Abstract
Hairy cell leukemia (HCL) is a rare neoplasm of mature small B lymphoid cells with characteristic circumferential ‘hairy projections’ involving the peripheral blood, bone marrow and splenic red pulp. With the advent of immunophenotyping and newer treatment modalities, prolonged remission can be achieved after a definitive diagnosis. Due to the rarity of this condition and presence of only a few case series from India, this work was undertaken. The aim was to study the clinico-pathologic and immunophenotypic features of all cases diagnosed as hairy cell leukemia. The cases were retrieved from Hematopathology records, between 1991 and 2012. The complete clinical details, investigations, treatment and follow-up were obtained from Medical Oncology records. The peripheral blood picture, bone marrow cytology and trephine sections along with special stains were reviewed. There were 12 cases of HCL during the study period with a M:F ratio of 11:1. Of these, ten were diagnosed as classical HCL and two as variant HCL. The most common clinical manifestations were fever, easy fatigability and weakness. Splenomegaly was present in 81.8 % cases. Though all the patients showed some form of cytopenia, there were three (25 %) patients with leucocytosis. The smears from all patients showed atypical lymphoid cells with circumferential hairy projections. TRAP was positive in 9 patients (81.8 %). Immunophenotyping was done in six cases, four were confirmed as HCL and two were diagnosed as HCL-v. The patients treated with Cladribine generally had a good response. The characteristic morphology of the hairy cells; along with correlation with the clinical features, TRAP positivity and immunophenotyping by flow cytometry is essential for diagnosis. Treatment response with Cladribine is good and has prolonged remission rates.
Keywords: Cytopenia, Splenomegaly, Hairy cells, Immunophenotyping
Introduction
Hairy cell leukemia (HCL) is an indolent neoplasm of small mature B lymphoid cells with oval nuclei and abundant cytoplasm with circumferential “hairy projections” involving peripheral blood (PB) and diffusely infiltrating the bone marrow (BM) and splenic red pulp. It is a disease of elderly males and accounts for 2 % of lymphoid leukemia. Accurate diagnosis at the right time is essential; as there are good treatment modalities that can induce prolonged remission. This study was undertaken, due to the rarity of the condition and the fact that there have not been many case series from India [1–3].
Aims and Objectives
To study the clinico-pathological and immunophenotypic features of all cases diagnosed as hairy cell leukemia.
Materials and Methods
The cases were retrieved from Haematopathology records, from 1991 to June 2012. The clinical details and follow up were obtained from Medical Oncology records. The peripheral smear, BM cytology and trephine sections were reviewed. Immunophenotyping reports were obtained from medical oncology records. The panel included CD 19, CD 20, CD 79a, CD 11c, CD 25, CD 103, CD 3 and CD 5. Bone marrow aspiration (BMA) done from posterior superior iliac spine using Islam (16 gauze) needle and bone marrow biopsy (BMB) was done using modified Jenson needle (8G). Peripheral smears (PS) were collected at the time of BMA/BMB. The PS and BMA were stained using Giemsa stain. Tartrate resistant acid phosphatase (TRAP) cytochemical stain was done on air-dried PS after fixation in citrate acetone formaldehyde by kit method (Sigma–Aldrich).
Bone marrow biopsy sections were stained with H&E stain after fixation in B 5 fixative, decalcification and routine processing. Reticulin staining was done in all cases to look for marrow fibrosis.
Results
There were 12 patients of HCL diagnosed over a period of 20 years indicating the rarity of the condition. There was marked male predominance with an M:E ratio of 11:1. The ages ranged from 38 to 75 years of age with a median age of 50 years. The most common clinical presentation included symptoms of anemia—weakness and easy fatigability, followed by fever. The rest of the presenting features are shown in Table 1. There was one patient who was asymptomatic, massive splenomegaly was detected during a Master Health check up and later on diagnosed as HCL.
Table 1.
Clinical presentation (n = 12)
| Symptoms | Number (%) | Examination findings | Number (%) |
|---|---|---|---|
| Weakness/easy fatigability/breathlessness | 7 (58.3) | Pallor | 7 (58.3) |
| Fever | 4 (33.3) | Splenomegaly | 9/11 (81.8) |
| Loss of weight/appetite | 3 (25.0) | Hepatomegaly | 6 (50) |
| Abdominal mass | 2 (16.6) | Lymphadenopathy, peripheral | 1 (8.3) |
| Loose motions | 2 (16.6) | Abdominal lymphadenopathy (on imaging) | 1 (8.3) |
| Bleeding | 2 (16.6) | ||
| Asymptomatic | 1 (8.3) |
On examination, seven patients (58.3 %) had pallor. There was palpable splenomegaly in nine cases and it was not enlarged in two cases. There was one patient who had a splenectomy 10 years earlier for an enlarged spleen, the exact indication and histopathology was not known. There was hepatomegaly in six patients. Only one patient had palpable peripheral lymphadenopathy and there was another patient where abdominal lymphadenopathy was detected on imageology.
Hematologic Parameters
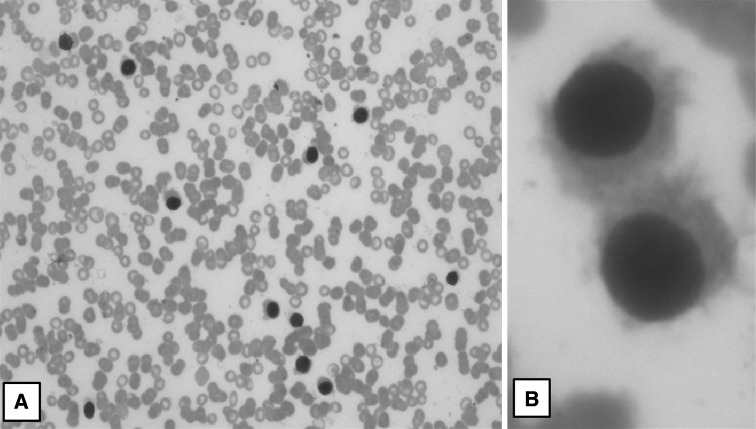
The PB, BM findings and immunophenotypic results are summarized in Table 2. The hemoglobin values ranged from 4.0 to 12.0 gm% with 83.3 % being anemic (<11.0 gm%). The total leucocyte counts ranged from 3,500 to 25,000/cmm, with three patients with leucopenia, six with normal counts and three with leucocytosis (13700, 24700 and 25000/cmm). One of the patients with leucocytosis had a splenectomy 10 years prior to this admission, the details of which are not known. The platelet counts ranged from 25,000/cmm to 1.7 lakh/cmm (Severe thrombocytopenia—1, moderate—3, mild—7 and 1 with normal counts). There were two patients with only anemia, seven with bicytopenia and three with pancytopenia. Atypical lymphoid cells with circumferential hairy projections were seen in all 12 cases. TRAP stain was performed on the peripheral smear in 11 cases (could not be done in 1 case), of which 9 showed strong positivity, 1 with weak positivity and 2 were negative. The ‘hairy cells’ and TRAP results are depicted in Figs. 1 and 2 respectively.
Table 2.
Hematologic parameters
| Blood | Number of patients | Bone marrow study | Number of patients | Immunophenotyping (done in six patients) | Results (pos/number done) |
|---|---|---|---|---|---|
| Hemoglobin | BM aspirate-particulate | 2 | CD 11c | 6/6 | |
| <5.0 gm% | 1 | Dry/scant | 10 | CD 103 | 5/6 |
| 5–10 gm% | 6 | Imprints-small lymphoid cells | 12 | CD 25 | 4/6 |
| 10–13.0 gm% | 5 | Trephine biopsy | CD 19 | 6/6 | |
| Total count (/cmm) | Diffuse | 3 | CD 20 | 6/6 | |
| <4,000 | 3 | Patchy/interstitial | 8 | CD 79a | 1/1 |
| 4,000–11,000 | 6 | Hypocellular, tiny foci | 1 | CD 3 | Neg |
| >11,000 | 3 | Reticulin | CD 5 | Neg | |
| Platelet count (/cmm) | Grade 2 condensation | 5 | CD 38 | 1/1 | |
| <30,000 | 1 | Grade 3 condensation | 7 | CD 10 | Neg |
| 30,000–1.0 lakh | 3 | ZAP 70 | 1/1 | ||
| 1.0–1.5 | 7 | ||||
| >1.5 | 1 | ||||
| Only anemia | 2 | ||||
| Bicytopenia | 7 | ||||
| Pancytopenia | 3 | ||||
| Hairy cells on PS | 12 | ||||
| TRAP strong positivity | 8/11 (81.8 %) | ||||
| Weak positive | 1 | ||||
Fig. 1.
Peripheral smear: a lymphocytic prominence with hairy cells (Giemsa ×40); b hairy cells with round nuclei, inconspicuous nucleoli, and circumferential hairy projections (Giemsa ×1,000)
Fig. 2.
a TRAP stain: peripheral smear; hairy cells showing acid phosphatase positivity (acid phosphatase: ×100), b diffuse and intense positivity in hairy cells after treatment with tartrate (TRAP: ×100)
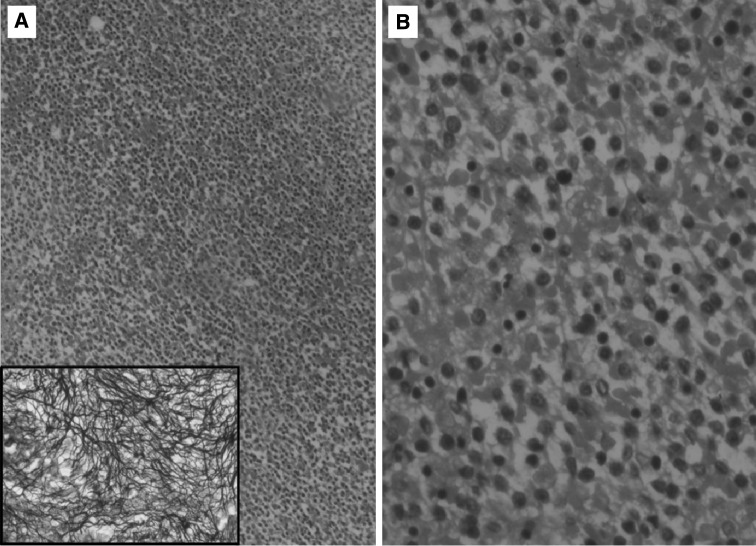
The BM aspirate was dry or only scant particulate in ten cases, with two cases having particulate aspirates. The imprints were of varying cellularity with the smears showing the prominence of small lymphoid cells. The ‘hairy projections’ were not prominent on the imprints. The trephine sections showed varying morphology, the biopsy showing diffuse involvement in three cases and patchy or interstitial in eight cases. The last case was initially reported as hypoplastic with small foci of fibrosis. It was only after a month when the ‘hairy cells’ became obvious on the PS, that the biopsy was reviewed and the lymphoid infiltrate in the small foci of fibrosis were noted. Fig. 3 shows the diffuse involvement of the marrow along pericellular reticulin condensation.
Fig. 3.
a Bone marrow biopsy: increased cellularity with interstitial expansion by monomorphic lymphoid cells (H&E ×40); Inset pericellular fibrosis highlighted by reticulin stain (reticulin stain ×400); b lymphoid cells have round to indented nuclei moderate cytoplasm and distinct cell borders (H&E ×100)
After the initial suspicion of HCL on morphologic grounds, samples of six patients were subjected to immunophenotyping by flow cytometry. Due to financial constraints, the other six patients could not have immunophenotyping done. In these six patients, the diagnosis was made mainly on the basis of classical ‘hairy cells’ in blood, BM morphology in correlation with the clinical picture and TRAP stain results. Of the six patients with immunophenotypic reports, four were diagnosed as HCL and 2 as HCL-variant (HCL-v). These two showed positivity for pan B cell markers, CD 11c, and were negative for CD 25 and one was positive, one negative for CD103. They also showed leucocytosis and particulate marrow aspirates. One was TRAP negative and one was weakly positive.
Treatment History
The treatment history and follow-up details are given in Table 3. Three of the patients diagnosed as HCL did well with Cladribine though they were complications like febrile neutropenia, alveolitis and initial persistence of disease. One patient died of an intracranial bleed during therapy. One of the patients diagnosed as HCL on morphology alone (diagnosis in 2001 when immunophenotyping was not easily available) was earlier put on Interferon. He did well initially, however relapsed after 9 years, was then given Cladribine and finally went into complete remission. There was one case of HCL-v which did poorly with Cladribine followed by Rituximab. The other six patients did not receive any therapy due to financial constraints.
Table 3.
Treatment and follow-up details (available in 6 cases)
| S No | Diagnosis | Treatment | Response | Complications during therapy | Follow-up (FU) |
|---|---|---|---|---|---|
| 1 | HCL by immunophenotyping | Cladribine | Complete remission | Febrile neutropenia | Complete remission on FU—2 years |
| 2 | HCL by immunophenotyping | Cladribine | Residual disease in BM, again put on cladribine | Alveolitis | Complete remission on FU—3 years |
| 3 | HCL by immunophenotyping | Cladribine | Complete remission | Febrile neutropenia | Complete remission on FU—1½ years |
| 4 | HCL by immunophenotyping | Cladribine | Died during treatment | Intracranial bleed | – |
| 5 | HCL by morphology, TRAP | Interferon | Relapsed after 9 years, started on cladribine | Miliary tuberculosis | Complete remission on FU—2 years |
| 6 | HCL-v by immunophenotyping | Cladribine | No response, rituximab added | Disease persisted | Lost to FU after 1½ years |
Discussion
HCL was first described in 1923 by Ewald who termed it ‘leukamische reticuloendotheliose’ [4]. It was later on characterized in 1958 by Bouroncle et al. [5] as a unique entity with distinct histopathologic and clinical characteristics. Schrek and Donnelly [6] coined the term ‘hairy cell’ in 1966 based on the microscopic observation of their hair-like cytoplasmic projections. The proposed pathomechanism for hairy cell morphology is due to overexpression and constitutive activation of members of Rho family of small GTPases and up-regulation of growth arrest specific molecules [7]. HCL is frequently misdiagnosed. It is essential to make an accurate diagnosis because it is one of the most successfully treated leukemias. Thus, recognizing its features and considering it in the differential diagnosis under the right clinical setting is crucial.
HCL makes up 2 % of all leukemias. A variant form (HCL-v) makes up 10–20 % of cases. In the present study there were only 12 cases over a period of 20 years indicating its rarity. Of these, two were diagnosed as HCL-v accounting for 16.6 %.
The clinical and hematologic features were compared to previous Indian case series [1–3] and depicted in Table 4.
Table 4.
Comparison between present study and other case series from India
| Clinical and laboratory features | Medhi et al. [3] (n = 23) |
Chatterjee et al. [2] (n = 15) |
Galani et al. [1] (n = 28) |
Present study (n = 12) |
|---|---|---|---|---|
| Median age (years) | 48.5 | 47 | 47 | 50 |
| M:F | 3.6:1 | 2:1 | 6:1 | 11:1 |
| Weakness and fatigue (%) | 82.6 | 60 | 80 | 58.3 |
| Pancytopenia, bicytopenia (%) | 87 | 60 | 54 | 83.3 |
| Thrombocytopenia (<1 lakh/cmm) (%) | NA | 60 | 77 | 33.3 |
| Leucocytosis (%) | NA | 1 | 8 (HCL-v not included) | 25 |
| Splenomegaly (%) | 95.6 | 100 | 96 | 81.8, 1 prior splenectomy |
| Hepatomegaly (%) | 65.2 | 53 | 28 | 50 |
| Lymphadenopathy (%) | 17.3 | 13 | 24 | 16.6 |
| TRAP positivity (%) | NA | 93 | 100 (n = 8) | 81.8 (n = 11) |
Hairy cell leukemia is a disease of middle-aged and elderly. Majority were males (11/12) which is similar to previous studies [8, 9]. The median age in the present study is 50 years which is similar to other studies [1–4, 7, 9].
At the time of initial diagnosis splenomegaly was present in 81.8 % (9/11) of patients which is slightly lower than other Indian studies [1–3].One of the patients (M/38 years) had a splenectomy done 10 years prior to admission, the indications, hematologic findings at that time and histopathology of spleen were not known. Incidently, he presented with leucocytosis and was diagnosed as classical HCL by flow cytometry. Hepatomegaly was seen in 50 % patients which is similar to few studies [2, 3]. Significant lymphadenopathy was noted in 16.6 % cases (one with palpable peripheral nodes, one with lymphadenopathy detected by imageology. This is similar to previous studies [1–4]. In fact, an article by Goodman et al. [4] says that though palpable lymphadenopathy is uncommon, significant internal lymphadenopathy can be detected by imageology in 1/3rd patients of HCL.
Majority of the cases presented with varying degrees of cytopenias, seven showing bicytopenias with three showing pancytopenia. There were three cases (25 %) showing significant leucocytosis. This is higher than most reported series. Of these, two were diagnosed as HCL-v, as described earlier. The 3rd was a case of classical HCL, who had an earlier splenectomy. We are not sure whether the prior splenectomy had anything to do with this or this was one of those rare things that occur [10]. The patient however responded well to Cladribine. All the cases showed lymphocyte prominence with hairy cells (12/12) on peripheral smear.
As most of the patients were elderly and presented with cytopenias, the differential diagnosis considered were:
Aplastic anaemia, marrow involvement by lymphomas, myelofibrosis, hypersplenism. However, the presence of hairy cells gave a morphological clue to the diagnosis of HCL in all cases. Apart from HCL, such cells may be seen in HCL-v and SLVL (Splenic lymphoma with villous lymphocytes).
The BM aspirates along with biopsy and imprint cytology aid in the diagnosis. As expected, the BM aspirates were dry or scant particulate in ten cases (diagnosed as HCL). It was particulate in two cases, which were diagnosed in HCL-v. The aspirates are dry in HCL because the hairy cells secrete basic fibroblast growth factor (bFGF) leading to reticulin fibrosis. Transforming growth factor β1 (TGF β1) also play a role [7]. In the six cases where immunophenotyping was not done, the diagnosis was made correlating the morphology-classic ‘hairy cells’, with the clinical features and positive TRAP (Tartrate-resistant acid phosphatase) stain. The TRAP stain was strongly positive in eight cases, weakly in one (diagnosed later as HCL-v), negative in two cases and not done in one case, giving a positivity rate of 81.8 %. The two cases which were negative were positive for HCL by flow cytometry. TRAP is sensitive in diagnosing cases and a useful cytochemical stain when immunophenotyping is not available. Although, current diagnostic laboratory methods rely less on TRAP, and more on immunophenotyping for greater diagnostic accuracy, it is sometimes difficult to obtain material for flow cytometry as most of the patients present with PB leucopenias and dry tap on aspiration. In such circumstances and places where immunophenotyping facilities are not available, TRAP is useful in arriving at the diagnosis which can be confirmed by IHC with B cell markers on trephine biopsy. However, it should be remembered that rare cases of HCL can be TRAP negative; also it can be positive in few neoplastic diseases other than HCL [11]. We had one case (not included in the study), which was TRAP positive and later diagnosed as SLVL.
Immunophenotype
The diagnosis is best made by integrating clinical, morphologic features with immunophenotyping by flow cytometry. The classic immunophenotypic profile includes positivity for sIg, CD 19, CD 20, CD 22, CD 25, CD 103, CD 11c and negativity for CD 3, CD 5. The newer markers CD 123 and annexin A1 are useful and annexin A1 has been used to differentiate HCL from other B cell lymphoproliferative diseases. These are yet not easily available in India. Of the six patients with immunophenotypic results, four were positive for B cell markers as well as CD 11c, CD 25 and CD 103 and were diagnosed as HCL.
Two of the cases were diagnosed as HCL-v on the basis of immunophenotypic profile, leucocytosis, particulate aspirates. Apart from classic HCL, HCL-v and Japanese variant have been described [12–14]. HCL-v is now thought to be biologically unrelated to HCL and the Japanese variant is not universally recognized [6, 12]. Resistance to cladribine therapy was an important problem with HCL-V. One of the patients with HCL-v did very poorly with Cladribine and the other refused therapy on being explained the prognosis.
Though HCL is an indolent disease, therapy is given if the patient is symptomatic. The therapy for HCL has been evolving. Prior to the advent of nucleoside analogues, interferon and splenectomy were the most effective therapies.
Splenectomy was one of the 1st used therapies; however, it is rarely used today. Around 40–60 % would have normalization of blood counts, post-splenectomy. Response to therapy was not uniform. One of the patients in this series had a splenectomy prior to diagnosis and presented with leucocytosis. We are not sure if the splenectomy or some other cause was responsible for this. One of the patients diagnosed earlier was treated with Interferon, did well at first and then relapsed after 9 years. He was then put on Cladribine and did well.
Cladribine is today considered the therapy of choice in HCL [15]. Five patients received Cladribine, as soon as they were diagnosed. Of these three responded well, and went into remission. One had an intracranial bleed during treatment and expired. The HCL-v, who received Cladribine, did not do well from the beginning.
Conclusion
HCL is a unique chronic indolent lymphoproliferative disorder of B-lymphocytes. It can mimic or coexist with other clonal hematologic disorders. The patients are generally middle-aged to elderly males presenting with cytopenias, splenomegaly, and typical ‘hairy cells’ in the periphery with dry marrow aspirates. Correlation between morphology, clinical features, TRAP stain results and immunophenotyping is essential for diagnosis. Response to Cladribine with prolonged remission rates is seen classic HCL, in contrast to the poor response seen with the variant type.
Awareness, suspicion and correlation between morphology, clinical features and immunophenotyping are essential for diagnosis.
Contributor Information
Kaumudi Konkay, Email: kaumudi_9@yahoo.com.
Megha S. Uppin, Email: megha_harke@yahoo.co.in
Shantveer G. Uppin, Email: drsuppin@yahoo.co.in
D. Raghunadha Rao, Email: telerama@redifmail.com
CH. Geetha, Email: drgeetha_nims@yahoo.com
T. Roshni Paul, Phone: 9394073281, Email: troshnip@yahoo.co.in.
References
- 1.Galani KS, Subramanian PG, Gadage VS, et al. Clinico-pathological profile of hairy cell leukemia: critical insights gained at a tertiary care cancer hospital. Indian J Pathol Microbiol. 2012;55:61–65. doi: 10.4103/0377-4929.94858. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee T, Panigrahi I, Mahapatra M, et al. Hairy cell leukemia: clinical, pathological and ultrastructural findings in Asian–Indians. Indian J Cancer. 2008;45:41–44. doi: 10.4103/0019-509X.41768. [DOI] [PubMed] [Google Scholar]
- 3.Medhi K, Kumar L, Sharma A, et al. Hairy cell leukemia: experience at a Tertiary Cancer Centre in Northern India. Indian J Med Pediatr Oncol. 2006;27:8–14. [Google Scholar]
- 4.Goodman GR, Bethel KJ, Saven A. Hairy cell leukemia: an update. Curr Opin Hematol. 2003;10:258–266. doi: 10.1097/00062752-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bouroncle BA, Wiseman BK, Doan CA. Leukemic reticuloendotheliosis. Blood. 1958;13:609–630. doi: 10.1182/blood-2016-01-696179. [DOI] [PubMed] [Google Scholar]
- 6.Schrek R, Donnelly WJ. Hairy cells in blood in lymphoreticular neoplastic disease and flagellated cells of normal lymph nodes. Blood. 1966;27:199–211. [PubMed] [Google Scholar]
- 7.Foucar K, Falini B, Catovsky D, Stein H, et al. Hairy cell leukemia. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of hematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 188–193. [Google Scholar]
- 8.Flandrin G, Collado S. Is male predominance (4/1) in hairy cell leukaemia related to occupational exposure to ionizing radiation, benzene and other solvents? Br J Haematol. 1987;67:119–120. doi: 10.1111/j.1365-2141.1987.tb02310.x. [DOI] [PubMed] [Google Scholar]
- 9.Polliack A. Hairy cell leukemia: biology, clinical diagnosis, unusual manifestations and associated disorders. Rev Clin Exp Hematol. 2002;6:366–388. doi: 10.1046/j.1468-0734.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 10.Adley BP, Sun X, Shaw JM, Variakojis D. Hairy cell leukemia with marked lymphocytosis. Arch Pathol Lab Med. 2003;12:253–254. doi: 10.5858/2003-127-253-HCLWM. [DOI] [PubMed] [Google Scholar]
- 11.Yam LT, Janckila AJ, Li CY, Lam WK. Cytochemistry of tartrate-resistant acid phosphatase: 15 years’ experience. Leukemia. 1987;1:285–288. [PubMed] [Google Scholar]
- 12.Wu ML, Kwaan HC, Goolsby CL. Atypical hairy cell leukemia. Arch Pathol Lab Med. 2000;124:1710–1713. doi: 10.5858/2000-124-1710-AHCL. [DOI] [PubMed] [Google Scholar]
- 13.Sainati L, Matutes E, Mulligan S, et al. A variant form of hairy cell leukemia resistant to alpha-interferon: clinical and phenotypic characteristics of 17 patients. Blood. 1990;76:157–162. [PubMed] [Google Scholar]
- 14.Blasinska-Morawiec M, Robak T, Krykowski E, et al. Hairy cell leukemia variant treated with 2-chlorodeoxyadenosine: a report of three cases. Leuk Lymphoma. 1997;25:381–385. doi: 10.3109/10428199709114177. [DOI] [PubMed] [Google Scholar]
- 15.Robak T, Blasinska-Morawiec M, Blonski J, et al. 2-Chlorodeoxyadenosine (cladribine) in the treatment of hairy cell leukemia and hairy cell leukemia variant: 7-year experience in Poland. Eur J Haematol. 1999;62:49–56. doi: 10.1111/j.1600-0609.1999.tb01114.x. [DOI] [PubMed] [Google Scholar]