Abstract
Triclosan (TCS) is a synthetic antibacterial chemical that is used in personal care products and is measurable in urine. Urinary TCS has been associated with allergy in children in Norway and the United States. A reasonable degree of temporal reliability of TCS urinary concentrations has been reported among U.S. children as well as for Puerto Rican pregnant women. We examined the reliability of TCS measures in urine among Norwegian pregnant women. Triclosan was measured in spot urine samples collected in gestational weeks 17, 23, and 29 from 45 women in The Norwegian Mother and Child Cohort Study (MoBa) enrolled in 2007 and 2008. Spearman’s rank correlation coefficient (rs) and intraclass correlation coefficient (ICC) statistics were calculated. Fifty-six percent of the 45 women had a least one sample with a value above the method limit of detection (2.3 µg/L). The correlation coefficients were 0.61 for TCS concentrations at 17 and 23 weeks and 0.49 for concentrations at 17 and 29 weeks. For the three time points, the ICC was 0.49. The reliability of TCS concentrations in repeated urine samples from pregnant Norwegian women was reasonably good, suggesting a single urine sample can adequately represent TCS exposure during pregnancy.
Keywords: biomarkers, MoBa, intraclass correlation coefficient, pregnancy, reliability, triclosan
Introduction
Triclosan (TCS) is a synthetic antibacterial chemical that is used in personal care products such as toothpaste, cosmetics, skin care creams and lotions, soaps and dental products, as well as in toys and kitchen utensils1. TCS does not accumulate in the body and has a urinary half-life of about 21 hours2. A biomarker of TCS is measurable in urine. Urinary concentrations of TCS have been found to be associated with allergy in Norwegian children3 and in children and adolescents in the U.S. National Health and Nutrition Examination Study (NHANES)4.
In environmental epidemiology, collection of biological specimens is often limited to one single sample for each participant, and therefore knowledge about the temporal variability of urinary concentrations of the biomarkers is of importance in planning studies and interpreting results. The temporal variability of urinary concentrations of TCS in urine has been evaluated in 6–10 year old healthy U.S. children5, and in pregnant women in Puerto Rico6. However, differences between countries in the regulations and recommended use of TCS-containing consumer products7–9 may influence the dominant sources of exposure and thus, the reliability of triclosan measures. In the present study, we examined the temporal variability of TCS concentrations in urine collected from Norwegian pregnant women across the gestational period.
Materials and Methods
The Norwegian Mother and Child Cohort Study (MoBa) is a prospective population-based pregnancy cohort initiated and maintained at the Norwegian Institute of Public Health10.
MoBa participants were recruited from all of Norway from 1999–2008, and 38.5% of the invited women consented to participate. The cohort now includes more than 108,000 children and 90,700 mothers. Follow-up is conducted by questionnaires at regular intervals and by linkage to national health registries, among them the Medical Birth Registry of Norway11. The study protocol was approved by The Regional Committee for Medical and Health Research Ethics in southeastern Norway. Informed consent was obtained from each MoBa participant upon recruitment. The current study is based on version 7 of the quality-assured data files released for research in June 2012.
All women in the MoBa cohort study were asked to provide a set of biologic specimens in approximately the 17th week of pregnancy. For women in the reliability substudy of MoBa (n=671), sets of biologic specimens were also collected at weeks 23 and 29 of pregnancy from November 2007 to December 2008. The rationale for this substudy was to examine whether biomarker concentrations in the 17-week specimens were representative for the gestational period. Subjects in the reliability substudy were enrolled from four hospitals, each representing a different region of Norway [northwest (Sunnmøre Hospital HF Ålesund), southwest (Stavanger University Hospital HF), central (St Olavs Hospital HF), and eastern (Østfold Hospital HF Fredrikstad) Norway]. The present study includes data from 45 women selected for TCS analysis from the MoBa reliability substudy. Fifteen women were included in this study because a previous study had found evidence of high total bisphenol-A (BPA) concentrations in at least one of their urine samples, and an additional 30 women were randomly sampled from the population of women in the reliability substudy. The 15 women with a previously measured high total BPA concentration had been initially selected at random from the reliability substudy. The high total BPA concentration was found to be caused by a urine preservative in the urine collection tubes12.
To replicate the collection and handling of MoBa samples, urine was transferred to a 8-mL BD vacutainer® urinalysis preservative tube containing a mixture of chlorhexidine, ethyl paraben and sodium propionate (BD Diagnostics, Franklin Lakes, NJ, USA). Total and free TCS concentrations were measured by online solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry13 at the Centers for Disease Control and Prevention (CDC, Atlanta, USA). The samples were processed by procedures adopted by CDC to avoid external contamination with TCS14. The method limit of detection (LOD) was 2.3 µg/L. To account for differences in urinary analyte concentrations caused by urine dilution, the TCS concentration were divided by creatinine concentrations15.
Statistics
We calculated Spearman’s rank correlation coefficient (rs) for TCS urinary concentrations between pairs of samples from week 17 and 23, week 23 and 29, and between week 17 and 29. We calculated the intraclass correlation coefficient (ICC) statistics for the three pairs. The ICC is a measure of consistency or agreement of the same measurement within an individual. The ICC was calculated using a one-way random-effects model on natural log transformed TCS concentrations. For concentrations below the LOD, instead of imputed values we used the actual instrumental values16. The Spearman’s rank correlation coefficients and the ICC statistics are presented for urinary TCS concentrations by uncorrected (µg/L) and creatinine-corrected (µg/creatinine) values. We also calculated the ICC among the subset of participants who had TCS concentrations below the method limit of detection in all three urine samples, to evaluate whether the instrumental values would provide any additional information compared to random values or substitution methods. All statistics were performed with Statistical Package for Social Sciences (SPSS version 19.0; SPSS Inc., Chicago, IL, USA).
Results
Approximately half of the 45 women in the present study were primiparous and 30 years or older at the time of delivery (Table 1). The majority of the pregnant women were married or lived with a partner (95%), had a college degree (71%), and had a pre-pregnancy body mass index (BMI) between 20 and 25 kg/m2 (60%) (Table 1).
Table 1.
Characteristics of women in the MoBa TCS reliability substudy by TCS concentration
| Characteristics | n (%) | Women (n) with ≥1 sample with TCS > LOD |
Geometric mean of mean TCS for all 3 samples (µg/L)1,2 |
Geometric mean of meanTCS for all 3 samples (µg/g creatinine)1,2 |
|---|---|---|---|---|
| Marital status3 | ||||
| Married | 20 (46) | 9 | <LOD | <LOD |
| Cohabitated | 21 (49) | 14 | 4.5 | 4.6 |
| Single | 2 (5) | 0 | <LOD | <LOD |
| Maternal education† | ||||
| Less than high school | 4 (10) | 0 | <LOD | <LOD |
| High school | 8 (19) | 4 | <LOD | <LOD |
| Up to 4 years of college | 24 (57) | 14 | 3.4 | 3.9 |
| > 4 years of college | 6 (14) | 4 | <LOD | 3.0 |
| Maternal income (in 1000 Nok/year)3 | ||||
| <150 | 7 (17) | 2 | <LOD | <LOD |
| 150–300 | 11 (26) | 8 | <LOD | 2.8 |
| 300–400 | 17 (40) | 9 | 4.0 | 4.0 |
| >400 | 7 (17) | 5 | 4.3 | 5.7 |
| Parity | ||||
| Primiparous | 23 (51) | 19 | 5.3 | 5.8 |
| Multiparous | 22 (49) | 6 | <LOD | <LOD |
| Smoking during pregnancy3 | ||||
| None | 38 (90) | 20 | <LOD | 2.5 |
| Sometimes | 2 (5) | 2 | 19.4 | 16.9 |
| Daily | 2 (5) | 0 | <LOD | <LOD |
| Maternal age at delivery, years | ||||
| <25 | 5 (11) | 1 | <LOD | <LOD |
| 25–29 | 12 (33) | 10 | 3.7 | 4.1 |
| 30–34 | 12 (27) | 8 | 3.9 | 4.5 |
| >35 | 13 (29) | 6 | <LOD | <LOD |
| Pre-pregnancy BMI, kg/m23 | ||||
| <20 | 7 (17) | 4 | <LOD | 2.5 |
| 20–24.9 | 25 (60) | 15 | 2.7 | 3.3 |
| 25–29.9 | 6 (14) | 4 | 3.8 | 3.2 |
| ≥ 30 | 4 (9) | 0 | <LOD | <LOD |
(<LOD) indicates that the calculated GM concentration is below the LOD = 2.3 µg/L.
No statistically significant difference between the groups (ANOVA p>0.05)
GM calculations for the woman’s mean concentration based on her 3 urine sample.
Information missing for maternal education (n=3), marital status (n=2), maternal income (n=3), smoking during pregnancy (n=3), and pre-pregnancy BMI (n=3)
Overall, 36% of the urine samples (48/135) had detectable total TCS concentrations and 13% of the urines samples (18/135) had detectable free TCS concentrations. Given the low detection of free TCS, these results will not be discussed further. Fifty-six percent of the women had a least one urine sample with a detectable concentration and 27% had detectable TCS concentrations in all three urine samples. The number of women with at least one urine sample with detectable TCS concentrations, as well as the geometric mean of TCS showed some increase with higher maternal education and income (Table 1), but there was no statistically significant difference between the various categories. The geometric mean TCS concentration among women who reported smoking sometimes during pregnancy was higher than for the non-smokers and the daily smokers (Table 1), but the difference was mainly due to one woman with TCS concentrations above the 75th percentile at all three time points.
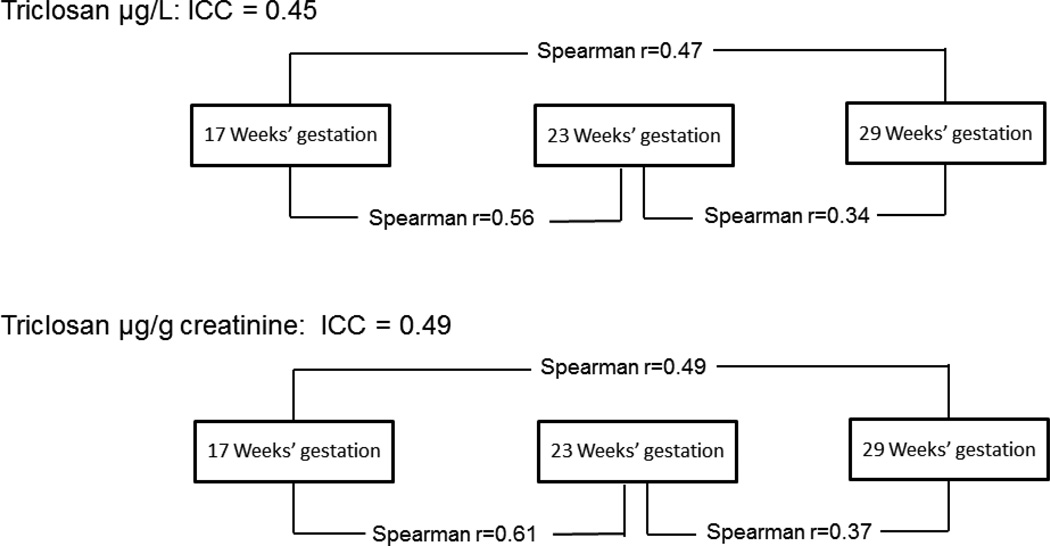
The 95th percentile of TCS concentrations among the 135 urine specimens was 387 µg/L (Table 2) (329 µg/g creatinine). The correlation of creatinine-corrected TCS concentrations in gestational weeks 17 and 23 was rs=0.61, and the correlation for weeks 17 and 29 was rs=0.49 (Figure 1). For the three time points, the ICC was 0.49 (95% CI: 0.31, 0.65), indicating moderate reliability. For women with undetectable TCS concentrations in all three urine samples (n=20), the ICC was 0.22 (p=0.06) for the creatinine-corrected TCS.
Table 2.
Urinary TCS concentrations (ng/mL) reported in different studies obtained using the same laboratory and analytical method.
| Percentiles | ||||||
|---|---|---|---|---|---|---|
| %>LOD | GM (95% CI) | 50th | 95th | Max | Reference | |
| Norwegian pregnant women1 | 36 | (<LOD) | (<LOD) | 387 | 970 | This work |
| Norwegian children | 47 | 2.4 | (<LOD) | 444 | 3610 | Bertelsen et al. 20133 |
| New York City children | 71.7 | 13.6 | 8.5 | 477 | 1040 | Teitelbaum et al. 20085 |
| Puerto Rican pregnant women | 88.9 | 29.9 (23.6, 37.9) | 26.2 | 944 | 2000 | Meeker et al. 20136 |
| NHANES 07–08 women (18–40 years) | 84.9 | 18.7 (14.7, 23.8) | 14.0 | 520 | 2780 | Meeker et al. 20136 |
| NHANES 09–10 women (18–40 years) | 79.0 | 16.9 (12.2, 23.4) | 13.0 | 577 | 2690 | Meeker et al. 20136 |
Includes TCS concentrations for 3 repeated samples per women (n=105 samples). (<LOD) for concentrations below the LOD = 2.3 µg/L
Figure 1.
Pairwise Spearman correlation coefficient and ICC measures for triclosan concentrations among 45 pregnant women in the MoBa cohort study
Discussion
The reliability of TCS concentrations in repeated urine samples from pregnant women was reasonably good (ICC=0.49). Despite the high proportion of participants with undetectable TCS concentration in our study the ICC is in line with results from a recent study of TCS reliability in urine samples from pregnant women in Puerto Rico (ICC=0.47)6, and with TCS measured in repeated first-morning urine samples from Danish men (ICC=0.56)17.
Only 36% of the urine samples from this sample of pregnant Norwegian women had detectable TCS concentrations as compared to 89% of the pregnant women from Puerto Rico6 and 80% of the NHANES 2007–2008 study population (6 years and older)18(Table 2). The 95th percentile (340 µg/L) was lower than the 95th percentile (571 µg/L) for women in NHANES 2007–20081 and considerably lower than the 944 µg/L among the pregnant women from Puerto Rico6. In the present study, the number of women with at least one urine sample with detectable TCS concentrations, as well as the geometric mean of TCS was somewhat higher among women with higher maternal education and income, although none of these differences were statistically significant. In the NHANES study urinary triclosan concentrations were reported to be highest during the third decade of life (20–30 years of age) and among those with the highest household income19.
Differences in detection frequencies are unlikely to be related to the analytical methodology. The between batch coefficient of variation (CV) for TCS at high concentration was 9.6% in the MoBa samples, very close to the reported CV for the analyses of NHANES 2009–2010 samples (9.3% at ~25 µg/L20), suggesting that instrumental performance unlikely impacted the TCS results, at least at the higher concentrations. Neither is our assessment method for urinary dilution likely to account for any differences in detection frequency. Although creatinine was used to account for urine dilution in the present study, a recently published paper reporting TCS in urine from pregnant women measured both creatinine and specific gravity, and found no difference in estimates of reliability regardless of the method used to account for urinary dilution21. That said, one potential concern is the presence of a highly concentrated preservative mixture that was previously found to influence BPA concentrations measured in urine samples12. While we cannot exclude the possibility that the preservative may have negatively impacted the mass spectrometer performance as a whole, the lower detection frequency of TCS among these MoBa samples is more likely to reflect pattern of use rather than poor instrumental performance. Moreover, although field blanks were not collected for this study, the concentration of free TCS relative to the total TCS was low, suggesting little, if any, contamination of the specimens.
The lower detection frequency and urinary concentrations of TCS among the Norwegian women are likely due to differences between countries in the regulations and recommended use of TCS-containing consumer products7–9. We reported a much lower TCS detection frequency in urine from Norwegian 10-year old children (47%)3 than what was reported by Teitelbaum et al. in a population of US minority children of similar age (71%)5 (Table 2). These studies report TCS concentrations measured using the same method and laboratory. In the EU the production and use of TCS in consumer products has declined during the last decade9, whereas there is little indication of a decline in TCS exposure in the U.S. population - according to biomonitoring data from NHANES18. In particular, due to the concern about the emergence of antibiotic-resistant bacteria, the Norwegian authorities have encouraged retailers and consumers to avoid routine use of products declared as antibacterial since the year 20008.
Body burden of TCS is probably influenced most by an individual’s use of TCS-containing products. The same brands of cosmetics and dental products are often used over an extended period of time, and thus, despite the short half-life, a single urine measure of TCS may be informative about longer-term average exposure, for example, over the course of a pregnancy, due to consistent patterns of product usage. Compared to non-persistent chemicals for which the main route of exposure is diet, the relative repeatability of urinary concentrations of the antibacterial agent triclosan among pregnant women suggest that the concentrations of triclosan in a single spot urine sample may be adequate to categorize exposure to triclosan during pregnancy. In particular, the ICC for TCS was one of the highest ICCs among 19 urinary biomarkers evaluated for the U.S. children5, and also comparable to the ICC for chemicals from other personal care products (e.g. parabens, benzophenone-3) in the women from Puerto Rico6.
The ICC of 0.22 for the concentrations below the method limit of detection suggests that the instrumental values do provide data that is not completely random. Using the actual instrumental values for concentrations below the LOD is at least as good as any other solution and may be the best approach16. Use of substitution methods such as LOD/√2 can result in an underestimate of correlation among measures22, but in this case lowered the ICC only slightly, to 0.48.
Despite the much higher proportion of participants with undetectable TCS concentrations in our study as compared to other studies - which is likely to be due to patterns of use and regulation of TCS-containing products in Norway - the ICC of 0.49 among the pregnant Norwegian women was similar to the ICC (0.47) among Puerto Rican pregnant women6. Although we cannot exclude the possibility that pregnancy influences the metabolism of TCS, the reliability of TCS urinary concentrations in repeated samples from pregnant women in Norway was reasonably good, suggesting that a single urine sample may be adequate in representing TCS exposure during pregnancy.
Acknowledgments
Acknowledgements and human subjects’ approval
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10). This study was supported in part by the grants from the National Institute of Environmental Health Sciences (P30-ES010126, R01-ES021777, and K12-ES019852), the Intramural Research Program of the National Institute of Health (NIH), National Institute of Environmental Health Sciences (NIEHS). The involvement of the CDC was determined not to constitute engagement in human subject research. Human subjects committees at NIEHS and at the University of North Carolina also approved this study protocol. We acknowledge X. Ye, X. Zhou, J. Kramer, and T. Jia (CDC) for technical assistance in measuring TCS. We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
References
- 1.CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta: Department of Health and Human Services. Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 2.Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- 3.Bertelsen RJ, Longnecker MP, Lovik M, Calafat AM, Carlsen KH, London SJ, et al. Triclosan exposure and allergic sensitization in Norwegian children. Allergy. 2013;68:84–91. doi: 10.1111/all.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savage JH, Matsui EC, Wood RA, Keet CA. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol. 2012;130:453–460. doi: 10.1016/j.jaci.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental research. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, Variability, and Predictors of Urinary Concentrations of Phenols and Parabens among Pregnant Women in Puerto Rico. Environ Sci Technol. 2013 doi: 10.1021/es400510g. e-pub ahead of print 2013/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Administration FUSFaD. Triclosan: What Consumers Should Know. 2012 [Google Scholar]
- 8.Eide AM. Analyse av triklosan i kosmetiske produkter. Oslo: The Norwegian Food Control Authority; 2003. [Google Scholar]
- 9.Scientific Committee on Consumer Safety. SCCS. Opinion on triclosan antimicrobial Resistance. [Accessed: November 18 2013];The European Commision. 2010 Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_023.pdf. [Google Scholar]
- 10.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 11.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–439. [PubMed] [Google Scholar]
- 12.Longnecker MP, Harbak K, Kissling GE, Hoppin JA, Eggesbo M, Jusko TA, et al. The concentration of bisphenol A in urine is affected by specimen collection, a preservative, and handling. Environmental research. 2013;126:211–214. doi: 10.1016/j.envres.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 14.Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential External Contamination with Bisphenol A and Other Ubiquitous Organic Environmental Chemicals during Biomonitoring Analysis: An Elusive Laboratory Challenge. Environmental health perspectives. 2013 doi: 10.1289/ehp.1206093. Advance publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environmental health perspectives. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Committee AM. Measurement of near zero concentration: recording and reporting results that fall close to or below the detection limit. Analyst. 2001;126:256–259. doi: 10.1039/b009590g. [DOI] [PubMed] [Google Scholar]
- 17.Lassen TH, Frederiksen H, Jensen TK, Petersen JH, Main KM, Skakkebaek NE, et al. Temporal variability in urinary excretion of bisphenol A and seven other phenols in spot, morning, and 24-h urine samples. Environmental research. 2013 doi: 10.1016/j.envres.2013.07.001. e-pub ahead of print 2013/08/13. [DOI] [PubMed] [Google Scholar]
- 18.CDC. National Health and Nutrition Examination Survey 2007–2008 Data Documentation, Codebook, and Frequencies, Environmental Phenols (EPH_E) 2010 [Google Scholar]
- 19.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. [Accessed: November 18 2013];Laboratory Procedure Manual. 2011 Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/EPH_F_met_phenols_parabens.pdf.
- 21.Philippat C, Wolff MS, Calafat AM, Ye X, Bausell R, Meadows M, et al. Prenatal Exposure to Environmental Phenols: Concentrations in Amniotic Fluid and Variability in Urinary Concentrations during Pregnancy. Environmental health perspectives. 2013 doi: 10.1289/ehp.1206335. e-pub ahead of print 2013/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nie L, Chu H, Liu C, Cole SR, Vexler A, Schisterman EF. Linear regression with an independent variable subject to a detection limit. Epidemiology. 2010;21(Suppl 4):S17–S24. doi: 10.1097/EDE.0b013e3181ce97d8. [DOI] [PMC free article] [PubMed] [Google Scholar]