Abstract
Objective:
The aim of the study was to validate the noninvasive resonance Raman spectroscopy (RRS) method in infants in comparison with the high-performance liquid chromatography (HPLC) method, and to evaluate the carotenoid status in preterm infants fed with mother’s milk or formula.
Study Design:
In the first phase of the study, resonance Raman measurements were made on male term infants’ skin and correlated with tissue harvested at the time of circumcision. Each baby’s foreskin was weighed, enzymatically digested, and the total carotenoids were extracted and quantitated by the HPLC. Next, to evaluate the carotenoid status of preterm infants (BW <1500 g), the skin and serum carotenoids in infants fed with either human milk or preterm formula were studied from the start of feedings and every 2 weeks until hospital discharge. Skin carotenoids were measured by RRS and the serum total carotenoids by HPLC.
Results:
Foreskin carotenoid levels measured by RRS correlated with HPLC measurements of total serum carotenoids (R=0.52, P<0.01, n=16). Forty preterm infants were studied for their carotenoid status. Thirty-two infants were fed mother’s milk, whereas 8 were fed a preterm infant formula that was not enriched with carotenoids. The gestation and birth weight of the 2 feeding groups were similar. The infants fed human milk had a higher serum total carotenoid concentration and skin Raman counts than formula-fed infants. The skin Raman counts and total serum carotenoid correlated (R=0.44, P=0.01). The human milk-fed infants’ serum total carotenoid concentrations and Raman values did not change during the study period; however, the formula-fed group’s total serum and skin carotenoid decreased significantly during the study.
Conclusion:
RRS of infant’s skin reliably assesses total carotenoid status noninvasively. Human milk-fed preterm infants have higher serum and skin carotenoids than formula-fed infants suggesting that formula-fed infants may benefit from carotenoid supplementation.
Keywords: carotenoids, formula, human milk, preterm infants, Raman spectroscopy
Carotenoids are fat-soluble, extensively conjugated polyene pigments synthesized exclusively by plants and microorganisms. Adults and children obtain carotenoids from the diet, especially from fruits and vegetables. Newborn infants, however, obtain their carotenoids via the placenta prenatally and then from mother’s milk or commercial formula. There are >600 known carotenoids, but only about 15 are routinely detected in human tissues, serum, and milk (1). The major carotenoids in human serum include β-carotene, lycopene, lutein, α-carotene, β-cryptoxanthin, and zeaxanthin. The various carotenoids are deposited with widely varying concentrations and distributions in many tissues of the body including retina, skin, liver, and fat.
Carotenoids have a variety of functions that may be of importance to human health and development. In adults, they appear to be physiologically important nutrients in preventing breast and lung cancer, heart disease, stroke, arteriosclerosis, and macular degeneration (2). In infants, carotenoids may function as antioxidants and anti-inflammatory mediators (3). They may also play important roles in preventing oxidative stress in chronic lung disorder, necrotizing enterocolitis, sepsis, intraventricularhemorrhage, and retinopathy of prematurity (4). In eye development, carotenoids lutein and zeaxanthin are postnatally concentrated in the macula of the eye and may be protective against harmful short-wave blue light (5) and be important in maturation of the visual system (6).
The standard method to measure an individual’s carotenoid status is to determine the blood levels. This method requires blood sampling, solvent extraction, and then quantitative analysis by high-performance liquid chromatography (HPLC). Resonance Raman spectroscopy (RRS) has been developed tomeasure the total carotenoid levels noninvasively in accessible tissues such as skin and the macula of the eye. The skin method is particularly attractive in infants and children because drawing sufficient blood for analysis may be a challenge. Raman spectroscopy is a laser spectroscopic technique that detects the characteristic vibrational energy of carotenoids in the tissue of interest. The carotenoid’s carbon-carbon single bonds and carbon double bonds each generate a spectrally sharp, resonantly enhanced, Raman scattered intensity when excited in any of the carotenoid’s vibronic absorption transitions in the visible wavelength region. The RRS measured the combined concentration of all or total carotenoids. The method cannot distinguish individual carotenoids that HPLC can. When blue laser light is used, the extremely large resonance enhancement readily permits total carotenoid (sum of lutein, carotene, lycopene, and so on) quantitation even in complex biological tissues. In the present study, all the infants were white; however, skin pigmentation may interfere with the Raman method. To date, the skin resonance Raman method has been used mainly in adults (7,8) and preschool age children (9) where it has been found to be a valid biomarker of fruit and vegetable consumption (8,9) but it has had limited use in neonates. The purpose of the study is to validate the Raman spectroscopy method in infants in comparison with the HPLC method and to evaluate the carotenoid status in preterm infants fed with mother’s milk or formula.
METHODS
To validate the Raman spectroscopy in infants, we collected foreskins from term infant’s circumcisions that were done within first 5 days of life. The excised skin was evaluated by RRS within 6 hours after the circumcision. The skin was then stored in sterile normal saline and the carotenoids determined by HPLC within 2 weeks using previously described method (10,11). Briefly, skin samples were suspended in phosphate buffered saline containing collagenase solution (50mg/mL; Sigma Cat # E-1644). The mixture was then vortex-mixed and incubated at 37°C for 60 minutes. After incubation, protease solution (20 mg/mL; Sigma catalog # 11360) was added, vortex-mixed, and mixture was incubated at 37°C for 30 minutes. Later, sodium dodecyl sulfate dissolved in ethanol was added and vortexed for 60 seconds. Samples were then extracted twice with hexane (2 × 500 μL), and dried down before HPLC injection. All the reagents were also added with antioxidant powder of butylated hydroxytoluene.
To evaluate the carotenoid status of preterm infants, we prospectively studied the skin and serum carotenoids in infants fed either human milk or preterm formula. As per our nursery protocol, all human milk was fortified with human milk fortifier (Similac Human Milk Fortifier). A commercially available premature formula (Similac Special Care) was selected for all formulafed infants. The human milk fortifier in the present study did not contain any carotenoids, whereas the formula had only β-carotene for nonnutritive purposes. An energy density of 24 kcal per oz was used for both human milk and formula. Supplementation of vitamin A, E, and/or carotenoids was not allowed.
Preterm infants with birth weights of 500 to 1500 g and <33 weeks gestation were enrolled. Infant feedings was based on maternal decision. Infants excluded from the study were infants who had serious congenital abnormalities that affected growth, infants with grade III or IV intraventricular hemorrhage, infants who had major surgeries, and infants with confirmed necrotizing enterocolitis. The study started at the initiation of enteral feedings until hospital discharge or 40 weeks postmenstrual age.
Skin carotenoids at the infant’s foot were measured by RRS and the serum total carotenoids by the HPLC method. Serum for total carotenoids was collected by venipuncture at the start of the study and every 2 weeks until discharge. Skin carotenoid was measured with an instrument developed for clinical use in infants using the RRS. Measurement occurred by exposing the sole of the infant’s foot to a 2 mm diameter low power (<9 mwatts) laser (monochromatic blue light, 488 nm) for about 30 seconds at the same time period as the blood draws. The skin carotenoid level was expressed by the measurement of Raman intensity or counts.
Standard statistical methods were used. Regression analyses were used to correlate the total skin carotenoids by the Raman method with the HPLC method. Comparison of the skin and serum carotenoids of the human milk- and formula-fed groups was evaluated using the t tests. Pearson correlations were applied for skin and serum carotenoids. All study procedures were approved by the University of Utah institutional review board and informed consent was obtained from the parents.
RESULTS
Sixteen foreskin specimens were collected from term white newborn infants to validate the skin Raman method on infant tissues. Total skin carotenoids as measured by RRS correlated with HPLC measurements of total carotenoids (R=0.52, P<0.01). Total carotenoids were determined by the sum of lycopene, lutein, crypotoxanthin, zeaxanthin, and carotenes.
Forty preterm infants were enrolled in the second part of the study. Thirty-two infants were fed mother’s milk, whereas 8 were fed a preterm infant formula. The gestation and birth weight of the 2 feeding groups were similar. Infants fed mother’s milk had gestation of 28±3 (mean±SD) weeks and birth weight of 1141±354 g and the formula-fed group had gestation of 29±3 weeks and birth weight of 1159±424 g.
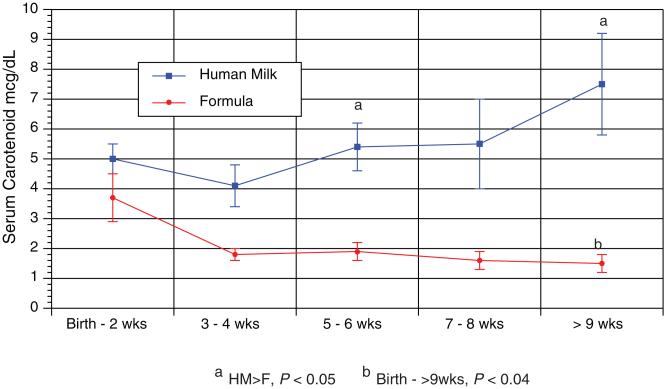
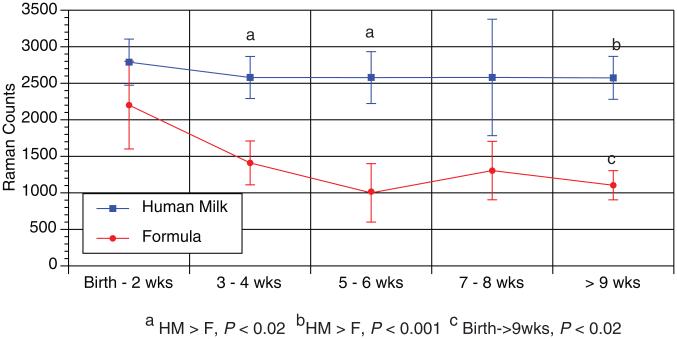
There was a correlation with skin Raman counts and total serum carotenoid across all time points (R=0.44, P=0.01). The human milk-fed infants’ serum total carotenoid concentrations and Raman values were not different during the study; however, the formula-fed group’s total serum and skin carotenoid decreased during the study. The human milk group had higher serum and skin carotenoids than the formula fed group at different time points of the study (Figs. 1 and 2).
FIGURE 1.
Serum carotenoid in relation to the infant’s age. During the study, the formula-fed infants (n=8) had decreasing serum carotenoids frombirth to 9 weeks of age (P<0.04, repeatedmeasures analysis of variance). The human milk-fed infants (n=32) had no change in their serum levels during the study. In the human milk-fed group, the serum carotenoid concentrations were higher than the formula-fed group at certain time points (P<0.05).
FIGURE 2.
Skin carotenoid level in relation to the infant’s age. During the study, the formula-fed infants (n=8) had decreasing skin carotenoids from birth to >9 weeks of age (P<0.02, repeated measures analysis of variance). The human milk-fed infants (n=32) had no change in their skin levels during the study. The skin carotenoid levels were higher in the human milk-fed group compared with the formula-fed group at certain time points (P<0.02).
DISCUSSION
The infants’ foreskin carotenoid measurement by RRS was valid when compared to the HPLC method. This is a consistent finding compared with adult abdominal skin biopsies as measured by RRS (12); however, the foreskin of an infant may present different tissue optics compared with the infant’s heel and the adult skin. We also found a good correlation between skin and serum carotenoids in premature babies. Skin carotenoid levels may accurately indicate carotenoid status since serum levels are influenced by postprandial fluctuations (13).
In our study, carotenoids were measured by HPLC and Raman spectroscopy. HPLC is the standard method in measuring carotenoids for its accuracy and precision (14). Raman spectroscopy is a method of identifying specific molecules that have similar chemical bonds. It cannot identify individual carotenoids. It relies on the scattering of monochromatic light from a laser in the visible or near infrared range. The laser light interacts with carotenoids, resulting in the energy of the laser photons being shift up. Carotenoids have a characteristic “fingerprint” from the vibrations of their long carbon backbone. Using theRRS, carotenoids can be easily measured on the top layer of the epidermis.
Our study showed that preterm infants fed with human milk had consistently higher carotenoid status than formula-fed infants. This finding was likely from the higher carotenoid content in human milk compared with the preterm formula that was not enriched with carotenoids. In mother’s colostrum, carotenoids are 5 times higher than in mature breast milk (15). Hind milk has higher carotenoid concentration compared with mid and fore milk (16); however, carotenoid concentrations decrease during 12 to 41 days of lactation (17,18). This decline of the carotenoids may have resulted from a lower maternal intake of carotenoid-containing foods (19) or prenatal vitamin intake that may contain insufficient amounts of carotenoids. Besides the lower human milk carotenoid content, the feeding volume in preterm infants may be limited and the carotenoid intake restricted. Also, it has been reported that the delivery of milk by tube feedings can decrease the carotenoid amount to the infant (20). Thus, human milk feedings to the preterm infant may require carotenoid supplementations because of the possible low maternal dietary intake of carotenoids, limited feedings, and the loss of carotenoid in the feeding method.
In our study, the preterm formula was not enriched with carotenoids. In one study, only β-carotene and β-cryptoxanthin were detected in some infant formulas (15). Thus, formulas with little carotenoids may contribute to the lower antioxidant activity than human milk (21) and the depletion of the preterm infant carotenoid status in the neonatal intensive care unit (22). This depletion may represent the low carotenoid intake of the infants and the oxidative stress the infant is exposed to. Enriching preterm formula with carotenoids is warranted to match the carotenoid status of the breast-fed infant.
Carotenoids play a vital role in eye development (5,23) and as antioxidants in the infant. Preterm infants may be at risk for oxidative stress because of their immature and deficient antioxidant system. Almost all preterm infants born have low stores of carotenoids because of the in utero transfer of carotenoids occurring mainly during the last trimester. Postnatally, the source of carotenoids is also limited for preterm infants and makes them at risk for oxidative stress. Oxidative stress in the neonatal intensive care unit may play a role in certain diseases of the preterm infant such as chronic lung disorder, necrotizing enterocolitis, sepsis, intraventricular hemorrhage, and retinopathy of prematurity (4,23).
In conclusion, skin carotenoids may be conveniently measured noninvasively by the RRS method. Human milk-fed preterm infants have higher skin and blood carotenoids than formula-fed infants. Carotenoid supplementation of infant formulas should be considered to bring these infants into the range of human milk-fed infants. A prospective study of the potential value of carotenoid supplementation of preventing oxidative stress and various medical complications in preterm infants is warranted.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Nellis H, De Leenheer A. Isocratic naonaqueous reversed phase liquid chromatography of carotenoids. Anal Chem. 1983;55:270–5. [Google Scholar]
- 2.Ribaya-Mercado JD, Blumberg JB. Lutein and zeaxanthin and their potential roles in disease prevention. J Am Coll Nutr. 2004;23:567S–87S. doi: 10.1080/07315724.2004.10719427. [DOI] [PubMed] [Google Scholar]
- 3.Lindeman JH, van Zoeren-Grobben D, Schrijver J, et al. The total free radical trapping ability of cord blood plasma in preterm and term babies. Pediatr Res. 1989;26:20–4. doi: 10.1203/00006450-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Thibeault DW. The precarious antioxidant defenses of the preterm infant. Am J Perinatol. 2000;17:167–81. doi: 10.1055/s-2000-9422. [DOI] [PubMed] [Google Scholar]
- 5.Jewell VC, Northrop-Clewes CA, Tubman R, et al. Nutritional factors and visual function in premature infants. Proc Nutr Soc. 2001;60:171–8. doi: 10.1079/pns200089. [DOI] [PubMed] [Google Scholar]
- 6.Lien E, Hammond B. Nutritional influences on visual development and function. Prog Retinal Eye Research. 2011;1:1–16. doi: 10.1016/j.preteyeres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Ermakov IV, Ermakova M, McClane RW, et al. Resonance Raman detection of carotenoid antioxidants in living human tissue. Optics Letters. 2001;26:1179–81. doi: 10.1364/ol.26.001179. [DOI] [PubMed] [Google Scholar]
- 8.Mayne ST, Cartmel B, Scarmo S, et al. Non invasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010;92:794–800. doi: 10.3945/ajcn.2010.29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarmo S, Heneberg K, Peracchio H, et al. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. Eur J Clin Nutr. 2012;66:555–60. doi: 10.1038/ejcn.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissenberg M, Schaeffler I, Menagem E, et al. Isocratic non-aqueous reversed-phase high-performance liquid chromatographic separation of capsanthin and capsorubin in red peppers (Capsicum annuum L.), paprika and oleoresin. J Chromatogr A. 1997;757:89–95. doi: 10.1016/s0021-9673(96)00665-6. [DOI] [PubMed] [Google Scholar]
- 11.Peng YM, Peng YS, Lin Y. A nonsaponification method for the determination of carotenoids, retinoids, and tocopherols in solid human tissues. Cancer Epidemiol Biomarkers Prev. 1993;2:139–44. [PubMed] [Google Scholar]
- 12.Hata TR, Scholz TA, Ermakov IV, et al. Non-invasive raman spectroscopic detection of carotenoids in human skin. J Invest Dermatol. 2000;115:441–8. doi: 10.1046/j.1523-1747.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 13.Zidichouski JA, Mastaloudis A, Poole SJ, et al. Clinical validation of a noninvasive, Raman spectroscopic method to assess carotenoid nutritional status in humans. J Am Coll Nutr. 2009;28:687–93. doi: 10.1080/07315724.2009.10719802. [DOI] [PubMed] [Google Scholar]
- 14.Talwar D, Ha TK, Cooney J, et al. A routine method for the simultaneous measurement of retinol, alpha-tocopherol and five carotenoids in human plasma by reverse phase HPLC. Clin Chim Acta. 1998;270:85–100. doi: 10.1016/s0009-8981(97)00224-6. [DOI] [PubMed] [Google Scholar]
- 15.Sommerburg O, Meissner K, Nelle M, et al. Carotenoid supply in breastfed and formula-fed neonates. Eur J Pediatr. 2000;159:86–90. doi: 10.1007/pl00013811. [DOI] [PubMed] [Google Scholar]
- 16.Jackson J, Lien E, White S, et al. Major Carotenoids in mature human milk: Longitudinal and diurnal patterns. J Nutr Biochem. 1998;9:2–7. [Google Scholar]
- 17.Jewell VC, Mayes CB, Tubman TR, et al. A comparison of lutein and zeaxanthin concentrations in formula and human milk samples from Northern Ireland mothers. Eur J Clin Nutr. 2004;58:90–7. doi: 10.1038/sj.ejcn.1601753. [DOI] [PubMed] [Google Scholar]
- 18.Gossage CP, Deyhim M, Yamini S, et al. Carotenoid composition of human milk during the first month postpartum and the response to beta-carotene supplementation. Am J Clin Nutr. 2002;76:193–7. doi: 10.1093/ajcn/76.1.193. [DOI] [PubMed] [Google Scholar]
- 19.Canfield LM, Clandinin MT, Davies DP, et al. Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr. 2003;42:133–41. doi: 10.1007/s00394-003-0403-9. [DOI] [PubMed] [Google Scholar]
- 20.Tacken KJ, Vogelsang A, van Lingen RA, et al. Loss of triglycerides and carotenoids in human milk after processing. Arch Dis Child Fetal Neonatal Ed. 2009;94:F447–50. doi: 10.1136/adc.2008.153577. [DOI] [PubMed] [Google Scholar]
- 21.Hanna N, Ahmed K, Anwar M, et al. Effect of storage on breast milk antioxidant activity. Arch Dis Child Fetal Neonatal Ed. 2004;89:F518–20. doi: 10.1136/adc.2004.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Zoeren-Grobben D, Kikkeschei L, van Lingen RA, et al. 2004;56:508. [Google Scholar]
- 23.Rubin L, Chan G, Barrett-Reiss B, et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol. 2012;32:418–24. doi: 10.1038/jp.2011.87. [DOI] [PubMed] [Google Scholar]