PAGE 15% polyacrylamide. Molecular weight markers were Precision Plus Protein Standards All Blue (Bio-Rad, Hercules, CA) and molecular masses are indicated in kDa.
Site-directed mutagenesis of Cyt1Aa toxin
Mutagenesis of pWF45 plasmid was performed by using QuikChange XL Site-Directed kit (Stratagene La Jolla, CA). The sequences of mutagenic oligonucleotides are shown in Table 1S. These oligonucleotides were synthesized by Sigma-Aldrich (St Louis, MO). Mutants were transformed in E. coli X-L1 blue strain selected in Luria Broth (LB) ampicillin 100 μg/ml at 25°C for 2 days. Plasmid DNA was extracted from selected colonies using a DNA extraction kit (Qiagen, Hilden, GER) and sequenced at the Institute of Biotechnology UNAM. These plasmids were transformed into acrystalliferous Bt 407 strain. Bt transformants were selected in LB erythromycin 10 μg/ml at 30°C. The sequence of selected clones was confirmed after PCR amplification of the selected colonies using IRE1d-IRE4r oligonucleotides that amplify a fragment of 750 pb of cyt1Aa gene (Table S1).
Preparation of small unilamellar vesicles (SUV)
Egg yolk phosphatidyl choline (PC), cholesterol (Ch) (Avanti Polar Lipids, Alabaster, AL) and stearylamine (S) (Sigma-Aldrich, St Louis, MO) from chloroform stocks, were mixed in glass vials in a 10:3:1 proportion, respectively, at 0.65 μmol final concentration of the total lipid mixture and dried by nitrogen flow evaporation, followed by overnight storage under vacuum to remove residual chloroform. The lipids were hydrated in 0.65 ml of 10 mM CHES, 150 mM KCl pH 9 by a 30 min incubation followed by vortex. To prepare SUV, the lipid suspension was sonicated three to five times during 20 s each in a Branson-1200 bath sonicator (Danbury, CT). SUV were used the same day upon their preparation.
Preparation of Brush Border Membrane Vesicles (BBMV)
Brush border membrane vesicles were isolated from dissected midguts of 1500 fourth instar A. aegypti larvae as reported (Nielsen-LeRoux and Charles, 1992) with some modifications; fourth instar larvae were chilled on ice, midguts were dissected, peritrophic membranes and Malpighian tubules removed, and midguts rinsed in ice-cold buffer A (0.3 M mannitol, 5 mM EGTA, 17 mM Tris/HCl, pH 7.4 supplemented with 1 mM PMSF and 5 mM DTT). Midguts were stored at −70°C until required. Midguts were thawed, pooled in 5 ml ice-cold buffer A and homogenized (eight strokes at 2250 r.p.m.) with Glas-Col 099C K64 homogenizer (Glas-Col LLC Terre Haute IN). The homogenate was adjusted to 9.5 ml with buffer A and made up to 12 mM MgCl2. The samples were allowed to stand on ice for 20 min. The mixture was then centrifuged at 3000 g for 15 min at 4°C. The supernatant was collected and kept on ice. The pellets were suspended in buffer A and treated as above for the first midgut homogenization. The resulting supernatant was pooled with the first supernatant. The pellet was again suspended in buffer A, incubated in ice, homogenized and centrifuged as above. This third supernatant was pooled with the first and second supernatant and centrifuged at 90 000 g for 10 min at 4°C. The resulting pellet was then finally suspended in buffer A and kept at −70°C until used. Protein content was determined by the Lowry DC protein assay (Bio-Rad) with bovine serum albumin as standard.
Oligomerization of Cyt1Aa and mutant toxins
Oligomerization of Cyt1Aa and mutants was induced in a final volume of 100 μl by incubation of 200 ng of Cyt1Aa solubilized protoxin, or that of Cyt1Aa mutants with 90 μl SUV liposomes and 10 ng of trypsin during 2 h at 30°C or with 20 ng of proteinase K during 1 h at 37°C and agitation at 350 r.p.m. PMSF, 1 mM final concentration, was added to stop the reaction. Samples were heated at different temperatures for 3 min, loaded in SDS-PAGE gels and transferred to PVDF Immobilon-P Millipore (Darmstadt, Germany) membranes in a wet chamber during 12 h, 150 mA, at 4°C. The PVDF membrane was blocked with 5% skimmed milk in phosphate-buffered saline (PBS) for 1h at room temperature with slow agitation and washed two times 5 min with PBS containing 0.1% Tween 20 (PBS-Tween). The membrane was then incubated in PBS-Tween containing polyclonal anti-Cyt1A antibody (1:30 000 dilution) for 1 h at room temperature, washed twice with PBS-Tween for 5 min and then incubated with goat anti-rabbit antibody coupled to horseradish peroxidase (Santa Cruz Biotechnology, Dallas, TX) (1:10 000 dilution in PBS-Tween). Finally, the peroxidase signal was visualized with SuperSignal chemiluminescent substrate (ECL; Amersham Pharmacia Biotech).
For the analysis of partition of Cyt1Aa or the mutants into the SUV liposomes, they were incubated as described above and finally centrifuged 30 min at 117 000 g to separate the membrane pellet from the supernatant. These samples were heated 3 min at 65°C before loading separation by SDS-PAGE and revealed by Western blot as above. Oligomerization assays were performed at least five times with different preparations of the Cyt1Aa or mutant toxins and different SUV preparations.
Alternatively, the oligomeric Cyt1Aa structure was produced by activation of 200 ng of soluble protoxin with 10 ng trypsin in the presence of 10 μg of BBMV of A. aegypti, in 50 μl of 50 mM Na2CO3 pH 10.5 for 2 h at 37°C. PMSF, 1 mM, was added to stop proteolysis. The membrane fraction was separated by centrifugation (30 min at 117 000 g), and the membrane pellet was heated 3 min at 65°C before loading SDS-PAGE and visualized by western blot using polyclonal anti-Cyt1A antibody as described above. These assays were done in duplicate. Molecular weight markers were Precision Plus Protein Standards All Blue (Bio-Rad), and molecular masses are indicated in kDa.
Haemolysis assays
Haemolytic assays were done as previously described (Rodríguez-Almazán et al., 2011). Briefly, rabbit red blood cells were washed three times in buffer A (0.1 M dextrose, 0.07 M NaCl, 0.02 M sodium citrate, 0.002 M citrate, pH 7.4) and finally diluted to a concentration of 2 × 108 cells ml−1 in the same buffer. The final volume of reaction mixture was 0.2 ml containing 20 μl of washed blood cells and various concentrations of Cyt1Aa toxin (20–200 ng) in the same buffer incubated at 37°C for 30 min in 96 wells microtitre plates. The supernatants were collected in a new microtitre plate by centrifugation at 2500 g for 5 min at 4°C and haemolytic activity was quantitated measuring the absorbance of the supernatant at 405 nm. Positive control showing 100% haemolysis was defined after incubation of the same volume of rabbit red blood cells with dechlorinated H2O. Negative controls were red blood cells incubated with buffer A. These assays were performed three times in triplicate each time. A t-test was performed using the statistical program GRAPHPAD PRISM (La Jolla, CA).
Insect bioassays
Aedes aegypti mosquitoes were reared at Instituto de Biotecnologia facilities, at 28°C, 75% humidity and a 12 h: 12 h, light: dark photoperiod. Mosquitocidal bioassays were performed against 10 early fourth instar larvae in 100 ml of dechlorinated water. Ten different concentrations (50 ng ml−1 to 10 000 ng ml−1) of spore/crystal suspensions of Cyt1Aa wild type or mutants were sonicated for 1 min in an ultrasonic processor (Cole-Palmer, Vernon Hills, IL) and immediately diluted into 100 ml water containers. Negative control (dechlorinated water) was included in the bioassay, and larvae viability examined 24 h after treatment. The mean lethal concentration (LC50) was determined by Probit analysis using statistical parameters (Finney, 1971) using data obtained from three independent assays (POLO-PLUS LEORA Software, Petaluma, CA).
We evaluated synergism as previously described (Fernandez-Luna et al., 2010) by testing for deviation from the null hypothesis of simple independent action, which assumes the proportion of larvae surviving to the exposure of mixture of toxins is the product of the proportions of larvae that survive to the exposure of each toxin separately. Briefly, we used the formula S(ab)EXP = S(a)OBS × S(b)OBS (Fernandez-Luna et al., 2010), where S(ab)EXP is the proportion of larvae expected to survive to the exposure of a mixture of toxins a and b, S(a)OBS and S(b)OBS are the observed proportion of larvae that survived to the exposure to toxin a or toxin b respectively. Thirty larvae were used per toxin and per mixture of toxins. The expected mortality for larvae that were exposed to the mixture of toxins a and b was calculated as (1 − S(ab)EXP) × 100%, and the expected numbers of dead and live larvae were calculated by multiplying the expected mortality and survival rates by the sample size used when each toxin was tested separately. These assays were done by triplicate. Finally, the Fisher’s exact test was used to determine if a significant difference occurred between observe and expected mortality data.
Supplementary Material
Alignment of the amino acid sequences of Cyt1Aa (P0A382), Cyt2Aa (Q04470) and VVA2 (Q6USC4). Alignments were done with ClustalW2 program (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The selected residues that were mutagenized in helix α-C of Cyt1Aa are indicated by underlined bold-letters. The consensus symbols of ClustalW2 alignment are: asterisk (*), indicates positions which have a single, fully conserved residue; colon (:), indicates conservation between groups of strongly similar properties (scoring > 0.5 in the Gonnet PAM 250 matrix); and period (.), that indicates conservation between groups of weakly similar properties (scoring = < 0.5 in the Gonnet PAM 250 matrix). The α-helices described in Cyt1Aa are represented by eeeee and β-strands by arrows (Cohen et al., 2011).
SDS-PAGE analysis of mutant proteins after solubilization and digestion with trypsin. SDS-PAGE performed with 15% polyacrylamide was stained with Coomassie Brilliant Blue. All Cyt1Aa mutants gave a 24-kDa fragment similar to the wild-type Cyt1Aa toxin, as a control we include Cyt1Aa protoxin without protease treatment. Molecular weight markers were Precision Plus Protein Standards All Blue (Bio-Rad) and molecular masses are indicated in kDa.
Oligomerization of Cyt1Aa after activation with pro-teinase K in solution. Samples were heated 3 min at different temperatures before loading into SDS-PAGE, transferred to PVDF and analysed by Western blot using polyclonal anti-Cyt1Aa antibody. Molecular weight markers were as in Fig. S2.
Sequence of mutagenic oligonucleotides. The sequence of mutagenic codon is underlined and labeled with bold letters.
Acknowledgments
We thank Claudia Rodríguez-Almazán, Lizbeth Cabrera, Blanca Ines García-Gómez and Jorge F. Sánchez-Quintana for technical assistance. This work was supported by grants from CONACyT 128883; DGAPA-UNAM IN201113; and NIH 1R01 AI066014.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- Al-yahyaee SAS, Ellar DJ. Maximal toxicity of cloned CytA δ-endotoxin from Bacillus thuringiensis subsp. israelensis requires proteolytic processing from both the N- and C-termini. Microbiology. 1995;141:3141–3148. [Google Scholar]
- Bond S. Appendix II commercially available proteases. In: Beyon RJ, Bond JS, editors. Proteolytic Enzymes a Practical Approach. Oxford, UK: IRL Press; 1990. pp. 239–240. [Google Scholar]
- Bravo A, Likitvivatanavong S, Gill SS, Soberón M. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol. 2011;41:423–431. doi: 10.1016/j.ibmb.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton PE, Reyes EZ, RuízdeEscudero I, Bravo A, Soberón M. Binding of Bacillus thuringiensis subsp. israelensis Cry4Ba to Cyt1Aa has an important role in synergism. Peptides. 2011;32:595–600. doi: 10.1016/j.peptides.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Yu YM, Dai SM, Law SK, Gill SS. High-level cryIVD and cytA gene expression in Bacillus thuringiensis does not require the 20-kilodalton protein, and the coexpressed gene products are synergistic in their toxicity to mosquitoes. Appl Environ Microbiol. 1993;59:815–821. doi: 10.1128/aem.59.3.815-821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hua G, Jurat-Fuentes JL, Abdullah MA, Adang M. Synergism of Bacillus thuringiensis toxins by a fragment of a toxin-binding cadherin. Proc Natl Acad Sci USA. 2007;104:13901–13906. doi: 10.1073/pnas.0706011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow E, Singh GJP, Gill SS. Binding and aggregation of the 25 kDa toxin of Bacillus thuringiensis subsp. israelensis to cell membranes and alteration by monoclonal antibodies and amino acid modifiers. Appl Environ Microbiol. 1989;55:2779–2788. doi: 10.1128/aem.55.11.2779-2788.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Albeck S, Ben-Dov E, Cahan R, Firer M, Zaritsky A, Dym O. Cyt1Aa toxin: crystal structure revels implications for its membrane-perforation function. J Mol Biol. 2011;413:804–814. doi: 10.1016/j.jmb.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Crickmore N, Bone EJ, Williams JA, Ellar DJ. Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. israelensis. FEMS Microbiol Lett. 1995;131:249–254. [Google Scholar]
- Crickmore N, Zeigler DR, Schnepf E, Van Rie J, Lereclus D, Baum J, et al. Bacillus thuringiensis toxin nomenclature. 2013 doi: 10.1128/mmbr.62.3.807-813.1998. [WWW document]. URL http://www.lifesci.sussex.ac.uk/Home/Neil_Crickmore/Bt/ [DOI] [PMC free article] [PubMed]
- Du J, Knowles BH, Li J, Ellar DJ. Biochemical characterization of Bacillus thuringiensis cytolytic toxins in association with a phospholipid bilayer. Biochem J. 1999;338:185–193. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Luna MT, Tabashnik B, Lanz-Mendoza H, Bravo A, Soberón M, Miranda-Rios J. Single-concentration tests show synergism among Bacillus thuringiensis subsp. israelensis toxins against the malaria vector mosquito Anopheles albimanus. J Invertebr Pathol. 2010;104:231–233. doi: 10.1016/j.jip.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Finney DJ, editor. Probit Analysis, A Statistical Treatment of the Sigmoid Response Curve. Cambridge, UK: Cambridge University Press; 1971. Adjustements for natural mortality; pp. 88–100. [Google Scholar]
- Gazit E, Burshtein N, Ellar DJ, Sawter T, Shai Y. Bacillus thuringiensis cytolytic toxin associates specifically with its synthetic helices A and C in the membrane bound state. Implications for assembly of oligomeric transmembrane pores. Biochemistry. 1997;36:15546–15554. doi: 10.1021/bi9707584. [DOI] [PubMed] [Google Scholar]
- Lailak C, Khaokhiew T, Promptas C, Promdonkoy B, Pootanakit K, Ch A. Bacillus thuringiensis Cry4Ba toxin employs two receptor-binding loops for synergistic interactions with Cyt2Aa2. Biochem Biophys Res Commun. 2013;435:216–221. doi: 10.1016/j.bbrc.2013.04.078. [DOI] [PubMed] [Google Scholar]
- Lereclus D, Agaisse H, Gominet M, Chaufaux J. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spo OA mutant. Bio/Technology. 1995;13:67–71. doi: 10.1038/nbt0195-67. [DOI] [PubMed] [Google Scholar]
- Li J, Koni PA, Ellar DJ. Structure of the mosquitocidal delta-endotoxin CytB from Bacillus thuringiensis sp. kyushuensis and implications for membrane pore formation. J Mol Biol. 1996;257:129–152. doi: 10.1006/jmbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- Nielsen-LeRoux C, Charles JF. Binding of Bacillus sphaericus binary toxin to a specific receptor on midgut brush-border membranes from mosquito larvae. Eur J Biochem. 1992;210:585–590. doi: 10.1111/j.1432-1033.1992.tb17458.x. [DOI] [PubMed] [Google Scholar]
- Pacheco S, Gómez I, Gill SS, Bravo A, Soberón M. Enhancement of insecticidal activity of Bacillus thuringiensis Cry1A toxins by fragments of a toxin-binding cadherin correlates with oligomer formation. Peptides. 2009;30:583–588. doi: 10.1016/j.peptides.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris M, Tetreau G, Laurent F, Lelu M, Despres L, David JP. Persistence of Bacillus thuringiensis israelensis Bti in the environment induces resistance to multiple Bti toxins in mosquitoes. Pest Manag Sci. 2011;67:122–128. doi: 10.1002/ps.2046. [DOI] [PubMed] [Google Scholar]
- Pérez C, Fernandez LE, Sun J, Folch JL, Gill SS, Soberón M, et al. Bti Cry11Aa and Cyt1Aa toxins interactions support the synergism-model that Cyt1Aa functions as membrane-bound receptor. Proc Natl Acad Sci USA. 2005;102:18303–18308. doi: 10.1073/pnas.0505494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C, Muñoz-Garay C, Portugal LC, Sánchez J, Gill SS, Soberón M, et al. Bacillus thuringiensissubsp. israelensisCyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell Microbiol. 2007;9:2931–2937. doi: 10.1111/j.1462-5822.2007.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promdonkoy B, Ellar DJ. Investigation of the pore-forming mechanism of a cytolytic δ-endotoxin from Bacillus thuringiensis. Biochem J. 2003;374:255–259. doi: 10.1042/BJ20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promdonkoy B, Rungrod A, Promdonkoy P, Pathaichindachote W, Krittanai C, Panyim S. Amino acid substitutions in α-A and α-C of Cyt2Aa2 alter hemolytic activity and mosquito larvicidal specificity. J Biotechnol. 2008;133:287–293. doi: 10.1016/j.jbiotec.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Almazán C, Ruíz de Escudero I, Cantón E, Muñoz-Garay C, Pérez C, Gill SS, et al. The amino- and carboxyl-terminal fragments of the Bacillus thuringiensis Cyt1Aa toxin have differential roles on toxin oligomerization and pore formation. Biochemistry. 2011;50:388–396. doi: 10.1021/bi101239r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberón M, López-Díaz JA, Bravo A. Cyt toxins produced by Bacillus thuringiensis: a protein fold conserved in several pathogenic microorganisms. Peptides. 2012;41:87–93. doi: 10.1016/j.peptides.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Thomas WE, Ellar DJ. Mechanism of action of Bacillus thuringiensisvar israelensis insecticidal δ-endotoxin. FEBS Lett. 1983;154:362–368. doi: 10.1016/0014-5793(83)80183-5. [DOI] [PubMed] [Google Scholar]
- Weng YP, Lin YP, Hsu CI, Lin J-Y. Functional domains of a pore-forming cardiotoxic protein Volvatoxin A2. J Biol Chem. 2004;279:6805–6814. doi: 10.1074/jbc.M308675200. [DOI] [PubMed] [Google Scholar]
- Wirth M, Georghiou GP, Federici BA. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito Culex quinquefasciatus. Proc Natl Acad Sci USA. 1997;9:10536–10540. doi: 10.1073/pnas.94.20.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MC, Park HW, Walton WE, Federici BA. Cyt of Bacillus thuringiensis delays evolution of resistance to Cry11A in the mosquito Culex quinquefasciatus. Appl Environ Microbiol. 2005;71:185–189. doi: 10.1128/AEM.71.1.185-189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Johnson JJ, Federici BA. Synergism of mosquitocidal toxicity between CytA and CryIV proteins using inclusions produced from cloned genes of Bacillus thuringiensis subsp. israelensis. Mol Microbiol. 1994;13:965–972. doi: 10.1111/j.1365-2958.1994.tb00488.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of the amino acid sequences of Cyt1Aa (P0A382), Cyt2Aa (Q04470) and VVA2 (Q6USC4). Alignments were done with ClustalW2 program (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The selected residues that were mutagenized in helix α-C of Cyt1Aa are indicated by underlined bold-letters. The consensus symbols of ClustalW2 alignment are: asterisk (*), indicates positions which have a single, fully conserved residue; colon (:), indicates conservation between groups of strongly similar properties (scoring > 0.5 in the Gonnet PAM 250 matrix); and period (.), that indicates conservation between groups of weakly similar properties (scoring = < 0.5 in the Gonnet PAM 250 matrix). The α-helices described in Cyt1Aa are represented by eeeee and β-strands by arrows (Cohen et al., 2011).
SDS-PAGE analysis of mutant proteins after solubilization and digestion with trypsin. SDS-PAGE performed with 15% polyacrylamide was stained with Coomassie Brilliant Blue. All Cyt1Aa mutants gave a 24-kDa fragment similar to the wild-type Cyt1Aa toxin, as a control we include Cyt1Aa protoxin without protease treatment. Molecular weight markers were Precision Plus Protein Standards All Blue (Bio-Rad) and molecular masses are indicated in kDa.
Oligomerization of Cyt1Aa after activation with pro-teinase K in solution. Samples were heated 3 min at different temperatures before loading into SDS-PAGE, transferred to PVDF and analysed by Western blot using polyclonal anti-Cyt1Aa antibody. Molecular weight markers were as in Fig. S2.
Sequence of mutagenic oligonucleotides. The sequence of mutagenic codon is underlined and labeled with bold letters.


