Abstract
Carbon dioxide (CO2) inhalation lowers brain pH and induces anxiety, fear, and panic responses in humans. In mice, CO2 produces freezing and avoidance behavior that has been suggested to depend on the amygdala. However, a recent study in humans with bilateral amygdala lesions revealed that CO2 can trigger fear and panic even in the absence of amygdalae, suggesting the importance of extra-amygdalar brain structures. Because the bed nucleus of the stria terminalis (BNST) contributes to fear- and anxiety-related behaviors and expresses acid-sensing ion channel-1A (ASIC1A), we hypothesized that the BNST plays an important role in CO2-evoked fear-related behaviors in mice. We found that BNST lesions decreased both CO2-evoked freezing and CO2-conditioned place avoidance. In addition, we found that CO2 inhalation caused BNST acidosis and that acidosis was sufficient to depolarize BNST neurons and induce freezing behavior; both responses depended on ASIC1A. Finally, disrupting Asic1a specifically in the BNST reduced CO2-evoked freezing, whereas virus-vector-mediated expression of ASIC1A in the BNST of Asic1a−/− and Asic1a+/+ mice increased CO2-evoked freezing. Together, these findings identify the BNST as an extra-amygdalar fear circuit structure important in CO2-evoked fear-related behavior.
Keywords: anxiety, ASIC1A, bed nucleus of the stria terminalis, CO2, panic, pH
Introduction
Carbon dioxide (CO2) inhalation causes anxiety, fear, and panic in humans, and these responses are heightened in patients with panic disorder (Esquivel et al., 2008). CO2 concentrations from 5% to 35% have been used to demonstrate CO2 sensitivity in panic disorder (Rassovsky and Kushner, 2003). Thus, CO2 provides a valuable experimental tool with which to provoke and study panic. A better understanding of the neuroanatomical and molecular basis of CO2 responses may provide critical insight into the mechanisms underlying panic.
The neuroanatomy underlying CO2-defensive responses remains unclear. The amygdala, in keeping with its role in other defensive responses (Phelps and LeDoux, 2005), has been hypothesized to play a critical role in panic and CO2-evoked responses (Gorman et al., 2000). Functional neuroimaging supports amygdala involvement, as both hypercapnia and dyspnea have been suggested to activate the amygdala (Brannan et al., 2001; Liotti et al., 2001; Evans et al., 2002). Further support for the amygdala in CO2 responses was also provided by recent mouse studies, in which amygdala manipulations altered CO2-evoked behavior (Ziemann et al., 2009). However, a 35% CO2 inhalation challenge surprisingly elicited panic attacks and feelings of fear, anxiety, and panic in human patients with bilateral amygdala lesions (Feinstein et al., 2013). Together, these observations suggest that the amygdala is key in regulating CO2-evoked defensive responses, but also underscore the possibility that structures outside of the amygdala may be critical.
The bed nucleus of the stria terminalis (BNST) is one extra-amygdalar structure that might contribute to CO2-evoked fear and anxiety. Supporting a possible role for the BNST in CO2-evoked behavior is the observation that the BNST exhibits relatively dense expression of acid-sensing ion channels (ASICs) (Coryell et al., 2007; Price et al., 2014), which have been implicated in CO2 responses (Ziemann et al., 2009). Deleting Asic1a or Asic2 in mice significantly attenuated CO2-evoked freezing and CO2 aversion (Ziemann et al., 2009; Price et al., 2014). These observations were consistent with previous studies, which found that Asic1a−/− and Asic2−/− mice have deficits in fear- and anxiety-related behaviors, such as cued and context fear conditioning and predator odor-evoked freezing (Wemmie et al., 2003; Coryell et al., 2007; Price et al., 2014; but see Pidoplichko et al., 2014). ASICs are trimeric, proton-gated cation channels of the DEG/ENaC family that are activated by extracellular acidosis (Waldmann et al., 1997; Wemmie et al., 2013). ASIC1A and ASIC2A are localized to dendritic spines and have previously been implicated in synaptic plasticity (Zha et al., 2006, 2009). Relatively little is known about the function of ASICs in humans, although polymorphisms in Asic1a and Asic2 have been recently associated with panic disorder, amygdala volume, and function (Gregersen et al., 2012; Smoller et al., 2014).
Because of the importance of the BNST in fear and anxiety, we hypothesized that the BNST contributes to CO2-evoked behavior. Furthermore, because ASIC1A is critical for CO2-evoked responses in mice, and ASIC1A is enriched in the BNST, we hypothesized that ASIC1A in the BNST may play a key role in these responses.
Materials and Methods
Mice.
Asic1a−/− mice were generated as previously described (Wemmie et al., 2002) and maintained on a C57BL/6J congenic background. Asic1aloxP/+ mice were generated by Xenogen Biosciences and intercrossed to produce Asic1aloxP/loxP mice. In brief, a targeting vector was designed in which exon 2 of Asic1a was flanked by loxP sites. A neomycin resistance cassette was also inserted to facilitate positive selection of electroporated embryonic stem cells and was later removed by flippase trasfection. Asic1aloxP/loxP mice were generated and maintained on a C57BL/6T congenic background. Because wild-type C57BL/6T mice froze more to 10% CO2 than their C57BL/6J counterparts, it was important that experimental groups were matched with controls of the same congenic background. All mice were kept on a 12 h light-dark cycle, with all experiments being performed during the light cycle. Mice were fed standard chow and water ad libitum. Both male and female mice were used, and all experimental groups were sex- and age-matched. Mice undergoing behavioral testing were 12–18 weeks in age. Animal care met the standards set by the National Institutes of Health, and all experiments were approved by the University of Iowa Animal Care and Use Committee.
Electrolytic lesions.
Mice were anesthetized with a mixture of ketamine and xylazine. A burr hole was drilled, and an electrode (an insect pin coated with epoxylite) was stereotaxically inserted into the BNST. The coordinates used to target the BNST were as follows: 0.4 mm anterior to bregma, 1.0 mm lateral to the midline, and 4.3 mm ventral from the pial surface. Bilateral lesions were made by passing 1.0 mA of DC through the electrode for 10 s per side. Mice were sutured and allowed to recover for at least 5 d before undergoing behavioral testing. Sham surgeries were performed as described above, but without insertion of the electrode into the BNST. Targeting was confirmed postmortem with Nissl staining and mapped (see Fig. 1A).
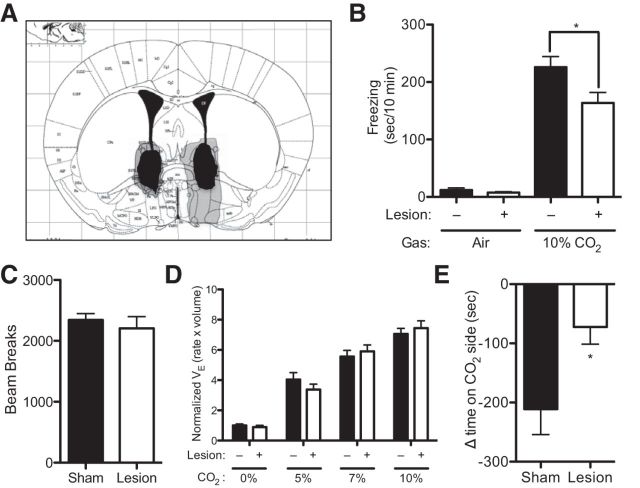
Figure 1.
BNST lesions reduce CO2-evoked behavior. A, Diagram representing location and size of the largest (gray) and smallest (black) lesions (Paxinos and Franklin, 2001). B, BNST lesions reduced CO2-evoked freezing. C, BNST lesions significantly reduced conditioned avoidance to CO2. D, BNST lesions did not alter minute ventilation (VE). E, BNST lesions did not alter locomotion in the open field.
CO2-evoked freezing.
CO2-evoked freezing was assessed as previously described (Ziemann et al., 2009; Price et al., 2014). Briefly, mice were placed in a Plexiglas chamber containing 10% CO2 (10% CO2, 21% O2, balanced with N2) for 10 min. Gas was infused at a flow rate of 5 L/min. Behavior was videotaped and scored by an observer blinded to genotype and condition. Freezing was defined as the absence of motion other than respiration.
CO2-conditioned place avoidance.
Custom avoidance chambers were constructed, each chamber with two sides (7.25 inches long × 5.5 wide × 7.5 tall), divided by a removable separator, and each side with distinct contextual cues: one side with black walls and a rod floor, and the other with white walls and a mesh floor. For gas flow, each side had an (0.25 inch) inlet and an (0.125 inch) outlet port. Gas was infused at a flow rate of 10 L/min. A 5 d protocol was used. On the first day, mice were allowed to freely explore both sides of the chamber for 20 min, and their behavior was videotaped. Time spent on each side was quantified by an observer blinded to genotype and condition; mice spending >70% of the time on either side at baseline were excluded. On days 2–4, mice underwent two training periods per day: one in the morning and one in the afternoon. During this time, mice were confined to one side of the chamber while being exposed to either 10% CO2 (10% CO2, 21% O2, balanced with N2) or compressed air. The context paired with CO2 and order of CO2 exposure were counterbalanced. On day 5, the mice were again allowed to freely explore both sides of the chamber, and the amount of time spent on the side previously paired with CO2 was quantified. Conditioned place avoidance was determined by subtracting the time spent on the CO2-paired side on day 1 from the time spent on the CO2-paired side on day 5.
Locomotor testing.
Total locomotion was assessed in the open field in room air as previously described (Coryell et al., 2007). In brief, mice were placed in an open field chamber measuring 40.6 × 40.6 × 36.8 cm (San Diego Instruments). Beam breaks were quantified over 30 min. Center activity was defined as the percentage of total beam breaks occurring in the center of the open field (25.5 × 25.5 cm) relative to total beam breaks.
Plethysmography.
Ventilation was measured using standard plethysmographic techniques as previously described (Hodges et al., 2008) in a Buxco chamber with continuous gas flow (700 ml/min). The protocol consisted of >10 min of baseline in 0% CO2/50% O2, balanced with N2, followed by exposures to 5%, 7%, and 10% CO2, all containing 50% O2, and balanced with N2. All data were acquired using custom-written MATLAB (MathWorks) software. All data segments ≥5 s in duration that did not contain sighs, sniffing, or movement artifacts were selected for analysis. At least 40 s of data was analyzed for each gas. All analyzed data were obtained after 60 s of gas exposure to ensure equilibrium in the chamber. Relative minute ventilation is reported, normalized to sham mice in 0% CO2/50% O2.
Immunohistochemistry and imaging.
Fresh tissue was embedded in OCT (Tissue-Tek) and frozen. The 18 μm coronal sections were sliced and mounted onto slides using the CryoJane system (Leica Biosystems). eGFP was imaged directly without use of antibodies. For ASIC1A immunohistochemistry, sections were postfixed in PBS with 4% PFA and 4% sucrose for 10 min, followed by three 5 min washes with PBS. Sections were then permeabilized with a 5 min exposure to 0.25% Triton X-100 in PBS, followed by three 5 min washes with PBS. Sections were blocked for 1 h in 5% goat serum and then incubated overnight at 4°C with rabbit polyclonal anti-ASIC1 antiserum (MTY19) (Wemmie et al., 2003) diluted 1:1000 in 5% goat serum Sections were washed three times for 5 min in PBS, followed by a 1 h incubation with goat-anti rabbit IgG antibody coupled to AlexaFluor-488 or AlexaFluor-568 (Invitrogen) diluted 1:500 in goat serum. Sections were washed three times for 5 min in PBS and mounted with Vectashield (Vector Labs). Sections were imaged at 10× using a Zeiss confocal microscope (Zeiss 710). Colabeling with ASIC1A and mouse monoclonal NeuN antibody (Millipore) and mouse monoclonal GFAP antibodies (Millipore) was done in a similar manner. NeuN was diluted 1:250, and GFAP was diluted 1:500 and then detected with a goat-antimouse antibody coupled to AlexaFluor-568 (Invitrogen). Sections were imaged at 20×.
Preparation of acute BNST slices.
Coronal BNST brain slices (300 mm) were prepared from 8- to 12-week-old mice, in accordance with the University of Iowa guidelines. Briefly, BNST slices were cut using a Vibratome 1000 Plus (Vibratome) in ice-cold slicing buffer (in mm as follows: 127 NaCl, 26 NaHCO3, 1.2 KH2PO4, 1.9 KCl, 1.1 CaCl2, 2 MgSO4, 10 d-glucose) bubbled with 95% O2 and 5% CO2. Slices were then transferred to a holding chamber containing oxygenated ACSF (in mm as follows: 127 NaCl, 26 NaHCO3, 1.2 KH2PO4, 1.9 KCl, 2.2 CaCl2, 1 MgSO4, 10 d-glucose) for 30 min at 34°C and for another 30 min at 22°C for recovery, and then transferred to a submersion recording chamber continually perfused with 32°C oxygenated ACSF (rate: 2 ml/min). Slices were equilibrated for at least 15 min before each recording.
Electrophysiology.
Whole-cell recordings from BNST neurons were made using an Axopatch 200B amplifier (Molecular Devices), sampled at 10 kHz, digitized by a DigiData 1322A, and later analyzed off-line by ClampFit (Axon software). Recording pipettes with resistances ranging between 3 and 6 Mohms were pulled using standard borosilicate capillaries by a Flaming-Brown electrode puller (P-97, Sutter Instruments) and were filled with a K-gluconate-based patch solution (in mm as follows: 125 K-gluconate, 20 KCl, 10 NaCl, 2 Mg-ATP, 0.3 Na-GTP, 2.5 QX314, 10 HEPES, 0.2 EGTA, pH 7.3 adjusted with KOH) for the measurement of acid-evoked currents. A different pipette solution (in mm as follows: 130 K-gluconate, 1 MgCl2, 5 Mg-ATP, 0.4 Na-GTP, 10 HEPES, 5 EGTA, pH 7.3 adjusted with KOH) was used for current-clamp experiments. A total of 20 mm CNQX was used to block AMPA receptors, 100 mm picrotoxin was used to block GABAA receptors, and 100 mm APV was used to block NMDA receptors.
Western blotting.
Tissue punches for Western blotting were taken bilaterally from the BNST, and homogenized. 7.5 μg lysate was run on a Bis-Tris gel and transferred to a PVDF membrane for Western blotting. Primary antibodies used were as follows: rabbit polyclonal anti-ASIC1 antiserum (MTY19) (Wemmie et al., 2003) diluted 1:500 and chicken polyclonal anti-GAPDH antibody (Millipore) diluted 1:10,000. Secondary antibodies used were as follows: IRDye 800CW donkey anti-rabbit IgG (LI-COR) and IRDye 680LT Donkey anti-chicken IgG (LI-COR), both diluted 1:10,000. The blots were imaged with the Odyssey imaging system (LI-COR).
Fiberoptic pH sensors.
Mice were anesthetized with a mixture of ketamine and xylazine. A burr whole was drilled and a fiberoptic pH sensor (World Precision Instruments) was implanted into the BNST. The following coordinates were used to target the BNST: 0.4 mm anterior from bregma, 1.0 mm lateral from the midline, and 4.3 mm ventral from the pial surface. After pH readings stabilized for at least 10 min, mice were then exposed to increasing doses of CO2 (5%, 10%, 20%) for 10 min, with a 10 min recovery period between doses. Simultaneous measurement of and delivery of acid into the BNST was accomplished by either affixing an injector <1 mm from the tip of the pH sensor and implanting it into the BNST, or cannulating the BNST for acid infusion and implanting a pH sensor into the amygdala. The following coordinates were used to target the amygdala: 1.5 mm posterior to bregma, 3.5 mm lateral to the midline, and 4.5 mm the from skull surface at bregma.
BNST cannulae.
Cannulae were sterotaxically implanted into the BNST in a manner similar to that which has been previously described (Coryell et al., 2009; Ziemann et al., 2009). Briefly, a 25 gauge guide cannula was implanted into the BNST. The following coordinates were used to target the BNST: 0.4 mm anterior to bregma, 1.0 mm lateral to the midline, and 3.5 mm ventral from pial surface. Mice recovered from surgery for at least 3 d. A 30 gauge injector was inserted into guide cannula, extending 1 mm past the cannula tip; 1 μl of ACSF (either pH 3.0 or pH 7.3) was infused over 5–10 s. Because of the rapid buffering capacity of the brain, it was necessary to inject such an acidic solution (pH 3) to lower the pH of the tissue to ∼6.8. Mice were immediately placed in a Plexiglas behavior chamber and the behavioral response was videotaped for 10 min. A blinded observer scored freezing, which was defined as an absence of movement except for respiration. Cannula targeting was confirmed postmortem with a dye infusion.
Viral injections.
Adeno-associated virus (AAV) vectors were obtained from the University of Iowa Gene Transfer Vector Core and injected as previously described (Coryell et al., 2008, 2009; Ziemann et al., 2009). All viruses used were AAV2/1 chimeric viruses, with a CMV promoter driving expression of ASIC1A, Cre recombinase, or eGFP. AAV-cre and AAV-Asic1a were coinjected with AAV-eGFP to aid with localization. A total of 0.5 μl of virus was injected into each site. The BNST coordinates were (relative to bregma): anteroposterior 0.4 mm, lateral ±1.0 mm, ventral 4.3 mm from the pial surface. Hippocampus coordinates were (relative to bregma): anteroposterior −1.5 mm, lateral 1 mm, and ventral 1.5 mm from pial surface. Mice were allowed to recover for at least 3 weeks before behavioral testing. Targeting was determined postmortem. Bilateral transduction in the BNST was required to be categorized as a hit and misses and unilateral hits were excluded from further analysis.
Statistical analysis.
All values are plotted as mean ± SEM. Significance between two groups with equal variance was tested using a Student's t test. Significance between two groups with unequal variance was tested using a Student's t test with Welch's correction. Significance between more than two groups was tested using an ANOVA. Within the context of the ANOVA, planned contrast testing (Student's t test) and post hoc testing (Bonferroni's multiple-comparison test) were performed to test for differences between two groups. For all tests, p < 0.05 was considered significant. Analyses were performed using Prism software (GraphPad).
Results
CO2-evoked behaviors depend on the BNST
Although the role of the BNST in humans is not fully understood, recent human functional imaging studies are consistent with BNST involvement in anxiety (Somerville et al., 2010, 2013; Alvarez et al., 2011; Grupe et al., 2013; Avery et al., 2014). Rodent studies have also implicated the BNST in fear-and anxiety-related behaviors, particularly unconditioned fear responses, such as to predator odors (LeDoux et al., 1988; Lee and Davis, 1997; Fendt et al., 2003; Sullivan et al., 2004). Much as predator odors herald the potential threat of predation, CO2 heralds the potential threat of suffocation; thus, CO2 might act as an unconditioned aversive stimulus that engages the BNST. Previous studies used bilateral electrolytic lesions to implicate the BNST in rodent models of fear and anxiety (Sullivan et al., 2004; Luyten et al., 2011). Therefore, to test the hypothesis that the BNST might contribute to CO2-evoked behaviors, we bilaterally lesioned the BNST. These lesions hit both the dorsal and ventral aspects of the BNST, including the oval and anterodorsal subnuclei (Fig. 1A). Because freezing is a widely used measure of fear-related behavior, and because previous studies suggested that 10% CO2 evokes a robust freezing response in wild-type C57BL/6J mice (Ziemann et al., 2009), we tested 10% CO2-evoked freezing in BNST-lesioned mice versus sham controls. We found that 10% CO2 induced prominent freezing behavior that was absent in compressed air (Fig. 1B) (F(1,37) = 152.1, p < 0.0001). Moreover, BNST lesions significantly attenuated CO2-evoked freezing, although they did not completely abolish it (Fig. 1B) (p = 0.0271).
To further probe the role of the BNST in CO2-evoked behavior, we developed a CO2 conditioned place avoidance assay (CO2-CPA), in which mice learn to associate 10% CO2 with a specific context in a two-sided box and subsequently avoid it when given a choice between the context previously paired with CO2 versus one previously paired with compressed air (see Materials and Methods). Because the BNST has been suggested to play a role in contextually conditioned fear (Sullivan et al., 2004), we hypothesized that BNST lesions would impair CO2-CPA. We found that both BNST- and sham-lesioned animals avoided the context previously paired with CO2. Consistent with our hypothesis, BNST lesions significantly reduced CO2-conditioned place avoidance (Fig. 1C) (t(11) = 2.580, p = 0.0256, n = 7, n = 6).
In light of a recent study implicating the BNST in control of respiratory rate (Kim et al., 2013) and because elevated CO2 concentrations (hypercapnia) potently stimulate breathing (Hodges et al., 2008), we further sought to determine whether BNST lesions might alter the hypercapnic ventilatory response. Inhaling 5%, 7%, and 10% CO2 increased minute ventilation (VE) to increasing degree (Fig. 1D) (F(3,68) = 422.8, p < 0.0001 n = 10, n = 9). However, BNST lesions did not interfere with this response (F(1,68) = 0.002381, p = 0.9657), and there was no CO2 × lesion interaction (F(3,68) = 3.435, p = 0.4471). We also assessed locomotion by quantifying total beam breaks in open field test and found that the BNST-lesioned mice were similar to their sham counterparts (Fig. 1E) (t(20) = 0.6284, p = 0.5369), and no difference was detected in center activity (sham 34.45 ± 1.451%, lesion 30.93 ± 1.680%, t(20) = 1.586, p = 0.1283). These results suggest that effects of BNST lesions on unconditioned and conditioned behavioral responses to 10% CO2 are not driven by differences in CO2-induced hyperventilation or by general differences in locomotion.
ASIC1A in BNST neurons is critical for acid-evoked currents and depolarization
Because previous studies suggested that ASIC1A contributes to CO2 detection in the amygdala and because ASIC1A was previously detected in the BNST (Coryell et al., 2007; Price et al., 2014), we hypothesized that ASIC1A in the BNST might contribute to CO2-evoked behavior. We confirmed the presence of ASIC1A in BNST neurons using ASIC1A immunohistochemistry in Asic1a+/+ versus Asic1a−/− mice (Fig. 2A,B) and by taking advantage of colocalization with the neuronal marker NeuN (Fig. 2C). We found that a substantial component of the ASIC1A signal overlapped with NeuN at neuron somata. We also observed an ASIC1A-specific extrasomatic signal that did not overlap with the astrocyte marker GFAP (Fig. 2D), which is most likely consistent with the previously established localization of ASIC1A in dendrites (Wemmie et al., 2002; Hruska-Hageman et al., 2004; Zha et al., 2006, 2009). Next, we tested for ASIC1A-dependent channel activity using a relatively severe acid challenge, pH 5.6. We found that this challenge evoked large currents from BNST neurons in Asic1a+/+ slices. Whereas BNST neurons from Asic1a−/− mice lacked this acid-evoked current (Fig. 2E) (t(6) = 4.364, p = 0.0048, n = 7, n = 6), membrane capacitance was not different across genotypes (Asic1a+/+ 49.09 ± 3.114 pF, n = 28; Asic1a−/− 49.04 ± 2.460 pF, n = 24: t(50) = 0.01259, p = 0.9900). Thus, even severe acidosis failed to evoke current in BNST neurons from Asic1a−/− mice. A more physiologic challenge, pH 6.8, induced depolarization and action potentials in Asic1a+/+ neurons assessed by whole-cell current clamp, but not in Asic1a−/− neurons (Fig. 2F) (t(5) = 7.385, p = 0.0007, n = 6, n = 7).
Figure 2.
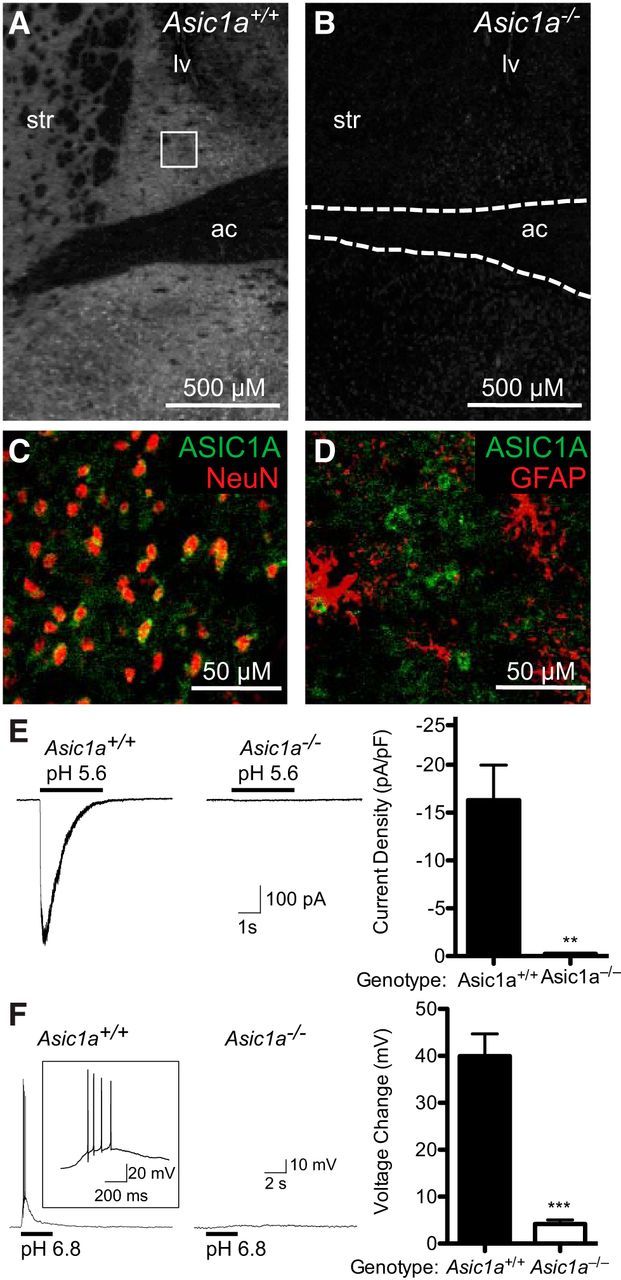
ASIC1A is expressed in the BNST and is critical for acid-evoked currents and depolarization. A, B, ASIC1A immunofluorescent labeling (white) in the BNST of Asic1a+/+ versus Asic1a−/− mice. The lateral venticle (lv), striatum (str), and anterior commissure (ac) are labeled for reference. Square represents region analyzed in different slices for NeuN and GFAP immunohistochemistry (bottom). C, Immunofluorescent labeling of ASIC1A (green) and NeuN (red) in the BNST of an Asic1a+/+ mouse. D, Immunofluorescent labeling of ASIC1A (green) and GFAP (red) in the BNST of an Asic1a+/+ mouse. E, Whole-cell voltage-clamp recording of BNST neurons in slices. pH 5.6 evoked currents in BNST neurons in slices from Asic1a+/+ but not Asic1a−/− mice. F, Whole-cell current-clamp recording of BNST neurons in slices. pH 6.8 elicited depolarization and action potentials in Asic1a+/+ neurons but not Asic1a−/− neurons.
CO2 causes freezing, conditioned avoidance, and BNST acidosis
Like BNST lesions, and consistent with previous observations (Ziemann et al., 2009; Price et al., 2014), we found that ASIC1A disruption attenuated both the freezing response to 10% CO2 (Fig. 3A) (F(1,39) = 10.02, p = 0.0030 gas × genotype interaction, p = 0.0049, n = 11, n = 11, n = 11, n = 10) and CO2-CPA (Fig. 3B) (t(36) = 1.977, p = 0.0279, n = 19, n = 19, one-tailed). To explore how CO2-induced behavior might depend on the BNST and ASICs, we measured pH in the BNST of anesthetized mice with a fiberoptic pH sensor. When the mice were breathing only compressed air, average pH was 6.93 ± 0.12 and 6.85 ± 0.08 in Asic1a+/+ and Asic1a−/− mice, respectively (t(4) = 0.5153, p = 0.63, n = 3 per group). These values are consistent with previous pH measurements in the lateral ventricle and amygdala using the same approach, and are less than what might be expected in awake animals (pH 7.29–7.39) (Mitchell et al., 1965; Mayhan et al., 1988) likely due to anesthesia-induced respiratory suppression and the associated hypercarbia (Ziemann et al., 2009). Increasing concentrations of inhaled CO2 to 5%, 10%, and 20% lowered pH in the BNST to increasing degree (Fig. 3C,D) (F(2,12) = 42.47, p < 0.0001, n = 3, n = 3). However, Asic1a disruption did not alter this response (F(1,12) = 0.3209, p = 0.5815), and there was no gas × genotype interaction (F(2,12) = 1.089, p = 0.3677). The magnitude of the acidosis in the two genotypes was not different, suggesting that ASIC1A disruption does not alter pH (Ziemann et al., 2009). Conceivably, respiratory suppression due to anesthesia may obscure the absolute pH changes evoked by CO2 inhalation. Nevertheless, these measurements grossly confirm CO2-induced acidosis in the BNST and provide a useful estimate of the pH changes.
Figure 3.
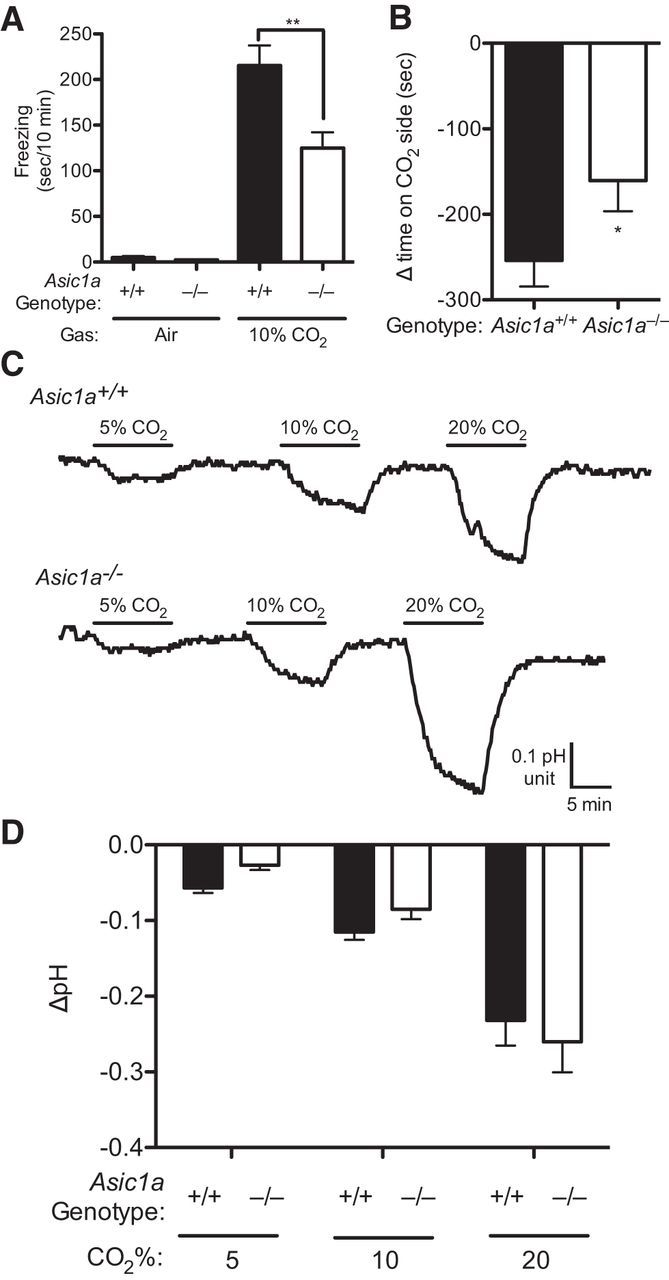
CO2 inhalation causes freezing, avoidance, and BNST acidosis. A, Freezing behavior in air versus 10% CO2. CO2 elicited freezing, which was attenuated in the Asic1a−/− mice. B, CO2-conditioned place avoidance, measured as a change in time spent on target side after conditioning relative to pretest, was significantly attenuated in Asic1a−/− mice relative to Asic1a+/+ mice. C, Representative traces of pH recordings measured by fiberoptic pH sensor implanted in the BNST. Inhaling the indicated concentrations of CO2 induced acidosis in both Asic1a+/+ and Asic1a−/− mice. D, Mean pH responses to CO2 were similar in Asic1a+/+ and Asic1a−/− mice.
BNST acidosis evokes freezing that depends on ASIC1A
To test whether acidosis in the BNST might be sufficient to trigger behavior, we directly infused acidic ACSF into the BNST of Asic1a+/+ and Asic1a−/− mice. We found that infusing ACSF pH 3 into the BNST of anesthetized mice lowered pH by a magnitude approximately similar to that observed during 10% CO2 inhalation (Fig. 4A). Importantly, delivering acidic ACSF to the BNST did not alter pH in the basolateral amygdala (Fig. 4A), a location where acidic pH has been previously shown to evoke freezing behavior (Ziemann et al., 2009). We next tested effects of infusions through guide cannulae into the BNST of awake, behaving mice (Fig. 4B) and found a significant genotype × infusion interaction (Fig. 4C) (F(1,18) = 60.81, p < 0.0001). Like CO2 inhalation, direct acidification of the BNST evoked freezing behavior in Asic1a+/+ mice relative to vehicle pH 7.3 (Fig. 4C) (t(8) = 7.81, p < 0.001). However, this response was absent in Asic1a−/− mice (t(10) = 0.347, p = 0.74). Acidic infusions that missed the BNST also failed to evoke freezing behavior (Fig. 4C) (t(6) = 1.40, p = 0.210). Together, these data suggest that the BNST is sensitive to pH and that the ability of CO2 to evoke freezing behavior may come, at least in part, through direct acidification of the BNST.
Figure 4.
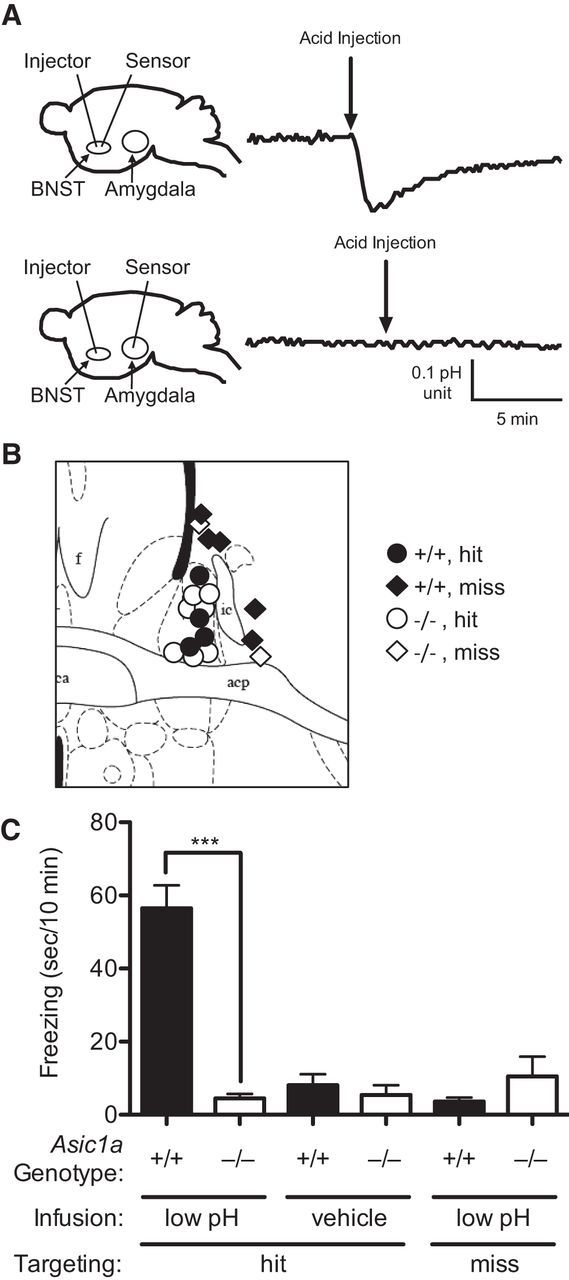
BNST acidosis evokes ASIC1A-dependent freezing. A, Schematic of experimental design with fiberoptic pH sensors implanted in BNST or amygdala, showing that infusion of low pH solution into the BNST acidifies the BNST but not the amygdala. B, Diagram of acid infusion sites; BNST (circles) versus extra BNST (diamonds) (Paxinos and Franklin, 2001). C, Freezing behavior after infusion of low pH solution or normal pH solution in cases that hit or missed the BNST. Infusing low pH solution evoked a freezing response in ASIC1A+/+ mice that was largely absent in Asic1a−/− mice. Low pH infusions into the BNST of Asic1a+/+ mice evoked significantly more freezing than in Asic1a−/− mice. In Asic1a+/+ mice, low pH infusions hitting the BNST elicited significantly more freezing than those that missed the BNST.
ASIC1A in the BNST is required for normal CO2-evoked freezing
To test whether the BNST is a key site of ASIC1A action in CO2-evoked behavior, we took advantage of a mouse with loxP sites inserted into the introns flanking the critical second exon of the Asic1a gene (Asic1aloxP/loxP). Expressing Cre recombinase in the BNST of these mice via an adeno-associated virus vector (AAV-Cre) removed exon 2 and disrupted ASIC1A as assessed by immunohistochemistry (Fig. 5A,B), Western blot (Fig. 5E), and acid-evoked currents (Fig. 5F,G) (t(8) = 5.086, p = 0.0009, n = 9, n = 8). Whereas ASIC1A expression levels were intact in Asic1aloxP/loxP mice transduced with the AAV-eGFP control vector (Fig. 5E). Within the BNST, the viruses tended to transduce both the oval and anterodorsal nuclei (Fig. 5B,C). In contrast to our lesion studies, where we cannot rule out effects of fibers of passage, in this approach no virus transduction was detected in the basolateral amygdala (Fig. 5D), suggesting that the virus was not taken up by fibers from cells connected to the BNST (Kim et al., 2013) or by fibers passing through the BNST or anterior commissure (Martínez-Lorenzana et al., 2004). We next tested the effects of these viral manipulations on freezing behavior evoked by 10% CO2. We found that CO2-evoked freezing was significantly attenuated in Asic1aloxP/loxP mice in which the BNST was transduced with AAV-Cre relative to those transduced with AAV-eGFP (Fig. 5H) (t(18) = 5.532, p < 0.0001, n = 12, n = 8). In contrast, general locomotor activity in the open field was unaffected by these manipulations (Fig. 5I) (t(18) = 0.2491, p = 0.8061), and center activity was not significantly different (AAV-GFP 36.23 ± 2.354%, AAV-Cre 29.81 ± 1.498%, t(18) = 2.037, p = 0.0567). These results suggest that CO2-evoked freezing depends on ASIC1A in the BNST.
Figure 5.
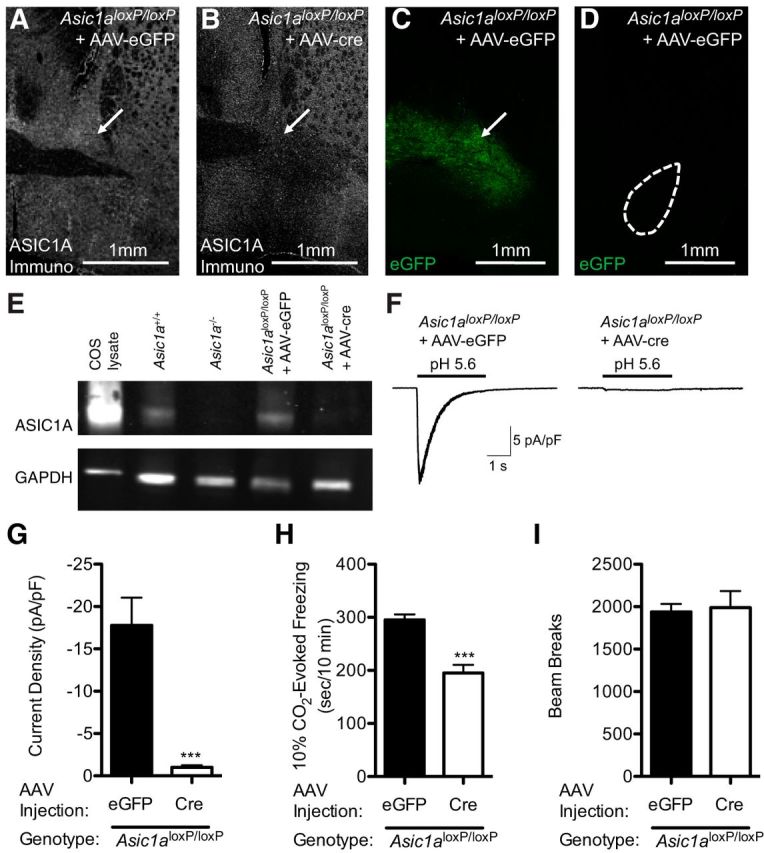
Disrupting ASIC1A in the BNST reduces acid-evoked currents and CO2-evoked freezing. A, B, Examples of ASIC1A immunofluorescent labeling (white) of Asic1aloxP/loxP mice injected with AAV-eGFP or AAV-cre into the BNST (arrow), showing a localized disruption of ASIC1A expression in the AAV-cre injected BNST. C, D, In the same mouse, AAV-eGFP transduction in the BNST produced eGFP expression (green) in the BNST (arrow), but not in the basolateral amygdala (dashed circle). E, Western blot of ASIC1A protein from Cos cells transfected with Asic1a and from BNST punches from mice of the indicated genotypes and virus transduction. AAV-cre injections eliminated the majority of the ASIC1A protein in the BNST. GAPDH immunoblotting was included as a loading control. F, G, Whole-cell voltage-clamp recordings of neurons from BNST slices. AAV-cre disrupted acid-evoked currents in the BNST of Asic1aloxP/loxP mice. H, Quantification of freezing behavior evoked by 10% CO2. AAV-cre injections into the BNST of Asic1aloxP/loxP mice significantly reduced freezing. I, AAV-cre transduction in the BNST of Asic1aloxP/loxP mice did not alter locomotion in the open field relative to AAV-eGFP transduced controls.
ASIC1A in the BNST is sufficient to increase CO2-evoked freezing
The previous data suggested that ASIC1A in the BNST is necessary for normal CO2-evoked freezing. To test whether ASIC1A in the BNST is also sufficient to increase CO2-evoked freezing, we used an AAV vector expressing ASIC1A (AAV-ASIC1A) to drive ASIC1A expression to the BNST of Asic1a−/− mice. We found that the AAV-ASIC1A vector produced prominent ASIC1A protein expression (Fig. 6A) and acid-evoked currents in transduced neurons (Fig. 6C) (t(6) = 2.894, p = 0.0275, n = 9, n = 7). Moreover, AAV-ASIC1A-injected Asic1a−/− mice had a significant increase in 10% CO2-evoked freezing relative to their AAV-eGFP-injected counterparts (Fig. 6D) (F(5,40) = 13.10, p < 0.0001, n = 5, n = 9, n = 7, n = 7, n = 7, n = 8, one-way ANOVA, t(12) = 3.165, p = 0.0081). To test for site specificity, we also transduced the dorsal hippocampus with AAV-ASIC1A or AAV-eGFP (Fig. 6B) and found no effect on 10% CO2-evoked freezing (Fig. 6D) (t(12) = 1.231, p = 0.2420). Finally, to test whether we could use this system to exaggerate CO2-evoked behavior, we injected AAV-ASIC1A versus AAV-eGFP into the BNST of Asic1a+/+ mice. We found that, in the background of normal ASIC1A expression, targeting AAV-ASIC1A to the BNST was sufficient to significantly increase CO2-induced freezing (Fig. 6D) (t(13) = 2.755, p = 0.0164), whereas these manipulations did not significantly affect general locomotor activity (Fig. 6E) (F(5,40) = 0.7138, p = 0.1547) or center activity (F(5,40) = 0.1.594, p = 0.1662) in the open field.
Figure 6.
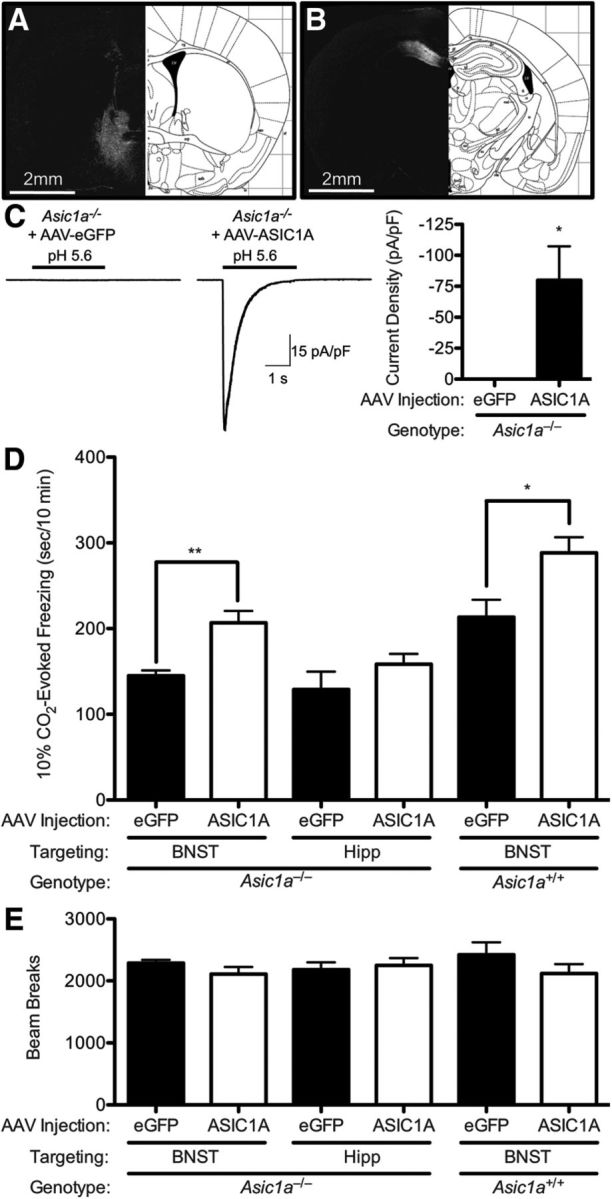
Expressing ASIC1A in the BNST is sufficient to increase CO2-evoked freezing. A, ASIC1A immunofluorescence in the BNST (white) of Asic1a−/− mice injected with AAV-ASIC1A. B, ASIC1A immunofluorescence in the dorsal hippocampus (white) of Asic1a−/− mice injected with AAV-ASIC1A. C, Whole-cell voltage-clamp recordings of neurons from BNST slices. AAV-ASIC1A produced acid-evoked currents in the BNST of Asic1a−/− mice. D, Quantification of freezing evoked by 10% CO2 in mice of indicated genotypes, viruses, and injection sites. In Asic1a−/− mice, AAV-ASIC1A in the BNST significantly increased freezing relative to AAV-eGFP injected controls, whereas AAV-ASIC1A in dorsal hippocampus (Hipp) did not. In Asic1a+/+ mice, AAV-ASIC1A in the BNST significantly increased CO2-evoked freezing relative to AAV-eGFP controls. E, Infusion of AAV-ASIC did not alter locomotion in the open field.
Discussion
Our results identify the BNST as a key structure in CO2-evoked fear-related behaviors. Not only does the BNST mediate CO2-evoked freezing and conditioned avoidance, our data suggest that CO2 acidifies the BNST and that the BNST can directly detect acidosis, through ASIC1A. These findings are consistent with a previously established role for the BNST in rodent models of unconditioned and conditioned fear (LeDoux et al., 1988; Lee and Davis, 1997; Fendt et al., 2003; Sullivan et al., 2004) and for ASICs in CO2-evoked behavior (Ziemann et al., 2009; Price et al., 2014).
Several lines of evidence suggest that the BNST is pH sensitive. CO2 inhalation lowers pH in the BNST. Explicitly and directly manipulating pH in the BNST evokes freezing behavior. A pH-sensitive channel, ASIC1A, is expressed in the BNST. And CO2- and acidosis-evoked behaviors depend on ASIC1A. Together, these observations suggest that ASIC1A in the BNST directly detects CO2-induced acidosis. However, we cannot rule out the possibility that acidosis activates some other pH-sensitive receptive mechanisms, which depend on ASIC1A in the BNST.
The observation that structures in the fear circuit are pH sensitive raises questions about the adaptive value of sensing pH deep within the CNS. By detecting rising CO2 levels, the BNST and amygdala might alert the animal to a threat of suffocation (Klein, 1993). But why is CO2 detection in the forebrain of value given that CO2 and its diverse physiological effects can be detected or inferred through a variety of other mechanisms, such as smell (Hu et al., 2007), taste (Chandrashekar et al., 2009), and interoception (e.g., chest wall movement) (Flume et al., 1996), or inspiratory resistance (Lang et al., 2011)? Perhaps CO2 sensitivity in the brain functions as a failsafe mechanism or as a last resort when other means of detecting suffocation and rising CO2 are inadequate. Or perhaps the pH sensitivity in the fear circuit helps the organism recognize the experience of CO2 inhalation as a danger. Or maybe pH sensitivity in deep CNS structures is a conserved vestige from simpler organisms (Cummins et al., 2014). Alternatively, pH-sensitive molecules may normally play other roles and might be serendipitously activated by CO2. For example, ASICs are localized on dendritic spines (Zha et al., 2006) and have been suggested to be activated by protons released during synaptic transmission (Wemmie et al., 2013). Regardless of these questions, the finding that multiple forebrain structures detect acidosis raises the possibility that brain pH may play a more general role in brain function and behavior.
One limitation of our lesion and viral studies is that they are not sufficiently precise to discern which BNST subnuclei are critical for CO2-evoked freezing and conditioned avoidance. This could be particularly important as recent studies have suggested opposing roles for BNST subnuclei in anxiety-related behavior (Haufler et al., 2013; Kim et al., 2013). For example, Kim et al. (2013) found that the oval nucleus of the BNST (ovBNST) promoted anxiety-like behavior, whereas the anterodorsal BNST (adBNST) reduced anxiety-like behavior in the elevated plus maze. Nevertheless, our findings are consistent with other en masse manipulations of the BNST (LeDoux et al., 1988; Lee and Davis, 1997; Fendt et al., 2003; Sullivan et al., 2004; Kim et al., 2013). Although our current methods did not hit the ovBNST without hitting surrounding structures, infusing smaller volumes of virus might limit spread and increase site specificity. However, the apparent anxiety-promoting effects on CO2-evoked behaviors detected here suggest that the ovBNST might be responsible.
The amygdala has also been implicated in CO2-evoked behaviors (Ziemann et al., 2009; Feinstein et al., 2013). This, coupled with our results herein, raises questions about the respective roles of the BNST and amygdala and their potential relationship. Are these roles duplicative, or do these two structures play unique roles that we have not yet been able to discern? Do these structures function independently, or do they function together, perhaps in series? Established interconnections between the BNST and amygdala suggest that the two structures may function together as a part of a network (Davis, 2002; Walker and Davis, 2008; Davis et al., 2010). However, because both the BNST and amygdala consist of multiple interconnected subnuclei, the relationship between nuclei in this network could be quite complicated. Other brain structures, such as the parabrachial nucleus, which receives input from the BNST, may also play a role (Kaur et al., 2013). Future studies will be necessary to further characterize these complex circuits.
Freezing is thought to represent an important defensive response to threat. For example, in a predator–prey confrontation, freezing probably serves the prey by helping to avoid detection. The finding that BNST lesions not only reduced CO2-evoked freezing, but also reduced conditioned place avoidance to CO2 is consistent with BNST-dependent responses as expression of negative emotion. However, it is not yet clear why freezing might be an adaptive in circumstances associated with rising levels of CO2. Perhaps when CO2 is high or pH is low, movement is risky from a cardiorespiratory standpoint. Or perhaps freezing is an orienting response to further assess any type of threat. Furthermore, under some conditions, freezing might be counteradaptive by slowing escape from dangerously high CO2 levels. It remains uncertain what CO2-evoked freezing behavior represents or how it might be related to the anxiety, fear, and panic evoked by CO2 in humans.
Nevertheless, our mouse studies suggest that the BNST might be important for CO2-evoked responses in humans and that ASICs may be critical. Our data raise the possibility that elevated ASIC1A expression or function in the BNST might exaggerate CO2-triggered responses in humans, thus suggesting a theoretically plausible mechanism for increased CO2 sensitivity in panic disorder patients. Similarly, our results suggest that the BNST might be a critical extra-amygdalar structure underlying elevated CO2 responses in humans with amygdala lesions (Feinstein et al., 2013; Smoller et al., 2014). Conversely, our results suggest that reducing ASIC1A expression or function in the BNST may reduce CO2 responses and that ASIC1A antagonists might have therapeutic potential for anxiety disorders, especially those accompanied by CO2 hypersensitivity.
Footnotes
J.A.W. was supported by the Department of Veterans Affairs (Merit Award), the National Institute of Mental Health (5R01MH085724), National Heart, Lung, and Blood Institute (R01HL113863), and a National Alliance for Research on Schizophrenia and Depression Independent Investigator Award. C.J.K. was supported by National Institute of Neurological Disorders and Stroke Training Grant T32NS045549. We thank John Freeman, George Richerson, Michael Welsh, Jason Allen, and Amanda Wunsch for their recommendations and assistance; and the University of Iowa Central Microscopy Facility and Gene Transfer Vector Core.
The authors declare no competing financial interests.
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU. BNST neurocircuitry in humans. Neuroimage. 2014;91:311–323. doi: 10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox PT. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry. 2007;62:1140–1148. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, McBride JL, Davidson BL, Wemmie JA. Restoring acid-sensing ion channel-1a in the amygdala of knock-out mice rescues fear memory but not unconditioned fear responses. J Neurosci. 2008;28:13738–13741. doi: 10.1523/JNEUROSCI.3907-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EP, Selfridge AC, Sporn PH, Sznajder JI, Taylor CT. Carbon dioxide-sensing in organisms and its implications for human disease. Cell Mol Life Sci. 2014;71:831–845. doi: 10.1007/s00018-013-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural circuitry of anxiety and stress disorders. In: Davis KL, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: the fifth generation of progress. Philadelphia: Lippincott, Williams and Wilkins; 2002. pp. 931–951. [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel G, Schruers K, Griez E. Experimental models: panic and fear. In: Blanchard RJ, Griebel G, Nutt D, editors. Handbook of anxiety and fear. New York: Elsevier; 2008. pp. 413–434. [Google Scholar]
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88:1500–1511. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, Wemmie JA. Fear and panic in humans with bilateral amygdala damage. Nat Neurosci. 2013;16:270–272. doi: 10.1038/nn.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flume PA, Eldridge FL, Edwards LJ, Mattison LE. Relief of the ‘air hunger’ of breathholding: a role for pulmonary stretch receptors. Respir Physiol. 1996;103:221–232. doi: 10.1016/0034-5687(95)00094-1. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- Gregersen N, Dahl HA, Buttenschøn HN, Nyegaard M, Hedemand A, Als TD, Wang AG, Joensen S, Woldbye DP, Koefoed P, Kristensen AS, Kruse TA, Børglum AD, Mors O. A genome-wide study of panic disorder suggests the amiloride-sensitive cation channel 1 as a candidate gene. Eur J Hum Genet. 2012;20:84–90. doi: 10.1038/ejhg.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20:633–641. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska-Hageman AM, Benson CJ, Leonard AS, Price MP, Welsh MJ. PSD-95 and Lin-7b interact with acid-sensing ion channel-3 and have opposite effects on H+-gated current. J Biol Chem. 2004;279:46962–46968. doi: 10.1074/jbc.M405874200. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions: an integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Wangelin BC, Bradley MM, Versace F, Davenport PW, Costa VD. Threat of suffocation and defensive reflex activation. Psychophysiology. 2011;48:393–396. doi: 10.1111/j.1469-8986.2010.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, Robillard R, Lancaster J, Zamarripa FE, Fox PT, Denton D. Brain responses associated with consciousness of breathlessness (air hunger) Proc Natl Acad Sci U S A. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, van Kuyck K, Vansteenwegen D, Nuttin B. Electrolytic lesions of the bed nucleus of the stria terminalis disrupt freezing and startle potentiation in a conditioned context. Behav Brain Res. 2011;222:357–362. doi: 10.1016/j.bbr.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Martínez-Lorenzana G, Jiménez JR, Condés-Lara M. Interamygdaloid connection of basolateral nucleus through the anterior commissure in the rat. Neurosci Lett. 2004;366:154–157. doi: 10.1016/j.neulet.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Faraci FM, Heistad DD. Effects of vasodilatation and acidosis on the blood–brain barrier. Microvasc Res. 1988;35:179–192. doi: 10.1016/0026-2862(88)90061-1. [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Carman CT, Severinghaus JW, Richardson BW, Singer MM, Shnider S. Stability of cerebrospinal fluid pH in chronic acid-base disturbances in blood. J Appl Physiol. 1965;20:443–452. doi: 10.1152/jappl.1965.20.3.443. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Ed 2. San Diego: Academic; 2001. [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Aroniadou-Anderjaska V, Prager EM, Figueiredo TH, Almeida-Suhett CP, Miller SL, Braga MF. ASIC1a activation enhances inhibition in the basolateral amygdala and reduces anxiety. J Neurosci. 2014;34:3130–3141. doi: 10.1523/JNEUROSCI.4009-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, Gong H, Parsons MG, Kundert JR, Reznikov LR, Bernardinelli L, Chaloner K, Buchanan GF, Wemmie JA, Richerson GB, Cassell MD, Welsh MJ. Localization and behaviors in null mice suggest that ASIC1 and ASIC2 modulate responses to aversive stimuli. Genes Brain Behav. 2014;13:179–194. doi: 10.1111/gbb.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y, Kushner MG. Carbon dioxide in the study of panic disorder: issues of definition, methodology, and outcome. J Anxiety Disord. 2003;17:1–32. doi: 10.1016/S0887-6185(02)00181-0. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Gallagher PJ, Duncan LE, McGrath LM, Haddad SA, Holmes AJ, Wolf AB, Hilker S, Block SR, Weill S, Young S, Choi EY, Rosenbaum JF, Biederman J, Faraone SV, Roffman JL, Manfro GG, Blaya C, Hirshfeld-Becker DR, Stein MB, et al. The human ortholog of acid-sensing ion channel gene ASIC1a is associated with panic disorder and amygdala structure and function. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.018. doi: 10.1016/j.biopsych.2013.12.018. Advance online publication. Retrieved Jan. 18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/S0896-6273(02)00661-X. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103:16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]