Abstract
Mutations in AChR subunits, expressed as pentamers in neuromuscular junctions (NMJs), cause various types of congenital myasthenic syndromes. In AChR pentamers, the adult ε subunit gradually replaces the embryonic γ subunit as the animal develops. Because of this switch in subunit composition, mutations in specific subunits result in synaptic phenotypes that change with developmental age. However, a mutation in any AChR subunit is considered to affect the NMJs of all muscle fibers equally. Here, we report a zebrafish mutant of the AChR δ subunit that exhibits two distinct NMJ phenotypes specific to two muscle fiber types: slow or fast. Homozygous fish harboring a point mutation in the δ subunit form functional AChRs in slow muscles, whereas receptors in fast muscles are nonfunctional. To test the hypothesis that different subunit compositions in slow and fast muscles underlie distinct phenotypes, we examined the presence of ε/γ subunits in NMJs using specific antibodies. Both wild-type and mutant larvae lacked ε/γ subunits in slow muscle synapses. These findings in zebrafish suggest that some mutations in human congenital myasthenic syndromes may affect slow and fast muscle fibers differently.
Keywords: acetylcholine receptors, neuromuscular diseases, zebrafish
Introduction
Congenital myasthenic syndrome (CMS) is a neuromuscular disorder caused by mutations of multiple genes involved in the synapse formation/function in the neuromuscular junction (NMJ), including agrin (Huzé et al., 2009), ache (Ohno et al., 2001), rapsyn (Ohno et al., 2002), musk (Chevessier et al., 2004), and chrn (Engel et al., 2012). Symptoms of CMS include severe muscle weakness and fatigue. Transmission of signal at these synapses is performed by AChRs. AChRs are pentamers, composed of α12β1δγ or α12β1δε, depending on the developmental age. Embryonic γ subunits are replaced by adult ε subunits as the animal develops (Mishina et al., 1986; Walogorsky et al., 2012b). Causal mutations of myasthenic syndromes have been reported in all genes encoding AChR subunit (Engel et al., 2003). Mutations in the γ subunit lead to phenotypes that change with development (Robinson et al., 2013). In addition, we previously showed that a single mutation in the α1 subunit of zebrafish shows effects on the synaptic current that change with development. As the γ subunit is replaced by ε, the synaptic phenotype is alleviated and the swimming of larvae recovers (Walogorsky et al., 2012a). However, mutations of AChR subunits are expected to affect synapses of both slow and fast muscle fibers equally.
Zebrafish have NMJs comparable with those of mammals. They are nicotinic, and postsynaptic AChRs are composed of subunits homologous to mammalian counterparts. Subunits corresponding to mammalian α1, β1, γ, δ, and ε express and assemble in zebrafish. Some genes in zebrafish are duplicated because of the genome-wide duplication specific to teleosts (Meyer and Van de Peer, 2005). Among muscle-type AChR subunit genes in zebrafish, only β1 has two duplicated copies, β1a and β1b. However, only β1b is functional (Papke et al., 2012). Henceforth in this paper, we refer to α1 as α and β1b as β for simplicity.
The skeletal system in larval zebrafish trunks contains slow and fast muscle fibers: two muscle types that are also found in mammalian skeletal systems. Whereas slow and fast muscle fibers in mammals are intermingled and can be distinguished only by using histological techniques, such as ATPase staining (Brooke and Kaiser, 1970) or anti-myosin heavy chain antibody (Weiss et al., 1999), slow and fast muscle fibers of teleosts are spatially segregated and can easily be distinguished by their location and the orientation relative to the body axis (Bone, 1978; Luna and Brehm, 2006). The segregation and anatomical distinction of muscle fibers in zebrafish provide a unique opportunity to study the difference between these two types of muscle fibers, by analyzing the NMJs in preidentified types of muscle fibers.
Here, we isolated a novel mutant of the zebrafish AChR that expresses functional AChRs only in slow muscles. By analyzing this mutant, we showed that a single δ subunit mutation has different effects on AChRs of slow muscle and fast muscle fibers.
Materials and Methods
Fish lines.
Zebrafish colonies were maintained following animal protocols at the National Institute on Alcohol Abuse and Alcoholism and the Oregon Health & Science University. The love sofa mutant was generated by ENU mutagenesis by Dr. Alex Nechiporuk, Oregon Health and Science University, Portland, OR (Drerup and Nechiporuk, 2013) and was kindly provided. The allele sop tj19d was used in the study (Granato et al., 1996). Mutants were crossed with the TAB strain (RRID:ZIRC_ZL1438) and maintained as male or female heterozygotes. Embryonic lethal homozygous larvae of sofa potato or love sofa were obtained by crossing male and female heterozygotes and used for experiments before their sex was determined.
Swimming analysis.
High-speed image capturing of larval zebrafish escape response was performed with Photron camera at 1000 frames/s (Epley et al., 2008). Captured images were saved as JPEG files and processed with Photoshop (Adobe System).
Clones and expression.
Constructs for expression of wild-type AChR δ subunit were previously described (Ono et al., 2004). It has a muscle-specific α-actin promoter, and the expression was restricted to muscle cells. A point mutation for L332P was introduced to the clone with the QuikChange Lightening Site-Directed Mutagenesis Kit (Agilent Technologies). The construct was later confirmed to contain no other mutation than L332P by sequencing.
Electrophysiology.
Miniature endplate current (mEPC) recordings from slow and fast muscles of zebrafish larvae were performed as previously described, with some modifications (Ono et al., 2001). The pipette solution contained Lucifer yellow (Sigma-Aldrich) at 1 mg/ml. To minimize the movement of muscle fibers during the recording, an L-type calcium channel blocker, nifedipine (Sigma-Aldrich), was used instead of osmolality shock with formamide (Ono et al., 2001). It was shown recently that synaptic transmission of zebrafish NMJ depends on the P/Q type calcium channel (Wen et al., 2013), and 10 μm nifedipine indeed did not inhibit mEPCs. Calcium channels in the excitation–contraction coupling were blocked by nifedipine, and stable recordings were possible. 100 nm TTX was added to the bath solution to block the spontaneous firing of motor neurons (Won et al., 2012). mEPC events were analyzed in MiniAnalysis (Synaptosoft). Events with slow rise and slow decay, which are reflections of synaptic events in neighboring cells, were excluded from analysis (Luna et al., 2004). For application of ACh, a glass electrode (opening ∼30 μm; filled with bath solution containing 30 μm ACh and Lucifer yellow) was placed near the voltage-clamped muscle cell and positive pressure was applied by Picospritzer II (Parker Hannifin; 30 ms, 1 psi).
Immunohistochemistry.
Immunohistochemistry methods, including information on antibodies, were previously described (Ikenaga et al., 2011; Park et al., 2012). F59 antibody (Santa Cruz Biotechnology, catalog #sc-32732 RRID:AB_670118) was used as a slow muscle marker, and nerve terminals were labeled with SV-2 antibody (Developmental Studies Hybridoma Bank, catalog #sv2 RRID:AB_528480). For mAB35 staining, which recognizes an epitope in the AChR α subunit, the incubation period for the primary antibody was 48 h (Sigma-Aldrich, catalog #M217 RRID:AB_260473). A polyclonal antibody was raised against an epitope of NLISLNEKEETLTT, which is conserved between ε and γ subunits (GenScript). The specificity of the antibody was tested on the ε knock-out fish, which was generated using Transcription Activator-Like Effector Nuclease and will be reported elsewhere. The antibody staining was abolished at NMJs of the ε knock-out fish (data not shown). α-BTX staining was performed as described previously (Ono et al., 2001). All images were taken on the Zeiss 510 Meta Confocal microscope (Carl Zeiss Microimaging) with 40× C-Apo objective (NA 1.2) and analyzed in Photoshop (Adobe System).
Results
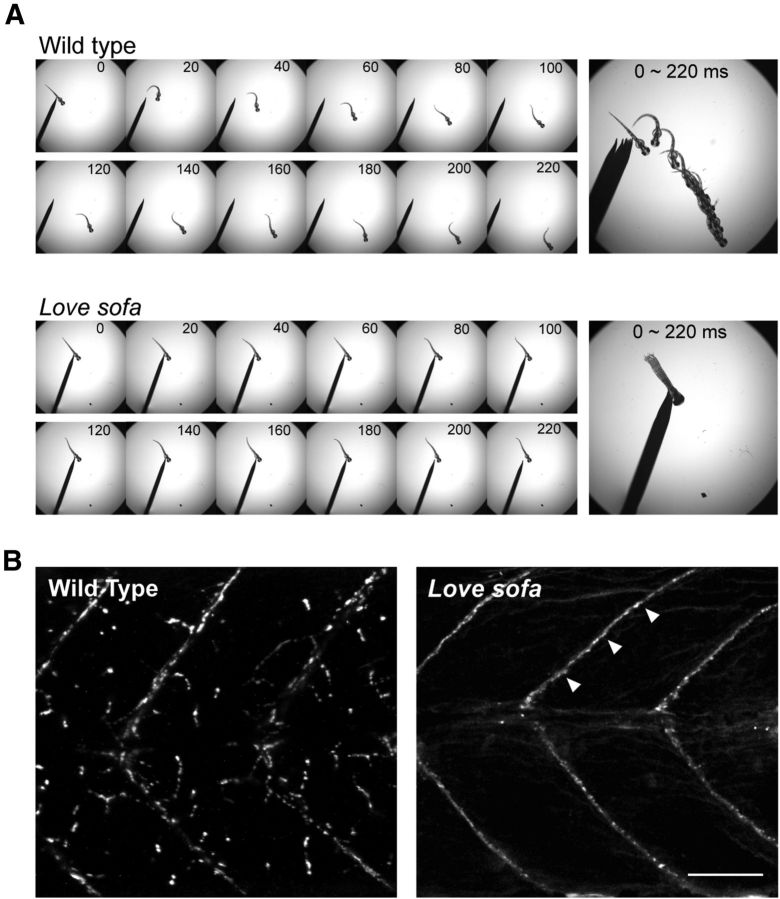
Through ENU mutagenesis, a new locomotion mutant was isolated. A complementation analysis with known locomotion mutants was performed, and the mutation failed to complement the sofa potato mutant, suggesting that the two mutants have a common affected gene. The sofa potato mutant has a missense mutation, L28P, in the AChR δ subunit (Ono et al., 2004). As a result of the L28P mutation, AChR pentamers do not form on the plasma membrane, and subunits are retained in the endoplasmic reticulum (Park et al., 2012). Because of the genetic commonality to sofa potato, we tentatively named the new mutant love sofa. Unlike the sofa potato mutant, which remains paralyzed until its death ∼6–8 d post fertilization (dpf), the love sofa mutant can move, although its tail beat and propulsion are weak (Fig. 1A).
Figure 1.
Phenotypes of the love sofa mutant. A, Time lapse images of a wild-type larva (top) and a love sofa mutant larva (bottom) at 2 dpf. Escape response was elicited by a gentle touch on the tail. Images at 0–220 ms after the start of the movement are shown with 20 ms intervals. B, Trunk regions of a wild-type larva (left) and a love sofa mutant larva (right) were stained with α-BTX conjugated with Alexa-555. The love sofa mutant lacks staining at distributed synapses, and only myoseptal synapses (arrowheads) are visualized. Scale bar, 50 μm.
When stained with α-BTX, the love sofa mutant displayed a unique pattern of AChR distribution (Fig. 1B). In 3 dpf wild-type larvae, AChR clusters are observed at the edge of muscle cells and in the central regions of muscle cells as round, punctate spots (Fig. 1B). The former are called “myoseptal synapses” and the latter “distributed synapses” in previous studies (Lefebvre et al., 2007). The myoseptal synapses seem to be the only existent synapse in the love sofa mutant (arrowheads), and AChR clusterings at distributed synapses, in contrast, are almost completely absent. Therefore, synaptic transmission presumably occurs only at myoseptal synapses, which causes weak muscle contraction and compromised swimming. This pattern of α-BTX staining is very different from that of sofa potato, which completely lacked AChRs on muscle cell surfaces (Ono et al., 2001).
We sequenced exons of the δ subunit gene in the love sofa mutant. We identified a putative missense mutation at L332, changing leucine to proline. The 332nd amino acid is either leucine (L), isoleucine (I), or phenylalanine (F) in the δ subunit of other species (Fig. 2A). The L332P mutation is located in the M3-M4 cytoplasmic loop, close to the M3 transmembrane region, and segregates strongly with the phenotype (Fig. 2A). It is therefore presumed causal to the phenotype.
Figure 2.
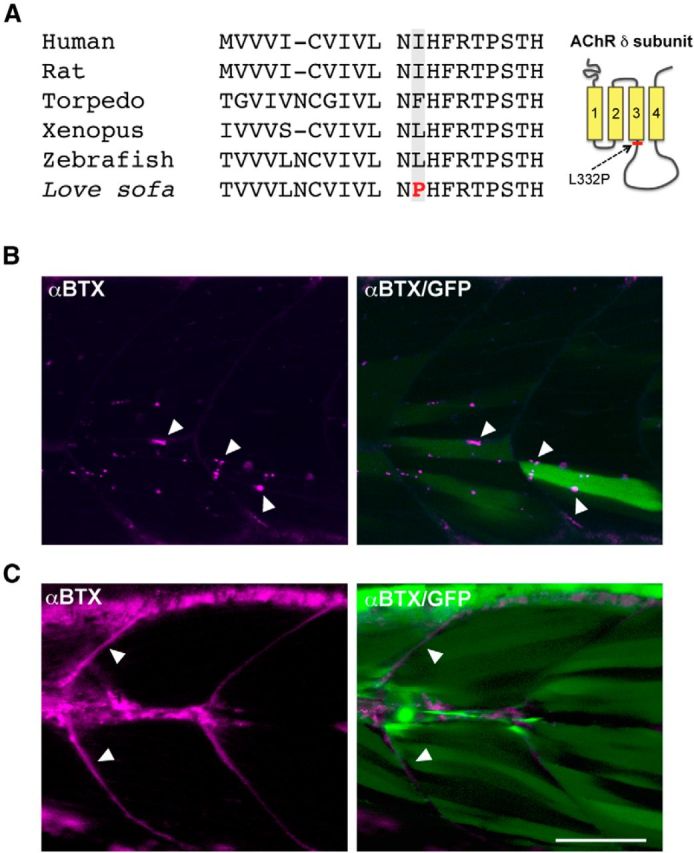
Genetic identification of the love sofa mutation. A, Alignment of amino acid sequences of AChR δ subunit from human, rat, torpedo, Xenopus, wild-type zebrafish, and the love sofa mutant. The putative mutation of love sofa is located at the shadowed amino acid. In wild-type zebrafish, the amino acid is leucine, whereas in other species isoleucine or phenylalanine is found. In love sofa, it is changed to proline. The location of the L332P mutation in the δ subunit protein is shown in the right panel. B, The wild-type δ subunit was expressed in mosaic fashion by injecting the gene construct into the love sofa mutant at one cell stage. Muscle fibers expressing the transgene, as marked by the cytoplasmic GFP (green), displayed distributed synapses (arrowheads) as marked by α-BTX (magenta). C, When the δ subunit harboring the L332P mutation was expressed in the sofa potato mutant, α-BTX staining visualized myoseptal synapses (arrowheads) but failed to exhibit distributed synapses. Magenta represents α-BTX; green represents cytoplasmic GFP. Scale bar, 50 μm.
To confirm that the δ subunit is the mutated gene, we performed a rescue experiment. Wild-type δ subunit was expressed in the love sofa mutant stochastically by injecting the DNA construct into fertilized eggs. After 3 d of incubation, some muscle cells in the developing larvae expressed wild-type δ subunits resulting from the transgene, as indicated by the cytoplasmic expression of GFP (green), and these cells formed distributed synapses (Fig. 2B, arrowheads). These data matched the complementation study and confirmed that the δ subunit gene is the responsible gene in the love sofa mutant.
To confirm that the L332P mutation is the causal mutation, we introduced a δ subunit harboring the L332P mutation (δL332P) into sofa potato mutants and examined whether we could phenocopy the love sofa mutant. Muscle cells without the δL332P transgene are genetically sofa potato, expressed no AChRs, and displayed no signal with α-BTX staining (Ono et al., 2004). In contrast, muscle cells expressing the δL332P transgene, as indicated by the cytoplasmic GFP (green), showed α-BTX-positive signals (magenta), but only along muscle cell edges (arrowheads), suggesting that these cells expressed only myoseptal synapses (Fig. 2C). Therefore, we propose the L332P mutation in the δ subunit is the causal mutation in love sofa.
Myoseptal synapses are found only in the superficial region of the trunk in zebrafish, whereas distributed synapses are found in deeper layers of muscle cells. This raises the possibility that myoseptal synapses are specific to slow muscles because slow muscle fibers form a single, superficial layer at 3 dpf (Devoto et al., 1996). To test this possibility, we examined α-BTX staining in wild-type larvae counterstained with the F59 antibody, which specifically labels slow muscle fibers (Fig. 3A) (Devoto et al., 1996; Bryson-Richardson et al., 2005). In muscle cells positive for F59 signals, myoseptal signals of α-BTX were observed (Fig. 3B). In deeper muscle cells negative for F59 signals, α-BTX showed a pattern of distributed synapses (Fig. 3C). However, at the interface between slow muscles and fast muscles, distributed synapses were observed, which we could not assign to either of the two muscle fiber types. We therefore expressed wild-type δ subunit in the sofa potato mutant. The stochastic nature of the wild-type δ subunit expression allowed the observation of a single slow muscle fiber, whereas neighboring muscle cells were genetically sofa potato and completely lacked surface AChR expression. In slow muscle fibers expressing the wild-type δ subunit, only myoseptal synapses were observed (Fig. 3D). We conclude from these experiments that slow muscles have only myoseptal synapses and fast muscles have only distributed synapses. This suggests that, in the love sofa mutant, only slow muscles may have functional NMJs.
Figure 3.
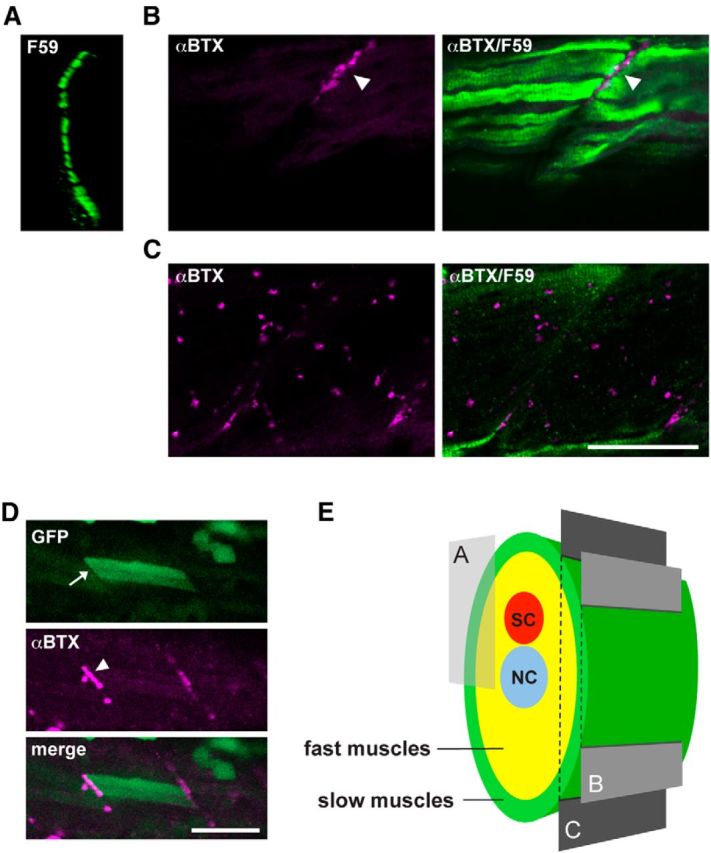
Different distribution of AChRs in slow and fast muscle fibers. A, Cross section of a wild-type trunk stained with the F59 antibody. The displayed region is indicated in E. F59-positive slow muscle cells form a single layer at the most superficial region. B, C, Optical slices of a wild-type larva near the surface (B) or deeper (C). Magenta represents α-BTX staining; green represents the F59 antibody signal. Scale bar, 50 μm. D, Stochastic expression of wild-type δ subunit in the sofa potato mutant. A slow muscle that expresses the transgene displayed cytoplasmic GFP (arrow; green) as well as α-BTX-positive AChR (arrowhead; magenta). Because fast muscles in the neighboring layer lacked the transgene expression, only myoseptal α-BTX staining was observed. Scale bar, 50 μm. E, Schematic cross section of the trunk of a larval zebrafish. Slow muscles are marked with green. Optical sections corresponding to A–C are indicated with a box and planes. SC, Spinal cord; NC, notochord.
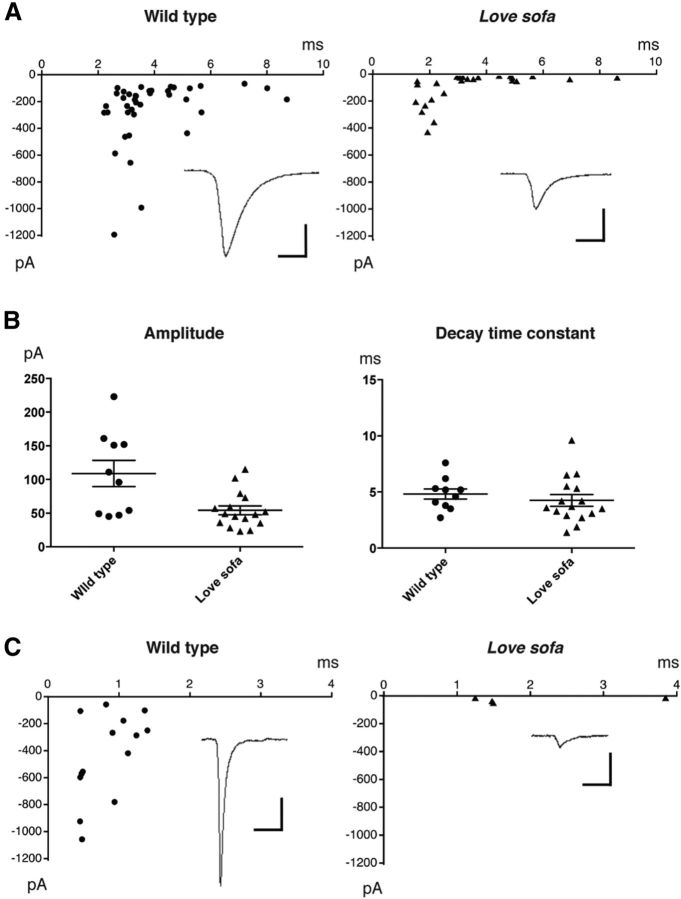
We recorded mEPCs from muscle fibers of the love sofa mutant using the whole-cell configuration patch clamp. The identification of fast or slow muscles was confirmed after patch-clamp recordings by the Lucifer yellow fluorescence contained in the pipette solution. Slow muscle fibers on the body surface are oriented parallel to the body axis, whereas deeper fast muscle fibers are oriented at an angle of ∼30°. In slow muscle cells, robust mEPCs were observed in both love sofa and wild-type larvae. Decay time constant (10%–90%) was fit to a single exponential, and the obtained time constant was plotted against the peak amplitude for each event. Representative plots from a single cell of love sofa and wild-type are shown in Figure 4A. When compared between groups of cells (Fig. 4B), the current amplitude was smaller (unpaired t test; p < 0.01) in love sofa (54.2 ± 6.6 pA; n = 16 cells) compared with wild-type (108 ± 19 pA; n = 10 cells), whereas the decay time constant was not different (p = 0.26) between love sofa (4.25 ± 0.51 ms) and wild-type (4.82 ± 0.45 ms). mEPCs recorded from fast muscles showed a striking difference between love sofa and wild-type. A majority of fast muscle cells in the love sofa mutant failed to exhibit any mEPCs (n = 13). In some fast muscles (n = 4), mEPCs with small amplitudes (< 60 pA) were observed. These events were highly infrequent, and statistical analysis from a single cell was difficult. When events from multiple fast cells were pooled, however, the smaller amplitude was evident compared with wild-type (Fig. 4C).
Figure 4.
AChR functions in slow and fast fibers of the love sofa mutant. A, Decay time constants (x) plotted against current peak amplitudes (y) for mEPCs in a slow muscle of wild-type (left) or love sofa (right). Averaged traces for all mEPCs are shown as insets. Calibration: 100 pA, 5 ms. B, Accumulated data of current amplitudes and decay time constants recorded from slow muscle cells. Each dot represents an average for a single cell. Averages with SEs are shown. C, Decay time constants (x) plotted against current peak amplitudes (y) for mEPCs in fast muscle cells of wild-type (left) and love sofa (right). Averaged traces are shown as insets. Calibration: 100 pA, 5 ms.
The lack of α-BTX staining and the strong suppression of mEPCs in fast muscles raise three possibilities. First, terminals of motor neurons may not contact fast muscles or do not differentiate to release ACh. Second, pentamers may not assemble and express at synapses in fast muscles. Third, pentamers in fast muscles may assemble and reach the synapse, but not bind to α-BTX or ACh. To examine these possibilities, we performed immunohistochemistry, with antibodies reacting with a synaptic vesicle protein (SV2) or the AChR α1 subunit (mAb35) (Tzartos et al., 1981). Anti-SV2 antibody displayed signals at synapses of slow muscles and fast muscles, both in the love sofa mutant and the wild-type larvae. This indicates that nerve terminals of the love sofa mutant reached both slow and fast muscles and are differentiated. Staining with anti-α1 AChR in love sofa was observed in slow muscles (Fig. 5A, dashed box), which was expected from the α-BTX staining. Interestingly, anti-α1 AChR staining (mAb35) exhibited signals also in fast muscles of the love sofa mutant (Fig. 5A, arrowheads) at locations colocalizing with nerve terminal endings (merged panel). This suggests that pentamers assemble and express at synapses in fast muscles of the love sofa mutant, but these pentamers cannot function, rendering the synapses almost silent.
Figure 5.
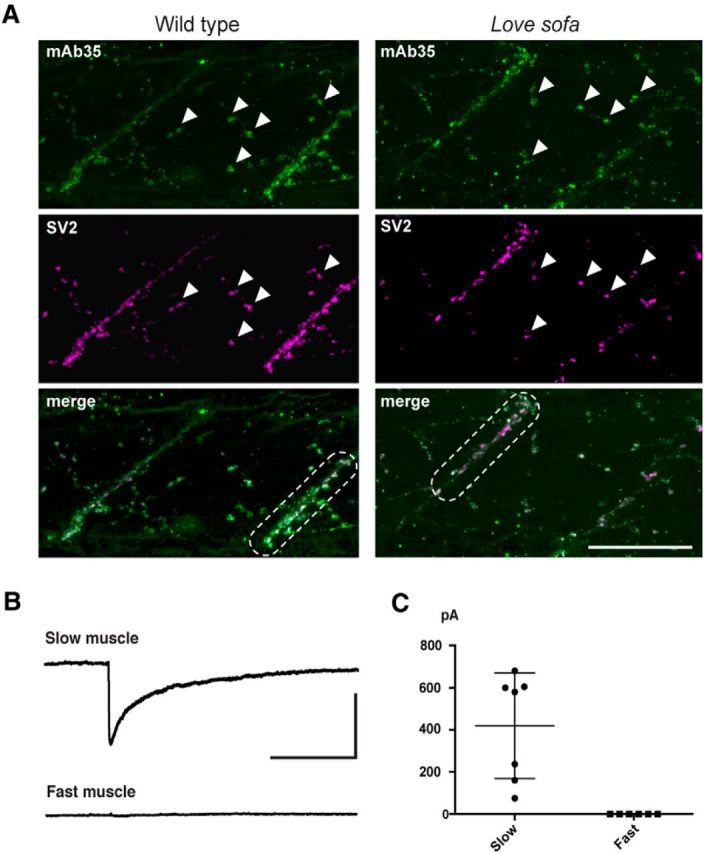
Nonfunctional AChRs in fast muscles of the love sofa mutant. A, Wild-type (left) and love sofa (right) larvae were doubly labeled with mAb35 antibody (green) and SV2 antibody (magenta). Boxes of dashed lines indicate myoseptal synapses in slow muscles. Arrowheads indicate distributed synapses in fast muscles. Scale bar, 50 μm. B, Representative traces of voltage-clamped slow and fast muscles in response to the application of 30 μm ACh. Calibration: 5 s, 500 pA. C, Amplitudes of ACh-induced currents in slow (n = 7) and fast muscles (n = 6) are shown. Each dot represents a muscle cell.
To further confirm that the functional defect of fast muscles in the love sofa mutant is postsynaptic, we perfused slow and fast muscles of the love sofa mutant under voltage clamp with a solution containing 30 μm ACh. Slow muscles showed robust inward currents with the ACh application (n = 7). In contrast, fast muscles without mEPCs failed to display any inward current in response to the ACh application (n = 6). These data confirmed that the absence of mEPCs in the love sofa mutant results from the AChRs in fast muscle cells.
Why does a single δ subunit mutation have different effects on AChRs of slow muscles and fast muscles? We proposed previously that slow muscle fibers in zebrafish have subunit compositions lacking ε or γ, based on the comparison of channel properties between native AChRs in zebrafish muscles and heterologously expressed AChRs in Xenopus oocytes (Mongeon et al., 2011). We therefore hypothesized that the δL332P subunit contained in both types of pentamers has distinct functional effects because of pentamer subunit compositions (i.e., αβδ in slow fibers and αβδε/γ in fast fibers). αβδL332P in slow muscles are functional, whereas αβδL332Pε/γ are nonfunctional.
To test this hypothesis, we generated an antibody against an epitope specific to the ε and γ subunit. In wild-type, fast muscles revealed the colocalization of the ε/γ subunit and the α-BTX staining (Fig. 6A). In sharp contrast, slow muscles displayed the α-BTX staining at muscle cell edges, but these locations did not have matching signals for the ε/γ subunit. Likewise, fast muscles of love sofa showed positive clusters of the ε/γ subunit, although α-BTX staining is absent (Fig. 6B). Slow muscles in love sofa mutant again lacked ε/γ subunits at synapses indicated by the α-BTX staining (Fig. 6B). These results support our hypothesis that AChRs in slow muscles lacked the ε/γ subunit in both wild-type and the love sofa mutant, and the presence of the ε/γ subunit in the pentamer causes the specific effect of the δL332P mutation in fast muscles.
Figure 6.
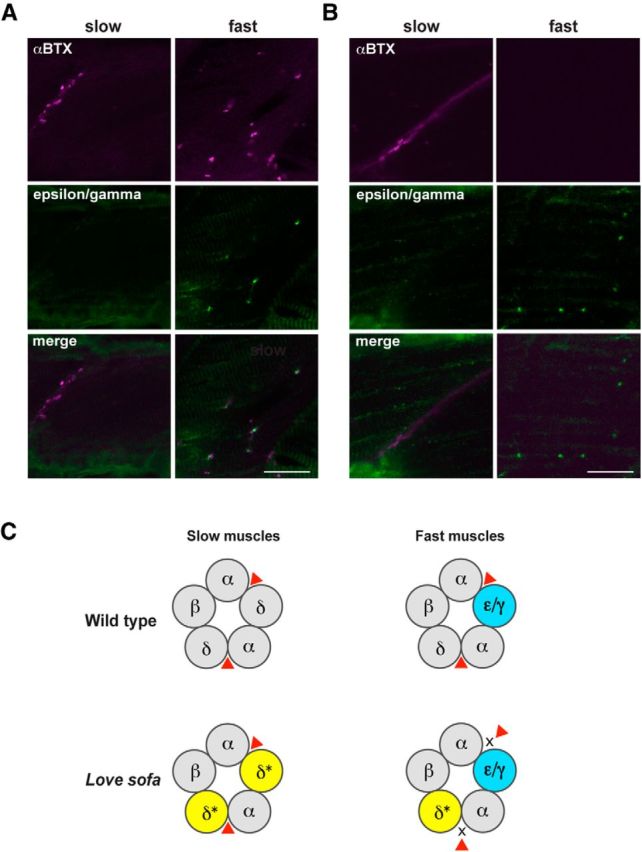
Distinct subunit compositions of AChRs in slow and fast muscles underlie the fiber-type specific phenotypes in the love sofa mutant. Wild-type (A) and love sofa (B) larvae were doubly labeled with an antibody for ε/γ subunits (green) and α-BTX (magenta). Optical sections corresponding to slow muscle fibers and fast muscle fibers are shown. Scale bar, 20 μm. C, A schematic depicting the hypothesis. In wild-type larvae (top), pentamers of slow muscles comprise αβδ, whereas those in fast muscle comprise αβδε/γ. Red triangle represents the ligand (ACh or α-BTX). In the love sofa mutant (bottom), the δ subunit harbors a L332P mutation (shown with an asterisk and yellow). As a result, the binding of ligand to the pockets between α and δ is inhibited in fast muscles.
Discussion
We showed that a single mutation of the δ subunit, L332P, allows the synapse in slow muscles to function but renders those in fast muscles almost nonfunctional. Because of the distinct patterns of AChR distribution in slow and fast muscles of zebrafish (Fig. 3), which was first described in this paper, the love sofa mutant exhibits α-BTX staining that is restricted to the myoseptal region (Fig. 1B). The characteristic pattern of α-BTX staining restricted to the myoseptal region in love sofa larvae was observed as early as 1 dpf (data not shown). Interestingly, the movement of the love sofa mutant at 1 dpf was indistinguishable from that of wild-type, in contrast to the clearly defective swimming at 2 dpf or later. This is in agreement with a previous study that showed that coiling, a type of movement specific to 1 dpf embryos, depends on slow fibers (Naganawa and Hirata, 2011).
The identity of infrequent, small mEPCs in fast muscles of love sofa is unclear (Fig. 4C). Some of them may be reflections of mEPCs arising from neighboring slow muscle fibers transmitted through gap junctions (Luna et al., 2004). However, decays of mEPCs through gap junctions are usually prolonged, whereas some recorded mEPCs have decay time constants shorter than 2 ms, suggesting that the synaptic transmission occurred not in neighboring slow muscles but in the voltage-clamped fast muscle. In addition, we observed a very small number of distributed synapses in love sofa with α-BTX staining (Fig. 1B). These cells may represent a special subpopulation of fast muscles, such as intermediate fibers. Muscle fibers with mixed metabolic characteristics of slow and fast fibers, called intermediate fibers, are reported in teleosts, including zebrafish adults (van Raamsdonk et al., 1982). Their distribution in embryos/larvae, however, remains unclear (Buss and Drapeau, 2000). In the stochastic rescue experiment (Fig. 3D), we also looked for a muscle fiber that exhibited a mixture of two types of AChR distribution, myoseptal and distributed. We did not find cells with a mixed distribution (data not shown). The identity of rare fibers that express distributed synapses in love sofa and their relationship to the infrequent mEPCs require further investigation.
An attractive hypothesis to explain the distinct effects of a single δ subunit mutation on two types of synapses is that the pentamer compositions are different. A parsimonious explanation is that the δ subunit is expressed in fast muscle fibers but not in slow muscle fibers. However, we reason that δ subunits are contained in synapses of both slow and fast muscle fibers. First, the δL28P mutation in the sofa potato mutant leads to the abolition of AChRs both in slow and fast muscle fibers (Ono et al., 2004). Second, the δL322P mutation in the love sofa mutant had stronger effects in fast muscle fibers, but it also affected slow muscle fibers, reducing their current amplitude (Fig. 4B). Third, a combination of zebrafish AChR subunits lacking the δ subunit failed to form functional receptors when expressed heterologously in Xenopus oocytes (Mongeon et al., 2011). These data strongly suggest that pentamers in both fiber types contain the δ subunit. By using an antibody specific for ε/γ subunits, we confirmed the previous study by showing that AChRs in slow muscles lack the ε/γ subunit in wild-type. This difference of subunit composition was also observed in the love sofa mutant. These data support our hypothesis that different subunit compositions underlie distinct effects of the δL332P mutation in slow and fast muscles.
How can δL332P make αβδε/γ pentamers nonfunctional while the function of αβδ pentamers is retained? The δL332P subunit in the αβδL332Pε/γ pentamer may affect the AChR function (Fig. 6C). In human congenital myasthenic syndromes, AChR mutations change the channel gating, the binding of ligand, or the expression of AChR to the membrane surface (Ohno et al., 1996; Wang et al., 2000). A combination of these effects can also be observed by a single mutation. The lack of binding to α-BTX in love sofa fast muscles suggests that the binding of ligand is changed because the binding domains for ACh and α-BTX are identical (Balass et al., 1997; Harel et al., 2001). The expression of AChR, on the other hand, does not seem to be strongly affected because mAb35 staining revealed the existence of pentamers at synapses of fast muscles (Fig. 5A). In the αβδε/γ composition, two αs and a single subunit of β, δ, and ε (or γ) make up the pentamer, and the binding with ACh as well as α-BTX occurs with amino acid residues located between αδ and αε (Fig. 6C) (Blount and Merlie, 1989). The β subunit is a structural subunit that does not contribute to ligand binding. The αβδ composition, in contrast, likely contains two αs and two δs (Fig. 6C) (Charnet et al., 1992). It is expected that slow muscles of love sofa pentamers contain two δL332P s, whereas fast muscles contain only one δL332P. It is therefore surprising and somewhat counterintuitive that the pentamer with two mutant δs is functional, whereas that with one mutant δ is strongly affected. However, the location of the L332P mutation is not close to the ligand-binding domain, and the mutation will not only affect local areas surrounding the mutated amino acid. Instead, the effect of the mutation on the overall AChR structure will be the result of a global change. While the crystal structure of acetylcholine binding protein (AChBP) elucidated how extracellular components of AChRs assemble (Brejc et al., 2001), the contribution of the M3-M4 intracellular loop to the overall structure/function of pentamers remains less well understood. This region generally allows larger flexibility, making it a preferable site of insertion for fluorescent molecules (Nashmi et al., 2003; Epley et al., 2008). The L332 may be too close to the M3 to allow for such flexibility. There are also examples of M3-M4 loop mutations that lead to low AChR expressions (Engel et al., 1999). Interestingly, a six residue in-frame duplication in the M3-M4 cytoplasmic loop close to M3 leads to a mode switching of channel kinetics in the human ε subunit (Milone et al., 1998). The effect of L332P mutation on the ligand binding and channel kinetics, which is dependent on the subunit composition, may provide new information on the structure of pentamers.
Subunit compositions of AChRs in human slow muscles have not been studied separately from fast muscles; and to the best of our knowledge, a type of human myasthenic syndrome that predominantly affects either slow or fast muscles has not been reported. Interestingly, in ε knock-out mice, the proportion of slow muscle fibers increases, suggesting a functional link between the receptor subunit composition and the fiber type (Jin et al., 2008). A generally held belief was that the only two possible combinations of subunits in vertebrate NMJs were αβδε and αβδγ. However, the combination of αβδ leads to a functional channel in heterologous expression systems (Charnet et al., 1992), and we recently proposed that this combination works in NMJs in vivo, which was further confirmed in the present study (Mongeon et al., 2008). It will be highly intriguing to examine whether αβδ pentamers also express in (sub)populations of human slow muscle fibers. Moreover, some muscle fibers found in the mammalian extraocular muscle show special characteristics, including the lack of action potentials (Chiarandini and Stefani, 1979) or the location of NMJs at myotendinous junctions (Zimmermann et al., 2011). Molecular identity of AChRs in these extraocular fibers remains unknown. If they have AChR subunit compositions distinct from fast muscle fibers, patients with certain types of mutations may present symptoms caused by phenotypes specific to such fibers. We therefore propose that consideration of NMJs containing αβδ pentamers may be beneficial to better understand congenital myasthenic syndromes.
Footnotes
This work was supported by the intramural program at the National Institute on Alcohol Abuse and Alcoholism. We thank Drs. Alex Nechiporuk and Paul Brehm for kindly providing the love sofa mutant; and members of the Laboratory of Molecular Physiology for helpful discussions.
The authors declare no competing financial interests.
References
- Balass M, Katchalski-Katzir E, Fuchs S. The alpha-bungarotoxin binding site on the nicotinic acetylcholine receptor: analysis using a phage-epitope library. Proc Natl Acad Sci U S A. 1997;94:6054–6058. doi: 10.1073/pnas.94.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P, Merlie JP. Molecular basis of the two nonequivalent ligand binding sites of the muscle nicotinic acetylcholine receptor. Neuron. 1989;3:349–357. doi: 10.1016/0896-6273(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Bone Q. Locomotor muscle. In: Randall H, editor. Fish physiology Vol. II. New York: Academic; 1978. pp. 1–34. [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Bryson-Richardson RJ, Daggett DF, Cortes F, Neyt C, Keenan DG, Currie PD. Myosin heavy chain expression in zebrafish and slow muscle composition. Dev Dyn. 2005;233:1018–1022. doi: 10.1002/dvdy.20380. [DOI] [PubMed] [Google Scholar]
- Buss RR, Drapeau P. Physiological properties of zebrafish embryonic red and white muscle fibers during early development. J Neurophysiol. 2000;84:1545–1557. doi: 10.1152/jn.2000.84.3.1545. [DOI] [PubMed] [Google Scholar]
- Charnet P, Labarca C, Lester HA. Structure of the gamma-less nicotinic acetylcholine receptor: learning from omission. Mol Pharmacol. 1992;41:708–717. [PubMed] [Google Scholar]
- Chevessier F, Faraut B, Ravel-Chapuis A, Richard P, Gaudon K, Bauché S, Prioleau C, Herbst R, Goillot E, Ioos C, Azulay JP, Attarian S, Leroy JP, Fournier E, Legay C, Schaeffer L, Koenig J, Fardeau M, Eymard B, Pouget J, et al. MUSK, a new target for mutations causing congenital myasthenic syndrome. Hum Mol Genet. 2004;13:3229–3240. doi: 10.1093/hmg/ddh333. [DOI] [PubMed] [Google Scholar]
- Chiarandini DJ, Stefani E. Electrophysiological identification of two types of fibres in rat extraocular muscles. J Physiol. 1979;290:453–465. doi: 10.1113/jphysiol.1979.sp012783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto SH, Melançon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–3380. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- Drerup CM, Nechiporuk AV. JNK-interacting protein 3 mediates the retrograde transport of activated c-Jun N-terminal kinase and lysosomes. PLoS Genet. 2013;9:e1003303. doi: 10.1371/journal.pgen.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG, Ohno K, Sine SM. Congenital myasthenic syndromes: recent advances. Arch Neurol. 1999;56:163–167. doi: 10.1001/archneur.56.2.163. [DOI] [PubMed] [Google Scholar]
- Engel AG, Ohno K, Sine SM. Sleuthing molecular targets for neurological diseases at the neuromuscular junction. Nat Rev Neurosci. 2003;4:339–352. doi: 10.1038/nrn1101. [DOI] [PubMed] [Google Scholar]
- Engel AG, Shen XM, Selcen D, Sine S. New horizons for congenital myasthenic syndromes. Ann N Y Acad Sci. 2012;1275:54–62. doi: 10.1111/j.1749-6632.2012.06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epley KE, Urban JM, Ikenaga T, Ono F. A modified acetylcholine receptor δ-subunit enables a null mutant to survive beyond sexual maturation. J Neurosci. 2008;28:13223–13231. doi: 10.1523/JNEUROSCI.2814-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nüsslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Harel M, Kasher R, Nicolas A, Guss JM, Balass M, Fridkin M, Smit AB, Brejc K, Sixma TK, Katchalski-Katzir E, Sussman JL, Fuchs S. The binding site of acetylcholine receptor as visualized in the X-ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron. 2001;32:265–275. doi: 10.1016/S0896-6273(01)00461-5. [DOI] [PubMed] [Google Scholar]
- Huzé C, Bauché S, Richard P, Chevessier F, Goillot E, Gaudon K, Ben Ammar A, Chaboud A, Grosjean I, Lecuyer HA, Bernard V, Rouche A, Alexandri N, Kuntzer T, Fardeau M, Fournier E, Brancaccio A, Rüegg MA, Koenig J, Eymard B, et al. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet. 2009;85:155–167. doi: 10.1016/j.ajhg.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenaga T, Urban JM, Gebhart N, Hatta K, Kawakami K, Ono F. Formation of the spinal network in zebrafish determined by domain-specific pax genes. J Comp Neurol. 2011;519:1562–1579. doi: 10.1002/cne.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin TE, Wernig A, Witzemann V. Changes in acetylcholine receptor function induce shifts in muscle fiber type composition. FEBS J. 2008;275:2042–2054. doi: 10.1111/j.1742-4658.2008.06359.x. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Jing L, Becaficco S, Franzini-Armstrong C, Granato M. Differential requirement for MuSK and dystroglycan in generating patterns of neuromuscular innervation. Proc Natl Acad Sci U S A. 2007;104:2483–2488. doi: 10.1073/pnas.0610822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna VM, Brehm P. An electrically coupled network of skeletal muscle in zebrafish distributes synaptic current. J Gen Physiol. 2006;128:89–102. doi: 10.1085/jgp.200609501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna VM, Wang M, Ono F, Gleason MR, Dallman JE, Mandel G, Brehm P. Persistent electrical coupling and locomotory dysfunction in the zebrafish mutant shocked. J Neurophysiol. 2004;92:2003–2009. doi: 10.1152/jn.00454.2004. [DOI] [PubMed] [Google Scholar]
- Meyer A, Van de Peer Y. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD) Bioessays. 2005;27:937–945. doi: 10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- Milone M, Wang HL, Ohno K, Prince R, Fukudome T, Shen XM, Brengman JM, Griggs RC, Sine SM, Engel AG. Mode switching kinetics produced by a naturally occurring mutation in the cytoplasmic loop of the human acetylcholine receptor epsilon subunit. Neuron. 1998;20:575–588. doi: 10.1016/S0896-6273(00)80996-4. [DOI] [PubMed] [Google Scholar]
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986;321:406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Mongeon R, Gleason MR, Masino MA, Fetcho JR, Mandel G, Brehm P, Dallman JE. Synaptic homeostasis in a zebrafish glial glycine transporter mutant. J Neurophysiol. 2008;100:1716–1723. doi: 10.1152/jn.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeon R, Walogorsky M, Urban J, Mandel G, Ono F, Brehm P. An acetylcholine receptor lacking both and subunits mediates transmission in zebrafish slow muscle synapses. J Gen Physiol. 2011;138:353–366. doi: 10.1085/jgp.201110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganawa Y, Hirata H. Developmental transition of touch response from slow muscle-mediated coilings to fast muscle-mediated burst swimming in zebrafish. Dev Biol. 2011;355:194–204. doi: 10.1016/j.ydbio.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Dickinson ME, McKinney S, Jareb M, Labarca C, Fraser SE, Lester HA. Assembly of alpha4beta2 nicotinic acetylcholine receptors assessed with functional fluorescently labeled subunits: effects of localization, trafficking, and nicotine-induced upregulation in clonal mammalian cells and in cultured midbrain neurons. J Neurosci. 2003;23:11554–11567. doi: 10.1523/JNEUROSCI.23-37-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Wang HL, Milone M, Bren N, Brengman JM, Nakano S, Quiram P, Pruitt JN, Sine SM, Engel AG. Congenital myasthenic syndrome caused by decreased agonist binding affinity due to a mutation in the acetylcholine receptor epsilon subunit. Neuron. 1996;17:157–170. doi: 10.1016/S0896-6273(00)80289-5. [DOI] [PubMed] [Google Scholar]
- Ohno K, Tsujino A, Brengman JM, Harper CM, Bajzer Z, Udd B, Beyring R, Robb S, Kirkham FJ, Engel AG. Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc Natl Acad Sci U S A. 2001;98:2017–2022. doi: 10.1073/pnas.98.4.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Engel AG, Shen XM, Selcen D, Brengman J, Harper CM, Tsujino A, Milone M. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am J Hum Genet. 2002;70:875–885. doi: 10.1086/339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F, Higashijima S, Shcherbatko A, Fetcho JR, Brehm P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J Neurosci. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F, Mandel G, Brehm P. Acetylcholine receptors direct rapsyn clusters to the neuromuscular synapse in zebrafish. J Neurosci. 2004;24:5475–5481. doi: 10.1523/JNEUROSCI.0851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Ono F, Stokes C, Urban JM, Boyd RT. The nicotinic acetylcholine receptors of zebrafish and an evaluation of pharmacological tools used for their study. Biochem Pharmacol. 2012;84:352–365. doi: 10.1016/j.bcp.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Ikeda H, Ikenaga T, Ono F. Acetylcholine receptors enable the transport of rapsyn from the Golgi complex to the plasma membrane. J Neurosci. 2012;32:7356–7363. doi: 10.1523/JNEUROSCI.0397-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KG, Viereck MJ, Margiotta MV, Gripp KW, Abdul-Rahman OA, Akins RE. Neuromotor synapses in Escobar syndrome. Am J Med Genet A. 2013;161:3042–3048. doi: 10.1002/ajmg.a.36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos SJ, Rand DE, Einarson BL, Lindstrom JM. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981;256:8635–8645. [PubMed] [Google Scholar]
- van Raamsdonk W, van't Veer L, Veeken K, Heyting C, Pool CW. Differentiation of muscle fiber types in the teleost Brachydanio rerio, the zebrafish: posthatching development. Anat Embryol. 1982;164:51–62. doi: 10.1007/BF00301878. [DOI] [PubMed] [Google Scholar]
- Walogorsky M, Mongeon R, Wen H, Mandel G, Brehm P. Acetylcholine receptor gating in a zebrafish model for slow-channel syndrome. J Neurosci. 2012a;32:7941–7948. doi: 10.1523/JNEUROSCI.0158-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walogorsky M, Mongeon R, Wen H, Nelson NR, Urban JM, Ono F, Mandel G, Brehm P. Zebrafish model for congenital myasthenic syndrome reveals mechanisms causal to developmental recovery. Proc Natl Acad Sci U S A. 2012b;109:17711–17716. doi: 10.1073/pnas.1215858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Ohno K, Milone M, Brengman JM, Evoli A, Batocchi AP, Middleton LT, Christodoulou K, Engel AG, Sine SM. Fundamental gating mechanism of nicotinic receptor channel revealed by mutation causing a congenital myasthenic syndrome. J Gen Physiol. 2000;116:449–462. doi: 10.1085/jgp.116.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Schiaffino S, Leinwand LA. Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implications for functional diversity. J Mol Biol. 1999;290:61–75. doi: 10.1006/jmbi.1999.2865. [DOI] [PubMed] [Google Scholar]
- Wen H, Linhoff MW, Hubbard JM, Nelson NR, Stensland D, Dallman J, Mandel G, Brehm P. Zebrafish calls for reinterpretation for the roles of P/Q calcium channels in neuromuscular transmission. J Neurosci. 2013;33:7384–7392. doi: 10.1523/JNEUROSCI.5839-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won YJ, Ono F, Ikeda SR. Characterization of Na+ and Ca2+ channels in zebrafish dorsal root ganglion neurons. PLoS One. 2012;7:e42602. doi: 10.1371/journal.pone.0042602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L, May PJ, Pastor AM, Streicher J, Blumer R. Evidence that the extraocular motor nuclei innervate monkey palisade endings. Neurosci Lett. 2011;489:89–93. doi: 10.1016/j.neulet.2010.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]