Many older adults are at risk for debilitating cognitive decline due to age-related neurodegenerative disease such as Alzheimer’s disease (AD) or other dementias. Because of their disease-related brain vulnerability, it is common for these individuals to experience delirium during episodes of acute illness (Inouye, 1999). When delirium is superimposed on dementia (DSD) there is a high probability for accelerated cognitive decline unless the delirium is resolved (Gross et al., 2012). DSD carries a high morbidity and mortality risk (Fick, Steis, Waller, & Inouye, In Press), precipitates institutionalization, and costs the US healthcare system between $125 and $157 billion annually (Hurd, Martorell, Delavande, Mullen, & Langa, 2013). Given the significant impact of rapid short-term cognitive decline on quality of life and healthcare costs, these individuals represent a population where intervention is critical to prevent the downward spiral toward more negative outcomes. Additionally, these characteristics make them a desirable group to study based on both clinical significance as well as the potential to observe cognitive changes (due to delirium resolution) in a shorter period of time.
Several programs have demonstrated efficacy in preventing delirium (e.g., Zaubler et al., 2013), but few studies have tested treatments to restore cognitive functioning in individuals with DSD. There are some data that demonstrate the potential of cognitively stimulating individualized activity interventions for treating DSD (Kolanowski, Fick, et al., 2011). We do not know, however, if these interventions are equally effective across all individuals. This exploratory study was designed to fill that scientific gap by identifying potential moderators of treatment effectiveness.
Cognitive stimulation is a generalized approach to cognition-focused intervention consisting of a range of activities that promote cognitive processing with the aim of enhancing overall mental and social functioning (Clare & Woods, 2003). Cognitive stimulation programs are typically designed to provide global cognitive stimulation in an implicit way, emphasizing cognitive processing rather than correct answers, in a multisensory approach with integrated elements of reminiscence. Activities are modified to match cognitive capacities and interests. Traditionally, cognitive stimulation has been implemented with individuals in early-stage dementia, and its benefits in that population are supported by a sizable body of evidence (Aguirre, Woods, Spector, & Orrell, 2013). Cognitive stimulation programs have demonstrated cognitive function benefits comparable to pharmacologic therapies, improvements in quality of life, and cost-effectiveness. Intervention approaches which target cognitive processing hold promise for restoring cognitive function in persons with DSD. In earlier pilot work we found that a cognitively stimulating activity intervention for individuals with DSD using simple games such as Name That tune or identify objects in a busy picture, during hospitalization demonstrated improvement in delirium prevalence, severity, and attention (blinded for review).
An intervention found effective in a particular sample may not be equally effective among individuals that make up that sample. Moderators of intervention outcomes are factors that characterize individuals more likely to respond to treatment and help identify best candidates for that particular treatment (Kraemer, Frank, & Kupfer, 2006). There is growing interest in including exploratory moderating analyses in clinical trials in order to inform future research design and to gain additional information regarding the circumstances under which a nursing intervention provides the best outcome (Bennett, 2000).
This study was guided by the theory of cognitive reserve (Stern, 2012). Cognitive reserve refers to the hypothetical ability of the brain, at varying individual capacities, to withstand a certain level of injury before the clinical manifestation of cognitive impairment. An active cognitive lifestyle, typically operationalized as higher engagement in educational, occupational, and complex cognitive activities across the lifespan, is associated with lower cognitive impairment risk and increased cognitive reserve (Valenzuela et al., 2013). The apolipoprotein E (ApoE) gene, and specifically the ApoE ε4 allele may also contribute to cognitive reserve, but evidence for its contribution to cognitive impairment is somewhat conflicting. The ApoE ε4 allele is the main genetic risk factor for AD (the frequency of ε4 among individuals with AD is approximately 40% compared to just under 14% in the general population) and is also associated with lower cognitive performance in cognitively intact carriers (Ertekin-Taner, 2007; Farrer et al., 1997; Greenwood, et al., 2005). It has also been shown, however, that ε4 carriers may be able to compensate for the deleterious genetic effects, at least partially, by building cognitive reserve through lifestyle activities (Garibotto, et al., 2012). Since higher cognitive reserve is thought to have a protective effect against the manifestation of cognitive impairment due to both dementia (Stern, 2012) and delirium (Yang et al., 2008), it is a factor that may influence the treatment effectiveness of cognitive stimulation for individuals with DSD.
Personality, or an individual’s relatively stable intrinsic structure of thinking, feeling, and behaving (Roberts & DelVecchio, 2000), affects behavior and preferences likely to influence activity and achievement during one’s lifetime (McCrae & Costa, 2002) and therefore cognitive reserve. Personality’s influence on health outcomes such as participation in and benefit from behavioral interventions in other populations is well-established. Multiple studies have demonstrated the moderating effect of personality on health and treatment effects as well as its relationship to motivation, performance, and treatment adherence (Franks, Chapman, Duberstein, & Jerant, 2009; Judge & Ilies, 2002). Personality can guide therapeutic activity interventions for individuals with dementia leading to increased engagement in those activities and resultant reduced passivity (Kolanowski, Litaker, Buettner, Moeller, & Costa, 2011). However, the influence of personality traits on cognitive stimulation outcomes in persons with DSD has not been examined. Cognitive reserve theory posits that participation in complex cognitive activities may result in compensatory use of alternate or additional neural networks in response to cognitive demand (Backman et al., 1999; Becker et al., 1996). Motivation to participate in such activities may support these compensatory mechanisms during a cognitive stimulation intervention.
Personality traits are considered largely stable throughout the life course (Roberts & DelVecchio, 2000), and personality stability has been demonstrated during the early stages of the dementia trajectory such that individuals maintain rank-order consistency with relatively modest mean-level changes (Twigg, Burgener, & Popovich, 2007). In other words, the relative strength of personality characteristics remains stable throughout the early dementia stages; for example, individuals who scored high on extraversion throughout their adult life tend to remain high in that trait compared to others even after the onset of AD.
Purpose
This exploratory study examined the moderating effects of personality traits on cognitive function following a cognitively stimulating individualized activity intervention delivered to individuals at high risk for short-term cognitive decline: those with DSD. Unlike intervention studies that target chronic cognitive impairment, the participants in this study were experiencing acute cognitive changes which gave us the opportunity to observe the effects of the intervention over short periods of time. The following research questions were explored:
Does personality moderate the relationship between a cognitive stimulation intervention and improvement in the cognitive functions of attention, delayed recall, orientation, and executive function during a period of acute cognitive decline in persons with dementia?
Is personality associated with engagement (time on task and level of participation) in a cognitive stimulation intervention during a period of acute cognitive decline in persons with dementia?
Method
Study Design
This study was approved by the university Institutional Review Board. To answer our research questions we used data from an ongoing randomized clinical trial (RCT), Recreational Stimulation for Elders as a Vehicle to Resolve Delirium Superimposed on Dementia (RESERVE-DSD, clinical trial identifier: blinded for review). In addition to including data collected within RESERVE-DSD, a personality assessment was added in order to test for a moderating effect not included in the original study design. These data were collected prospectively as participants were enrolled in the parent study. Participants were recruited and enrolled at admission to a post-acute care facility immediately following a hospitalization; all participants were community-dwelling prior to their hospital admission. Consenting participants were randomly assigned to intervention (cognitive stimulation) or control (usual care) groups.
Setting & Sample
The parent study from which our sample was drawn was conducted in seven Pennsylvania nursing homes with post-acute care services for rehabilitation following hospitalization. These sites represented a mix of for-profit, non-profit, county-owned, rural and urban, as well as large and medium community-based settings. Potential participants were screened for the following inclusion criteria: English speaking; 65 years of age or older; community-residing prior to most recent hospitalization; and having a responsible party (typically a spouse or adult child) to provide medical history, education, occupation, leisure, and personality data. Exclusion criteria were as follows: having any neurological or neurosurgical disease associated with cognitive impairment other than dementia including Parkinson’s disease with Lewy Body dementia; Huntingdon’s disease; normal pressure hydrocephalus; seizure disorder; subdural hematoma; head trauma; known structural brain abnormalities; frontotemporal dementia; nonverbal; having a life expectancy of six months or less; acute major depression; and severe hearing or vision impairment.
After initial eligibility determination, participants were screened for mild to moderate stage dementia through participant and informant interviews using the Modified Blessed Dementia Rating Scale (MBDRS; Blessed, Tomlinson, & Roth, 1968) and Clinical Dementia Rating Scale (CDR; Hughes, Berg, Danziger, Coben, & Martin, 1982), as well as for the presence of delirium using the Confusion Assessment Method (CAM; Inouye, et al., 1990). The Mini-Mental State Exam (MMSE; Folstein, et al., 1975) was also administered in order to inform tailoring of intervention content to cognitive status at the time of study enrollment; in this way, current impairment due to both delirium and pre-existing dementia was considered. Participants who scored a three or greater on the MBDRS and from 0.5 to 2.0 on the CDR were considered eligible as these two scores indicate the presence of mild to moderate stage dementia. Individuals with at least two features of delirium identified in the CAM were considered eligible. All dementia and delirium diagnoses were adjudicated by a panel of three experts: a geriatrician, neurologist and neuropsychologist.
The sample for this study consisted of 71 participants from the parent study; this constitutes a reasonable sample size for testing the specified aims in an exploratory study. A sample size of 70 participants (35 per group) yields 80% statistical power with a two-sided, 0.05 significance level test to detect an effect size of 0.68 standard deviation units (Piantadosi, 2005). The investigation of Aim 2 utilizes participants in the intervention group only (n=38); therefore, these analyses are considered preliminary in nature.
Intervention Protocol
RESERVE-DSD consists of mentally challenging recreational activities which incrementally increase in task difficulty and are tailored to each participant’s interests and functional abilities (See [blinded for review] for a full description of the intervention protocol). These recreational activities are delivered by trained Research Assistants (RAs) for 30 minutes once a day for 30 days or until discharge.
Measures
Sample Characteristic and Covariates
Demographic data included age, gender, and race/ethnicity. Additionally, the Charlson Co-morbidity Index (van Doorn et al., 2001), a weighted index that takes into account the number and seriousness of co-morbid diseases, was used to further characterize the study sample at baseline. The Lifetime of Experiences Questionnaire (LEQ) provides a measure of complex mental activities over the lifetime including educational, occupational, and leisure activities, a proxy measure for cognitive reserve (Valenzuela et al., 2013). ApoE ε4status was obtained by extracting DNA from buccal swab samples using the protocol of the Institute of Psychiatry in London (Freeman et al., 2003). Each individual may have up to two ε4 alleles; therefore, ApoE ε4status was quantified as the presence of 0, 1, or 2 ε4 alleles. Higher numbers of ε4 alleles are associated with greater risks for cognitive impairment (Ertekin-Taner, 2007).
Personality
The informant version of the NEO™ Personality Inventory-3 (NEO™-PI-3; McCrae & Costa, 2010) was used to assess personality. The 240-item NEO™-PI-3 measures five personality traits: neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness. Neuroticism is described as the chronic experience of distressing emotions; high scores include susceptibility to psychological distress as well as difficulty controlling impulses and poor reactions to stress, while low scores indicate emotional stability. Extraversion assesses two qualities: interpersonal involvement and energy, which represent an individual’s preferences for the quantity and quality of interpersonal interactions, level of activity, and capacity for joy. Openness to experience refers to an individual’s aesthetic sensitivity, attentiveness to inner feelings, preference for variety, active imagination, and intellectual curiosity. Agreeableness is a dimension of altruistic behavior and describes an individual’s attitudes toward others as well as their tendency toward cooperation or competition within interactions. Conscientiousness assesses goal-directed behavior, specifically the degree of organization, persistence, and motivation an individual possesses. NEO™-PI-3 scores are considered meaningful only when compared to appropriate norms (McCrae & Costa, 2010); therefore, raw scores were converted to T scores with a mean of 50 and standard deviation of 10 using adult normative data.
The NEO™ personality inventories are commonly used in the measurement of personality in individuals with dementia utilizing a retrospective assessment by the participant’s knowledgeable informant, typically a spouse, adult child, or sibling. The informant version of the NEO™-PI-3 demonstrates well documented reliability across studies (McCrae & Costa, 2010; Costa & McCrae, 1992). The reliability of the short version of the NEO has been investigated specifically among informants rating premorbid personality prior to dementia diagnosis. Average intraclass correlation coefficients ranged from 0.68 to 0.78 for inter-informant reliability and from 0.84 to 0.96 for intra-informant reliability across the five personality traits (Archer et al., 2006). All informants for this study met criteria specified by Ritchie and Fuhrer (1996) for knowledgeable informants: a person having had at least monthly contact with the participant for at least three years prior to dementia diagnosis. Informants were asked to picture their loved one as he/she was ten years prior to the onset of any cognitive impairment. Informants were 63.5 years of age on average, primarily female (74.3%), and were adult children of the participant (62.9%), spouses (18.6%), or other relatives (18.6%). Most informants did not live with the participant during the ten years prior to dementia onset (61.4%), but the majority had either daily (62.9%) or weekly (25.7%) contact during this period.
Cognitive Function
Four areas of cognitive function were measured each day during the participants’ participation in the study: attention, orientation, delayed recall, and executive function. RAs, blinded to condition assignment, were used to collect these data. Attention was measured using The Digit Span, a subtest of the Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1981). The Digit Span consists of asking the participant to repeat a series of numbers, increasing in length, first forward and then backward. The assessment ends when the participant fails to correctly repeat two sequences in a row. The reliability of the Digit Span has been demonstrated as .97 for the forward series and .96 for the backward series (Palmer & Meldon, 2003).
Orientation and delayed recall were measured using a shortened version of the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), a brief cognitive assessment that is frequently used in geriatric populations with cognitive impairment. The MoCA items utilized are brief assessments of orientation to person, time, and place, and two trials of delayed recall. It has demonstrated internal consistency reliability with a Cronbach’s alpha of up to .83 (Smith, Gildeh, & Holmes, 2007). Orientation scores were calculated based on the sum of seven orientation items. Delayed recall scores were calculated as the total correct responses (up to six) on two instances of three-word recall.
Executive function was measured using the CLOX (Royall, Cordes, & Polk, 1998), a clock drawing task consisting of both free drawing and copying tasks. Scores on each of the two parts of the CLOX range from 0 to 15 with higher scores indicating better executive function; these scores are added together to obtain a total CLOX score. The CLOX has demonstrated an internal consistency of .82 and inter-rater reliability of .93 to .94.
Intervention Engagement
Two instruments were used to measure engagement. Time on task was measured by the RA during the implementation of the intervention using a stop watch. Inter-rater reliability in a previous study demonstrated a percentage agreement of 93.6 and a weighted kappa of 0.91 (blinded for review). In addition, the RA determined a level of participation rating using a scale established by Kovach and Magliocco (1998) with scores ranging from 0 (dozing) to 3 (active participation). Previous inter-rater reliability on this scale was a percentage agreement of 98.2 and a weighted kappa of 0.96 (blinded for review). Total daily engagement was operationalized as the number of minutes engaged in the intervention multiplied by the level of participation.
Delirium Status
The CAM, a standardized delirium screening algorithm, was administered daily, for up to 30 days, by trained RAs. The CAM measures four features: 1) acute onset and fluctuating course, 2) inattention, 3) disorganized thinking, and 4) altered level of consciousness (Inouye et al., 1990). A subject is scored as having subsyndromal delirium if they exhibit any two features and full delirium if they exhibit features one and two and either three or four (Voyer, Richard, Doucet, & Carmichael, 2009). For the purposes of analysis, daily CAM scores were coded as 0 (no delirium), 1 (subsyndromal delirium), or 2 (full delirium). The CAM was validated against geriatric psychiatrists’ ratings using DSM-III-R criteria and has been shown to have a sensitivity between 94% and 100% and a specificity between 90% and 95% (Inouye, et al., 1990; Pompei, Foreman, Cassel, Alessi, & Cox, 1995). The CAM score was used as a time-dependent covariate measure within the analysis in order to control for the daily fluctuations of delirium status within subjects.
Data Analysis
Data were analyzed using SAS version 9.3 and IBM SPSS Statistics 21. Descriptive statistics for all variables measured at baseline were stratified according to randomized group to assess how the two groups compared qualitatively. These were assessed using chi-square tests for categorical variables or independent t-tests for continuous variables. A linear mixed-effects model was invoked to test each study aim. This model is most appropriate for hierarchical data with multiple observations collected over time on the same individuals; such was the case with the repeated outcome measures this study.
The statistical models included terms to account for the following baseline covariates, nested within each of the intervention and control groups: nursing home facility, age, gender, ApoE ε4status, MBDRS, and LEQ. Additionally, the daily CAM score was included as a time-dependent covariate in the analyses. For Aim 1, interaction terms for the five personality variables (neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness) by treatment group were included to test for the moderating effects of personality on each of the four cognitive outcomes. For Aim 2, which included the treatment group only, personality variables were included as main effects in the analysis.
Results
Baseline Characteristics
Baseline characteristics of the study sample are provided in Table 1. The study sample was primarily female (62%), Caucasian, and 85 years of age on average, with moderate level of cognitive impairment. Approximately one-third of the sample had at least one ApoE ε4 allele. On average, participants had a relatively low co-morbidity burden beyond their baseline dementia diagnosis. The mean LEQ score was 69.3(15.3). This is lower than scores reported for cognitively intact older adults, which range from approximately 90 (among women) to 98 (among men) on average (Valenzuela et al., 2013). There were no significant differences (p > .05) between treatment groups on any of the baseline characteristics, including personality trait score means.
Table 1.
Baseline Characteristics of the Study Sample
| Treatment Group (n=38) %/Mean(SD) |
Control Group (n=33) %/Mean(SD) |
Total (n=71) %/Mean(SD) |
p-value | Min | Max | |
|---|---|---|---|---|---|---|
| Age | 85.0 (5.2) | 85.0 (5.8) | 85.0 (5.4) | .96 | 65 | 95 |
| Gender (% female) | 63.2% | 60.6% | 62.0% | .82 | --- | --- |
| Race (% Caucasian) | 100% | 100% | 100% | -- | --- | --- |
| CDR Score | 1.2 (0.5) | 1.3 (0.6) | 1.3 (0.6) | .96 | 0.5 | 2.0 |
| MBDRS Score | 6.2 (2.7) | 7.1 (2.4) | 6.6 (2.6) | .14 | 3.0 | 13.0 |
| MMSE | 13.8 (7.0) | 13.1 (5.3) | 13.5 (6.2) | .63 | 0 | 30 |
| LEQ Score | 70.0 (15.2) | 68.5 (15.6) | 69.3 (15.3) | .68 | 38.8 | 117.5 |
| ApoE ε4 (% no ε4) | 68.4% | 64.5% | 66.7% | .34 | 0 | 2 |
| Charlson | 2.8 (1.5) | 2.8 (1.4) | 2.8 (1.5) | .97 | 1 | 6 |
CDR = Clinical Dementia Rating; MBDRS = Modified Blessed Dementia Rating Scale; LEQ = Lifetime of Experiences Questionnaire; Charlson = Charlson Co-Morbidity Index; MMSE = Mini-Mental State Examination; SD = standard deviation.
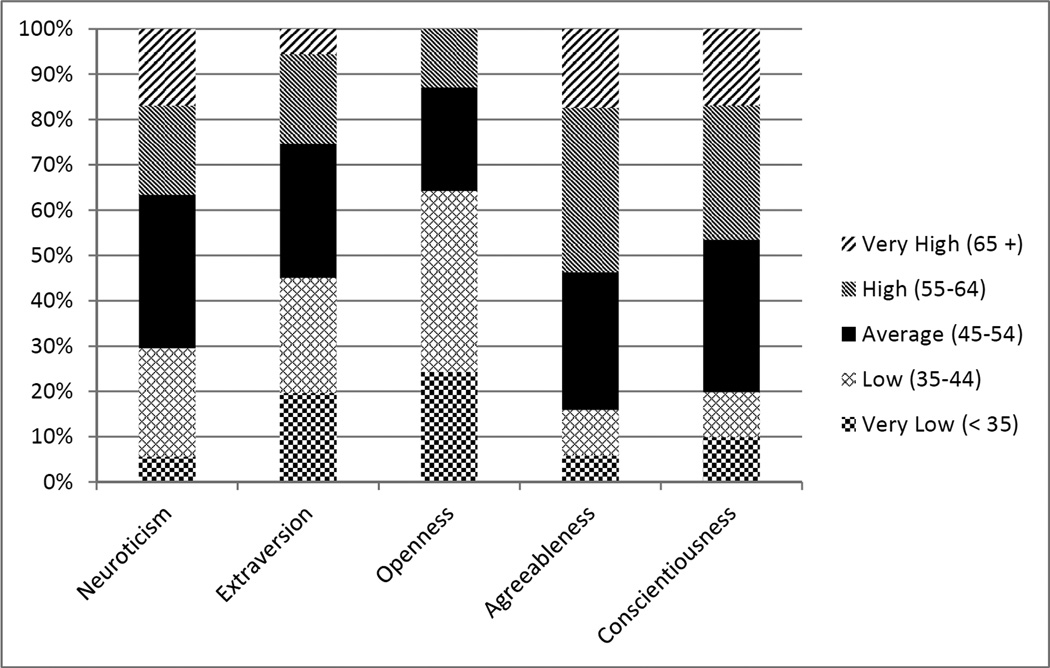
Figure 1 shows the cumulative percentages for the five personality trait intensity categories (ranging from very low to very high) for each trait score. The division of personality traits scores by intensity level as compared to a normative sample indicates several skewed distributions. This is most notable in regard to openness, with scores that tended to be lower than average, and agreeableness, with scores that tended to be higher than average.
Figure 1.
Effects of Personality on Cognitive Outcomes
Interactions of personality traits by treatment group reflect moderating effects of the personality variables on the association between treatment group assignment and the designated cognitive outcome. Since the effects of personality traits on cognitive outcomes among persons with DSD have not been previously explored in the scientific literature, main effects of the personality variables are also presented and discussed. For the purposes of this exploratory analysis, main effects were considered significant at the α=.05 level and interaction effects at the α=.10 level; all discussion of significant effects follows that designation. This decision was made due to the well-known difficulty in detecting moderating effects, particularly in regard to continuous moderator variables. Results of the mixed-effects linear model used to test the effects of the five personality traits on the cognitive outcomes are displayed in Table 2.
Table 2.
Effects of Personality on Cognitive Outcomes (n=71)
| Effect | Attention | Delayed Recall | Orientation | Executive Function | |||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | p-value | Estimate (SE) | p-value | Estimate (SE) | p-value | Estimate (SE) | p-value | ||
| Main Effects | |||||||||
| Neuroticism | Control | 0.10 (0.09) | .266 | 0.04 (0.04) | .393 | −0.03 (0.05) | .577 | 0.06 (0.18) | .745 |
| Treatment | −0.01 (0.06) | .936 | 0.05 (0.03) | .091* | 0.00 (0.03) | .924 | 0.01 (0.14) | .917 | |
| Extraversion | Control | −0.14 (0.07) | .037** | −0.08 (0.04) | .023** | −0.02 (0.03) | .581 | −0.07 (0.09) | .482 |
| Treatment | −0.03 (0.07) | .649 | −0.06 (0.03) | .049** | −0.06 (0.03) | .080* | −0.31 (0.11) | .004** | |
| Openness | Control | 0.03 (0.08) | .692 | 0.04 (0.04) | .201 | 0.07 (0.04) | .048** | −0.04 (0.13) | .743 |
| Treatment | −0.14 (0.07) | .047** | −0.02 (0.03) | .594 | −0.01 (0.03) | .821 | 0.00 (0.13) | .982 | |
| Agreeableness | Control | 0.12 (0.07) | .101 | −0.00 (0.04) | .974 | 0.02 (0.04) | .580 | 0.17 (0.14) | .220 |
| Treatment | 0.13 (0.07) | .060* | 0.09 (0.03) | .007** | 0.03 (0.04) | .387 | 0.05 (0.11) | .677 | |
| Conscientiousness | Control | −0.09 (0.05) | .085* | −0.02 (0.04) | .596 | 0.00 (0.03) | .898 | −0.12 (0.07) | .101 |
| Treatment | −0.08 (0.09) | .329 | −0.03 (0.04) | .466 | −0.06 (0.04) | .014** | −0.02 (0.14) | .869 | |
| Moderating Effects | |||||||||
| Neuroticism by Group | −0.10 (0.11) | .335 | 0.01 (0.05) | .836 | 0.03 (0.06) | .619 | −0.04 (0.22) | .847 | |
| Extraversion by Group | 0.11 (0.09) | .255 | 0.02 (0.05) | .692 | −0.04 (0.04) | .300 | −0.24 (0.13) | .064* | |
| Openness by Group | −0.17 (0.11) | .132 | −0.06 (0.05) | .202 | −0.08 (0.05) | .127 | 0.04 (0.19) | .813 | |
| Agreeableness by Group | 0.01 (0.10) | .904 | 0.09 (0.05) | .078* | 0.01 (0.06) | .853 | −0.12 (0.19) | .512 | |
| Conscientiousness by Group | 0.01 (0.11) | .928 | −0.01 (0.06) | .821 | −0.06 (0.05) | .256 | 0.10 (0.18) | .576 | |
p-value < .10;
p-value < .05;
SE = standard error.
Analyses controlled for the baseline covariates facility, age, gender, ApoE ε4status, MBDRS, and LEQ, as well as the time-dependent covariate daily CAM score.
Significant moderating effects of personality were found with regard to two cognitive outcomes: individuals with higher agreeableness were more likely to have improved delayed recall outcomes (p=.078), and those with lower extraversion more likely to have improved executive function outcomes (p=.064), as a result of the cognitive stimulation intervention. Additional significant main effects of personality were also identified. Lower extraversion scores in the control group (p=.037) and lower openness scores in the treatment group (p=.047) were associated with higher attention scores. Lower extraversion scores in both groups (p=.023; p=.049) and higher agreeableness scores in the treatment group (p=.007) were associated with higher delayed recall scores. Higher openness in the control group (p=.048) and lower conscientiousness in the treatment group (p=.014) were associated with higher orientation scores. And finally, lower extraversion scores were associated with higher executive function scores in the treatment group only (p=.004). ApoE ε4 status was not a significant predictor of any cognitive outcomes.
Effects of Personality on Intervention Engagement
Table 3 displays the results of the mixed-effects linear model used to test the effects of the five personality traits on engagement in the intervention. Since this analysis was conducted with the treatment group only (n=37, due to missing data for one participant), personality traits were included as main effects rather than interaction effects. Lower openness (p=.042), higher agreeableness (p=.001), and lower conscientiousness (p=.031) were significant predictors of increased engagement in the intervention.
Table 3.
Effects of Personality on Engagement in the Intervention (n=37)
| Effect | Estimate (SE) | p-value |
|---|---|---|
| Neuroticism | 0.42 (0.74) | .575 |
| Extraversion | −0.19 (0.84) | .822 |
| Openness | −1.86 (0.86) | .042** |
| Agreeableness | 3.45 (0.86) | .001** |
| Conscientiousness | −2.38 (1.03) | .031** |
p-value < .05;
SE = standard error.
Analyses controlled for the baseline covariates facility, age, gender, ApoE ε4status, MBDRS, and LEQ, as well as the time-dependent covariate daily CAM score.
Discussion
Personality is known to influence activity preferences, motivation for participation, and performance across populations. The influence of personality on activity participation among individuals with dementia has also been demonstrated (Kolanowski, Litaker, et al., 2011). In an RCT, the treatment effect of the population does not necessarily apply to any particular person or subgroup (such as males vs. females), but rather represents the average effect across all individuals in the population (Kraemer et al., 2006). Examining moderating variables, in this case personality traits, may help to identify whether cognitive stimulation for older adults at high risk for cognitive decline is differentially effective for individuals with certain personality characteristics. Results from this study identified significant moderating effects for delayed recall and executive function outcomes, indicating that personality traits, in part, differentially influenced the effectiveness of the intervention for some participants. Additionally, personality was associated with participation in the intervention.
Across all cognitive outcome measures, extraversion displayed the most consistent pattern of significant effects. High extraversion is associated with sociability, preference for excitement and stimulation (McCrae & Costa, 2010); however, in every case of a significant main or moderating effect, extraversion was negatively associated such that lower extraversion scores were associated with higher scores on the cognitive outcomes. In other words, it appears that introverts were more likely to benefit. While much attention is typically focused on the characteristics of extraverts (assertive, active, talkative), introverts can be more difficult to characterize. McCrae & Costa (2010) explain that introversion should be viewed as the absence of extraversion rather than its opposite; introverts are reserved but not unfriendly, even-paced rather than slow, and do not necessarily experience social anxiety. Among older adults with dementia, introverts have been found to be more likely to engage in one-on-one compared to group activities (Kolanowski & Richards, 2002). All activities delivered in the cognitive stimulation intervention were one-on-one between the participant and the research assistant, which may explain our findings.
More agreeable individuals benefitted to a greater extent from the intervention with regard to delayed recall. The direction of this effect is intuitive; agreeable individuals are eager to help others and tend to believe others are honest and well-intentioned (McCrae & Costa, 2010). These tendencies would favor participation in the intervention, and therefore benefit from it. Indeed, participants with higher agreeableness scores were more engaged in the intervention as well. While these results suggest that more agreeable individuals may be better candidates for the intervention due to greater engagement as well as delayed recall benefits, our previous work found that tailoring activity interventions to physical functional ability and individual interests may overcome some of the effects of low agreeableness (blinded for review).
Lower openness predicted higher engagement, which was unexpected considering high openness is associated with adventurousness, problem solving, and exploring new compensatory responses (McCrae & Costa, 2010). The distribution of openness scores in this sample was the most skewed of the five traits, with 64.3% of participants in the Low/Very Low categories. Anecdotally, the informants in this particular sample seemed to have difficulty answering some of items that measure openness such as “Sometimes when reading poetry or looking at a work of art, he felt a chill or wave of excitement,” and “He was intrigued by the patterns he found in art and nature.” Historically, studies using an informant-based, retrospective personality measure for persons with dementia have found lower openness scores compared to relative norms (Kolanowski, Litaker, et al., 2011).
In this sample, more highly conscientious individuals tended to engage in the intervention to a lesser extent than those who scored lower on this trait. High conscientiousness is associated with treatment success in other populations (Ozer & Benet-Martinez, 2006), but these tendencies were not supported in this study. An important consideration in interpretation of these results is the cognitive status of the participants: older adults with mild to moderate dementia as well as delirium. Some evidence suggests that awareness of cognitive impairment among older adults may interact with conscientiousness and its effects on cognitive intervention outcomes (Werheid, Ziegler, Klapper, & Kuhl, 2010). Lack of awareness of cognitive impairment is common in dementia, ranging from 31% to 67% in those with mild to moderate AD (Orfei et al., 2010), but some individuals remain aware of their cognitive deficits even through later disease stages. Conventional wisdom suggests that individuals with greater awareness of their impairment may be more motivated to participate in treatment efforts, which may in turn lead to better outcomes. However, Werheid, Ziegler, Klapper, & Kuhl (2010) found that older adults with mild cognitive impairment who were more aware of their impairment were less motivated to participate in a cognitive intervention. Conscientious individuals are used to achieving goals and performing at a high level. If they are unable to perform a cognitive task to their own standards, and are aware of this decreased ability (such as cognitive impairment due to dementia or delirium), they may withdraw from or participate less in the activity. It is possible that factors such as these played a role in the decreased intervention engagement of more conscientious individuals in this study, as well as the lack of moderating effects of conscientiousness on treatment outcomes.
Several limitations are important to consider in the interpretation of these study results. Although the study of moderating effects in RCTs has been championed by leaders in statistical methodology, efforts to detect these effects are often unsuccessful due to insufficient statistical power (Kraemer et al., 2006). However, the ability to detect statistically significant moderating effects for two of the five personality traits examined in this exploratory study is perhaps a testament to the pervasive influence of personality of treatment outcomes across populations and conditions. Additionally, the maximum possible time on task was 30 minutes, which ultimately led to right-censoring of these data due to the fact that most (70.2%) of the intervention sessions were conducted for the maximum amount of time. Although this may have limited variability in the engagement measure, these results suggest that a longer intervention time may be well-tolerated by those who consistently engage as well as necessary in order to comprehensively examine main and moderating effects on intervention engagement. Finally, this study has limited generalizability to the overall population of older adults with cognitive impairment. Although the statistical analyses included the CAM as a time-dependent covariate to control for the fluctuating effect of delirium on cognitive outcomes, it is unknown to what extent the presence of delirium influenced potential moderating effects of personality traits on the intervention outcomes. Despite these caveats, this study has many strengths including use of state of the art measures for personality in a novel population and a randomized clinical trial study design.
Recommendations for Future Research and Practice
The critical importance of utilizing multiple personality traits as predictors of health outcomes was illustrated by Friedman & Booth-Kewley (1987). In their meta-analysis of hundreds of studies linking personality and disease, they illustrated that a particular set of personality characteristics increased the risk of disease and that multiple characteristics must be examined simultaneously in order to determine disease predictors. Future research should examine the effects of combinations of personality traits or certain personality profiles for their potential moderating effects on cognitive stimulation outcomes. Studies which are appropriately powered to both detect moderating effects as well as examine personality trait interactions are needed in order to fully examine the role of personality in intervention outcomes among this vulnerable population. It is also unknown whether or to what extent personality plays a role in delirium manifestation or resolution. The body of literature exploring the relationship between delirium and personality is scant at best.
While previous research has demonstrated the benefits of tailoring activity interventions for older adults with cognitive impairment to personality traits (Kolanowski, Fick, & Buettner, 2009; Kolanowski, Litaker, Buettner, Moeller, & Costa, 2011), this study begins to suggest which individuals may be the best candidates for cognitive stimulation interventions, even when intervention content is personalized to individual interests. It is well-accepted in experimental research that an effective intervention may not be equally effective among individuals. Determining statistically significant moderators allows investigators, and ultimately clinicians, to identify individuals more likely to respond to a particular treatment (Baron & Kenny, 1986; Kraemer, Kiernan, Essex, & Kupfer, 2008). Although preliminary, these results do suggest practice implications that may be helpful to nurses working with cognitive impaired older adults, particularly individuals with or at risk for delirium (such as those experiencing a hospitalization or acute illness). While a body of evidence supports the potential benefit of cognitive stimulation among older adults with cognitive impairment, this approach to activity intervention may be more effective for certain personality types. The influence of personality is likely complex and much is yet to be investigated and understood, particularly among individuals with complex syndromes influencing cognitive function, such as delirium superimposed on dementia. This area of research is in its infancy, but is an important consideration for person-centered care as the science develops.
Conclusion
Identifying the best candidates for a particular treatment provides for targeting of that treatment as well as maximization of effect size in research. In a truly personalized intervention approach, the best option for each individual should be considered, rather than what may be best for a group of people, on average. Determining moderators of treatment effects provides a way to begin making these determinations. Within nursing science specifically, it has been recommended that moderators be considered to determine the circumstances in which a nursing intervention provides the best outcome (Bennett, 2000), and this study begins to address that gap in regard to cognitive interventions for older adults at high risk for cognitive decline. These findings suggest that the personality traits of extraversion and agreeableness are two factors that may be important to consider when selecting and tailoring cognitive interventions for individuals with dementia and delirium.
Acknowledgments
Nikki Hill, PhD, RN acknowledges support from National Institute of Nursing Research (NINR) grant number F31NR013304 and the National Hartford Centers of Gerontological Nursing Excellence Claire M. Fagin Fellow Award Program. Ann Kolanowski PhD, RN and Donna Fick PhD, RN acknowledge partial support from NINR grant number 5 R01 NR012242 02: Reserve For Delirium Superimposed on Dementia (DSD). The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH/NINR.
Contributor Information
Nikki L. Hill, Email: nikki.hill@psu.edu, National Hartford Centers of Gerontological Nursing Excellence Claire M. Fagin Fellow, The Pennsylvania State University College of Nursing, 201 Health & Human Development East, University Park, PA 16802.
Ann M. Kolanowski, College of Nursing, The Pennsylvania State University College of Nursing, University Park, PA.
Donna Fick, The Pennsylvania State University College of Nursing, University Park, PA.
Vernon M. Chinchilli, The Pennsylvania State University Department of Statistics, University Park, PA.
Rita A. Jablonski, The University of Alabama at Birmingham School of Nursing, Birmingham, AL.
References
- Aguirre E, Woods B, Spector A, Orrell M. Cognitive stimulation for dementia: A systematic review of the evidence of effectiveness from randomised controlled trials. Ageing Research Reviews. 2013;12(1):253–262. doi: 10.1016/j.arr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Archer N, Brown R, Boothby H, Foy C, Nicholas H, Lovestone S. The NEO-FFI is a reliable measure of premorbid personality in patients with probable Alzheimer's disease. International Journal of Geriatric Psychiatry. 2006;21(5):477–484. doi: 10.1002/gps.1499. [DOI] [PubMed] [Google Scholar]
- Backman L, Andersson JL, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer's disease. Neurology. 1999;52(9):1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Becker JT, Mintun MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease. Neurology. 1996;46(3):692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- Bennett JA. Mediator and moderator variables in nursing research: Conceptual and statistical differences. Research in Nursing & Health. 2000;23:415–420. doi: 10.1002/1098-240x(200010)23:5<415::aid-nur8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Clare L, Woods B. Cognitive rehabilitation and cognitive training for early-stage Alzheimer's disease and vascular dementia. Cochrane Database of Systematic Reviews. 2003;4:CD003260. doi: 10.1002/14651858.CD003260. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five Factor Inventory (NEO-FFI): Professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Ertekin-Taner N. Genetics of Alzheimer's disease: A centennial review. Neurologic Clinics. 2007;25(3):611–667. doi: 10.1016/j.ncl.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Journal of the American Medical Association. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Fick D, Steis M, Waller J, Inouye S. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. Journal of Hospital Medicine. doi: 10.1002/jhm.2077. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franks P, Chapman B, Duberstein P, Jerant A. Five factor model personality factors moderated the effects of an intervention to enhance chronic disease management self-efficacy. British Journal of Health Psychology. 2009;14(3):473–487. doi: 10.1348/135910708X360700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: Evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behavior Genetics. 2003;33(1):67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Booth-Kewley S. The "disease-prone personality": A meta-analytic view of the construct. American Psychologist. 1987;42:539–555. doi: 10.1037//0003-066x.42.6.539. [DOI] [PubMed] [Google Scholar]
- Garibotto V, Borroni B, Sorbi S, Cappa SF, Padovani A, Perani D. Education and occupation provide reserve in both ApoE epsilon4 carrier and noncarrier patients with probable Alzheimer's disease. Neurological Sciences. 2012;33(5):1037–1042. doi: 10.1007/s10072-011-0889-5. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005;19(2):199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Jones RN, Habtemariam DA, Fong TG, Tommet D, Quach L, Schmitt E, Yap L, Inouye SK. Delirium and long-term cognitive trajectory among persons with dementia. Archives of Internal Medicine. 2012;172(17):1324–1331. doi: 10.1001/archinternmed.2012.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. New England Journal of Medicine. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dementia and Geriatric Cognitive Disorders. 1999;10(5):393–400. doi: 10.1159/000017177. [DOI] [PubMed] [Google Scholar]
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- Judge TA, Ilies R. Relationship of personality to performance motivation: A meta-analytic review. Journal of Applied Psychology. 2002;87(4):797–807. doi: 10.1037/0021-9010.87.4.797. [DOI] [PubMed] [Google Scholar]
- Kolanowski A, Fick DM, Clare L, Steis M, Boustani M, Litaker M. Pilot study of a nonpharmacological intervention for delirium superimposed on dementia. Research in Gerontological Nursing. 2011;4(3):161–167. doi: 10.3928/19404921-20101001-98. [DOI] [PubMed] [Google Scholar]
- Kolanowski A, Hill NL, Clare L, Marx P. Practical protocol for implementing cognitive stimulation in persons with delirium superimposed on dementia. Non-Pharmacological Therapies in Dementia. 2012;2(2) [PMC free article] [PubMed] [Google Scholar]
- Kolanowski A, Litaker M, Buettner L, Moeller J, Costa PT., Jr A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. Journal of the American Geriatrics Society. 2011;59:1032–1041. doi: 10.1111/j.1532-5415.2011.03449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanowski A, Richards KC. Introverts and extraverts: Leisure activity behavior in persons with dementia. Activities, Adaptation & Aging. 2002;26(4):1–16. [Google Scholar]
- Kolanowski A, Fick DM, Buettner L. Recreational Activities to Reduce Behavioural Symptoms in Dementia. Geriatr Aging. 2009;12(1):37–42. [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychology. 2008;27(***2(Suppl)):S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. Journal of the American Medical Association. 2006;296(10):1286–1289. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- Kovach CR, Magliocco JS. Late-stage dementia and participation in therapeutic activities. Applied Nursing Research. 1998;11(4):167–173. doi: 10.1016/s0897-1897(98)80285-1. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr . NEO Inventories for the NEO Personality Inventory-3 (NEO-PI-3), NEO Five-Factor Inventory-3 (NEO-FFI-3), NEO Personality Inventory-Revised (NEO PI-R): Professional Manual. Lutz, FL: PAR, Inc.; 2010. [Google Scholar]
- McCrae RR, Costa PT., Jr . Personality in adulthood: A five-factor theory perspective. 2nd ed. New York: Guilford Press; 2002. [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Varsi AE, Blundo C, Celia E, Casini AR, Caltagirone C, Spalletta G. Anosognosia in Mild Cognitive Impairment and mild Alzheimer's disease: Frequency and neuropsychological correlates. American Journal of Geriatric Psychiatry. 2010;18(12):1133–1140. doi: 10.1097/JGP.0b013e3181dd1c50. [DOI] [PubMed] [Google Scholar]
- Ozer DJ, Benet-Martinez V. Personality and the prediction of consequential outcomes. Annual Review of Psychology. 2006;57:401–421. doi: 10.1146/annurev.psych.57.102904.190127. [DOI] [PubMed] [Google Scholar]
- Piantadosi S. Clinical Trials: A Methodologic Perspective, Second Edition. Hoboken, NJ: John Wiley & Sons, Inc; 2005. [Google Scholar]
- Palmer RM, Meldon SW. Digit span test in acute care. In: Hazzard WR, editor. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill Professional; 2003. pp. 157–168. [Google Scholar]
- Pompei P, Foreman M, Cassel CK, Alessi C, Cox D. Detecting delirium among hospitalized older patients. Archives of Internal Medicine. 1995;155(3):301–307. [PubMed] [Google Scholar]
- Ritchie K, Fuhrer R. The validation of an informant screening test for irreversible cognitive decline in the elderly: Performance characteristics within a general population sample. International Journal of General Psychiatry. 1996;11(2):149–156. [Google Scholar]
- Roberts B, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;126(1):3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Royall DR, Cordes JA, Polk M. CLOX: An executive clock drawing task. Journal of Neurology, Neurosurgery & Psychiatry. 1998;64(5):588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: Validity and utility in a memory clinic setting. Canadian Journal of Psychiatry. 2007;52(5):329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. The Lancet Neurology. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg P, Burgener S, Popovich A. Measurement and relevance of personality characteristics in persons with dementia: A longitudinal perspective. Research and Theory for Nursing Practice. 2007;21(1):13–31. doi: 10.1891/rtnpij-v21i1a004. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Leon I, Suo C, Piamba DM, Kochan N, Brodaty H, Sachdev P. Cognitive lifestyle in older persons: The Population-Based Sydney Memory & Ageing Study. Journal of Alzheimer's Disease. 2013;36(1):87–97. doi: 10.3233/JAD-130143. [DOI] [PubMed] [Google Scholar]
- van Doorn C, Bogardus ST, Williams CS, Concato J, Towle VR, Inouye SK. Risk adjustment for older hospitalized persons: A comparison of two methods of data collection for the Charlson index. Journal of Clinical Epidemiology. 2001;54(7):694–701. doi: 10.1016/s0895-4356(00)00367-x. [DOI] [PubMed] [Google Scholar]
- Voyer P, Richard S, Doucet L, Carmichael PH. Detecting delirium and subsyndromal delirium using different diagnostic criteria among demented long-term care residents. Journal of the American Medical Directors Association. 2009;10(3):181–188. doi: 10.1016/j.jamda.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scales -- Revised (WAIS-R) New York: Psychological Corporation; 1981. [Google Scholar]
- Werheid K, Ziegler M, Klapper A, Kuhl KP. Awareness of memory failures and motivation for cognitive training in mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2010;30(2):155–160. doi: 10.1159/000318755. [DOI] [PubMed] [Google Scholar]
- Yang F, Inouye S, Fearing M, Kiely D, Marcantonio E, Jones R. Participation in activity and risk for incident delirium. Journal of the American Geriatrics Society. 2008;56(8):1479–1484. doi: 10.1111/j.1532-5415.2008.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaubler TS, Murphy K, Rizzuto L, Santos R, Skotzko C, Giordano J, Bustami R, Inouye SK. Quality improvement and cost savings with multicomponent delirium interventions: Replication of the hospital elder life program in a community hospital. Psychosomatics. 2013;54(3):219–226. doi: 10.1016/j.psym.2013.01.010. [DOI] [PubMed] [Google Scholar]