Abstract
Failure of remyelination in diseases, such as multiple sclerosis (MS), leads to permanent axonal damage and irreversible functional loss. The mechanisms controlling remyelination are currently poorly understood. Recent studies implicate the cyclin-dependent kinase 5 (Cdk5) in regulating oligodendrocyte (OL) development and myelination in CNS. In this study, we show that Cdk5 is also an important regulator of remyelination. Pharmacological inhibition of Cdk5 inhibits repair of lysolecithin lesions. This inhibition is a consequence of Cdk5 disruption in neural cells because remyelination in slice cultures is blocked by Cdk5 inhibitors, whereas specific deletion of Cdk5 in OLs inhibits myelin repair. In CNP-Cre;Cdk5fl/fl conditional knock-out mouse (Cdk5 cKO), myelin repair was delayed significantly in response to focal demyelinating lesions compared with wild-type animals. The lack of myelin repair was reflected in decreased expression of MBP and proteolipid protein and a reduction in the total number of myelinated axons in the lesion. The number of CC1+ cells in the lesion sites was significantly reduced in Cdk5 cKO compared with wild-type animals although the total number of oligodendrocyte lineage cells (Olig2+ cells) was increased, suggesting that Cdk5 loss perturbs the transition of early OL lineage cell into mature OL and subsequent remyelination. The failure of remyelination in Cdk5 cKO animals was associated with a reduction in signaling through the Akt pathway and an enhancement of Gsk-3β signaling pathways. Together, these data suggest that Cdk5 is critical in regulating the transition of adult oligodendrocyte precursor cells to mature OLs that is essential for myelin repair in adult CNS.
Keywords: cyclin-dependent kinase 5, demyelination, myelination, oligodendrocyte, remyelination
Introduction
In the vertebrate CNS, demyelination is characterized by oligodendrocyte loss and axonal damage resulting in irreversible functional loss (Patrikios et al., 2006). In diseases, such as multiple sclerosis (MS), demyelination is generally a consequence of immune-mediated damage that early in the disease may be offset by effective remyelination (Jeffery and Blakemore, 1997; Franklin, 2002; Liebetanz and Merkler, 2006; Franklin and Ffrench-Constant, 2008), although remyelination eventually fails most likely because of the failure of recruitment or differentiation of adult oligodendrocyte precursor cells (OPCs) (Chang et al., 2000).
Several factors have been implicated in adult OPC responses to demyelinating insults. These include growth factors, such as PDGF-AA, neuregulin-1, IGF-1, and FGF, transcription factors (MRF, Sox-10, and Olig2), and signaling molecules, including retinoid X receptor γ, estrogen receptor β, and the androgen receptor (Huang et al., 2011; Kumar et al., 2013), PI3K/Akt/mTOR, Gsk3β (Flores et al., 2008; Narayanan et al., 2009; Galimberti et al., 2011). Cell-to-cell interactions mediated by LINGO-1, Wnt/β-catenin, and Notch1/Jagged have also been identified as regulators of myelination (Mi et al., 2008; Fancy et al., 2011; Galimberti et al., 2011; Huang and Franklin, 2011; Aparicio et al., 2013). Many of these pathways are simultaneously altered after injury, and how these multiple inputs are coordinated to modulate myelin repair remains unclear. It seems likely, however, there are a small number of signal integrators, one of which is cyclin-dependent kinase 5 (Cdk5).
Cdk5 is an atypical cell cycle kinase activated by binding its coactivators p35 or p39 (Lew et al., 1994; Tsai et al., 1994). Cdk5 is a master regulator of neural differentiation, cortical lamination, neuronal migration, axon guidance, membrane transport, and synaptic plasticity in the CNS (Tsai et al., 1993; Ohshima et al., 1996; Gilmore et al., 1998; Patrick et al., 1998; Gupta and Tsai, 2003; Gupta et al., 2003). Recently, oligodendrocyte development and myelination have been shown to be influenced by Cdk5 (Miyamoto et al., 2007, 2008; Bankston et al., 2013; Yang et al., 2013). Cdk5 activity increases during OPC differentiation and its loss results in reduced differentiation and migration of OPCs in vitro (Miyamoto et al., 2007, 2008). Furthermore, selective deletion of Cdk5 in Olig1+ cells delays OPC maturation and myelination (Yang et al., 2013) that is replicated in the p39 KO (Bankston et al., 2013).
Here we show that Cdk5 expression increases in a lysolecithin (LPC) dorsal spinal cord lesion. Localized pharmacological inhibition of Cdk5 activity resulted in reduced remyelination, and these data were confirmed in slice cultures. Oligodendrocyte lineage expression of Cdk5 influences remyelination. LPC lesions in CNP-Cre-mediated Cdk5 conditional knock-out mice (Cdk5 cKO) demonstrated significantly reduced myelin repair. Ultrastructural analyses confirmed a reduction in the number of myelinated axons in Cdk5 cKO accompanied by a more robust inflammatory response. Inhibition of remyelination in the absence of Cdk5 reflected modulation of signaling through Akt and Gsk-3β pathways, suggesting that Cdk5 acts as an integrator of multiple signals and modulates OPC behavior in demyelination/remyelination. Modulation of Cdk5 activity or its downstream targets may provide pharmacological potential for oligodendrocyte regeneration and repair for CNS demyelinating diseases.
Materials and Methods
Generation of conditional CNPCre/+;Cdk5fl/fl knock-out mouse.
All animal experiments were done in compliance with approved animal policies of the Institutional Animal Care and Use Committee at Case Western Reserve University School of Medicine. Floxed Cdk5 mouse breeding pairs were obtained from Dr. LiHui Tsai at MIT. CNPCre/+ mouse was obtained from Dr. K.A. Nave (Lappe-Siefke et al., 2003). To inactivate the Cnp1 gene in CNPCre/+ mouse, the CNP alle is disrupted by insertion of Cre recombinase ORF (Lappe-Siefke et al., 2003). CNP is a widely used maker of myelin-forming glial cells (Knapp et al., 1988; Yu et al., 1994) and is maintained in mature oligodendrocytes. Initially, Cdk5fl/fl mice were crossed with CNPCre/+ to generate double-transgenic CNPCre/+;Cdk5fl/+ mice, which were intercrossed to Cdk5fl/fl mice to produce a conditional knock-out mouse of CNPCre/+;Cdk5fl/fl (Cdk5 cKO). To determine the CNP Cre recombination rate, floxed Cdk5 homozygous were crossed with a Rosa;YFP reporter mouse (The Jackson Laboratory) to generate Cdk5fl/fl;Rosa YFP/YFP, bred onto CNPcre/+;Cdk5fl/+ to generate CNPcre/+;Cdk5fl/fl; RosaYFP/+ animals. For genotyping, genomic DNA was isolated from tail biopsies and mouse genotypes determined by PCR as described previously (Lappe-Siefke et al., 2003). Loss of Cdk5 protein in Cdk5 cKO was confirmed by immunoblot. Both CNPCre/+;Cdk5+/+ and CNPCre/+;Cdk5fl/+ heterozygotes served as controls because the heterozygotes of CNPCre/+;Cdk5fl/+ had no detectable phenotype compared with wild-type (WT) animals.
LPC-induced focal demyelination in dorsal spinal cord.
Eight- to 9-week-old conditional CNPcre/+;Cdk5fl/fl knock-out mice of either sex (Cdk5 cKO) and CNPcre/+; Cdk5+/+ WT littermate controls (WT) were used for LPC-induced focal demyelinating studies. Animals were deeply anesthetized with isoflurane, and a laminectomy was performed. After exposure of spinal cord, 1.5 μl of 1% LPC (L-a-lysophosphatidyl-choline, lysolectin) (Sigma) in 0.9% sodium chloride solution was microinjected using a pulled glass fine tip into the dorsal column at T11–T12 spinal cord at a rate of 0.25 μl/min. The needle was removed after a delay of 5 min to minimize back flow and the lesion closed. Postoperatively, animals received a subcutaneous injection of 2–3 ml of saline to prevent dehydration. The day of LPC injection was designated as day 0 (0 dpl). Mice were allowed to recover for 3, 7, 14, 21, and 28 dpl before death. All tissues were fixed with either 4% PFA or 2% glutaraldehyde for further histology and ultrastructural analysis. Control animals received an equivalent injection of saline, and tissues were collected according to the same paradigm.
For second injection of the Cdk5 inhibitor roscovitine (10 μg/1.5 μl), Akt activator SC79 (10 μg/1.5 μl), or 0.9% saline, animals were anesthetized 4 d after LPC lesion and Cdk5 inhibitor or Akt activator was delivered to the same area using the above paradigm. Animals were allowed to recover and killed at 12 dpl. Lesion sizes were determined by staining of serial sections with luxol fast blue.
LPC-induced demyelination in mouse cerebellar slice cultures.
The 300-μm-thick cerebellar slices were cut from P12 mouse Cerebellum using a Leica vibrating microtome (Leica, VT1000S) and immediately placed into cell-culture inserts (0.4 μm, Millicell-CM, Millipore). Slices were grown in medium containing 50% basal medium eagle medium, 25% heat-inactivated horse serum, 25% Hank's solution, 2.5% glucose, 1% glutamine, and penicillin-streptomycin. After 4 DIV, demyelination was induced by addition of 0.5 mg/ml LPC to the medium for 17–18 h. Slices were then incubated with medium ±10 μm roscovitine for 4 d, and remyelination assayed by MBP and NeuF200 visualization as below.
Slices were fixed with 4% PFA, delipidated, and incubated with primary antibodies anti-MBP (1:500, SMI-99, Covance) and anti-neurofilament (1:400, N4142, Sigma) overnight at 4°C followed by fluorescence-conjugated anti-mouse secondary antibody AlexaFluor-594 (1:500, Invitrogen) and anti-rabbit AlexaFluor-488 (1:500, Invitrogen) for 1 h. Cultured slices were mounted in Vectashield mounting medium with DAPI (Vector Laboratories) and analyzed using Leica DFC500 fluorescence microscope.
Immunohistochemistry.
Animals were perfused with 4% PFA. The LPC-lesioned spinal cords dissected and postfixed in 4% PFA overnight at 4°C, followed by equilibration in 20% sucrose. The 20 μm cryosections of spinal cord were collected and stored at −80°C. Antigen retrieval was performed by using Reveal Decloaker solution (Biocare Medical) before immunostaining. Sections were incubated with primary antibodies overnight at 4°C (MBP: SMI-99, 1:500, Covance; proteolipid protein [PLP]: 1:500, Abcam; Olig2: 1:250, Millipore; CC1: 1:250, Calbiochem; Caspase-3: 1:100, Cell Signaling Technology; Ki67: 1:100, BD Biosciences PharMingen; Iba1: 1:250, Wako Chemicals; GFAP: 1:500, DAKO; neurofilament 1:400, Sigma, Cdk5: 1:200, Santa Cruz Biotechnology), washed and incubated with fluorescently conjugated secondary goat anti-rabbit IgG AlexaFluor-594 or goat anti-mouse IgG 488 (1:500; Invitrogen) antibodies for 1 h at room temperature. Images were collected and analyzed using a Leica DFC500 fluorescence microscope.
Tissue preparation for electron microscopy analysis.
For ultrastructural analyses, animals were anesthetized and perfused with 2% glutaraldehyde/4% PFA in 0.1 m sodium carcodylate buffer, pH 7.4 (Electron Microscopy Sciences). The LPC-lesioned spinal cords were dissected and postfixed in 1% OsO4, dehydrated through a series of graded ethanol, and stained using saturated uranyl acetate and embedded in a Poly/Bed812 resin (Polysciences). The 1 μm coronal sections were cut and stained with toluidine blue and appropriate areas for ultrathin sections selected for EM. Ultrathin section (0.1 μm) from matching lesioned spinal cord areas of Cdk5 cKO and WT tissue blocks were cut and visualized using an electron microscope (JEOL100CX) at 80 kV. G ratios were calculated from at least 50–100 randomly selected myelinated axons. The percentage of unmyelinated of total axons was calculated from at least 5 or 6 samples from 3 or 4 different animals in each condition.
Biochemical analysis: Western blots.
Samples from LPC-lesioned spinal cords were micro-dissected, homogenized with RIPA lysis buffer containing protease inhibitor mixture and phosphatase inhibitor mixture. Equal amounts of protein were loaded and separated by 10% (for p-Akt and Akt), 12% (for p-Gsk-3β and Gsk-3β), and 15% (for MBP and PLP) SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in 5% BSA in PBS buffer containing 0.1% Tween20 overnight at 4°C and incubated with primary antibodies followed by secondary antibodies of anti-mouse or anti-rabbit IgG conjugated to HRP. The following primary antibodies were used: p-Akt (Ser473) (1:500, Cell Signaling Technology); Akt (1:1000, Cell Signaling Technology); p-Gsk-3β (Ser 9) (1:500, Cell Signaling Technology); Gsk-3β (1:1000, Millipore); mTOR (1:1000, Cell Signaling) and P-mTOR (1:500, Cell Signaling); MBP (1:1000, Covance Compay); PLP (1:1000, Abcam) and β-tubulin (1:1000, Sigma). The density of immunoblotting was quantified using ImageJ software against appropriate loading controls.
Statistical analyses.
For cell counts, 3 or more nonadjacent sections from each animal taken from a total of 3–5 animals from WT and CKO were counted and analyzed. Lesion volumes were calculated by the lesion area from serial sections throughout the entire lesion based on the equation for volume of cylinder (V= lesion area × length of lesion). Extracted proteins were collected from at least 3 or 4 animals from WT and cKO groups. Data are presented as mean ± SEM followed by p value. Data statistical analysis was performed using the two-way ANOVA tests for comparison of Cdk5 cKO and WT groups. p values <0.05 were considered statistically significance.
Results
Emerging evidence suggests that Cdk5 is important for the development of oligodendrocytes (Miyamoto et al., 2007, 2008; Bankston et al., 2013; Yang et al., 2013) in addition to its role in neuronal development (Tsai et al., 1993; Gilmore et al., 1998). Less is known about the role of Cdk5 in modulating remyelination in the adult CNS. To begin to address this issue, we used an LPC lesion model combined with targeted deletion of Cdk5 in the oligodendrocyte lineage and ex vivo analyses.
Alteration of Cdk5 expression in the spinal cord after LPC induced focal demyelination
To determine whether demyelinating insults to adult white matter alter the expression of Cdk5 and its activators p35 and p39, an LPC lesion was generated in the dorsal columns of adult C57BL/6 mice and the local levels of Cdk5 and its activators assayed 12 d after lesion induction. Compared with saline-injected controls, in LPC-lesioned animals, the levels of Cdk5 were significantly elevated in the region of the lesion (Fig. 1Aa,Ab), including in cells of the oligodendrocyte lineage (Fig. 1B), suggesting that Cdk5 may modulate myelin repair in the adult spinal cord. To begin to assess the role of Cdks in myelin repair, their function was blocked by localized delivery of the Cdks inhibitor roscovitine to the lesion area 4 d after LPC injection. This delay allowed the majority of the LPC induced immune response to diminish (Ghasemlou et al., 2007) and repair to be initiated before Cdk inhibition.
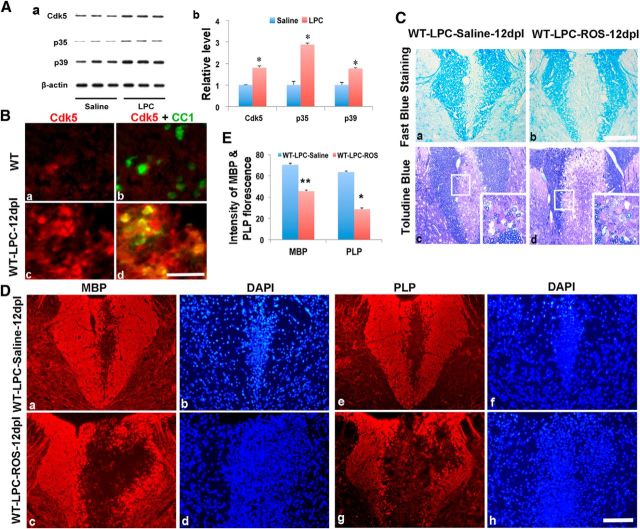
Figure 1.
Cdk5 is upregulated after LPC-induced focal lesion in C57BL6 WT animals. A, Western blot analyses showed increased expression of Cdk5 and its activators p35 and p39 in LPC-induced lesion of spinal cord at 12 dpl (Aa). B, Double immunostaining showed increased Cdk5 expression after LPC-induced spinal cord lesion in WT animals at 12 dpl (Bc) compared with nonlesioned controls (Ba). Cdk5 (red) expression colocalized with CC1+ cells (green) in LPC-lesioned area (Bd) compared with nonlesioned area (Bb). C, Luxol fast blue staining (Ca, Cb) and Toludine blue staining (Cc, Cd) of lesion areas of dorsal spinal cord showed larger lesion areas and reduced numbers of myelinated axons in the LPC-lesioned animals injected with roscovitine (ROS) (Cb, Cd) compared with saline-injected control animals (Ca, Cb) at 12 dpl. D, Immunostaining of MBP and PLP in LPC-lesioned spinal cord demonstrated a significantly larger area of demyelination in ROS-treated animals (Dc, Dd, Dg, Dh) compared with saline-injected control animals (Da, Db, De, Df) at 12 dpl. Insets, The lesion area at higher magnification. E, Quantification of immunofluorescent intensity of MBP and PLP in LPC-induced lesion areas of the lesioned spinal cord treated with ROS or saline. Values are mean ± SEM (n = 3). *p < 0.05. Scale bars, 25 μm.
Myelin repair was significantly impaired in animals that received local injection of roscovitine compared with animals receiving saline controls. Twelve days after lesion induction, the average lesion volume in roscovitine-treated animals was >3.5× larger 6.95 ± 0.06 mm3 (Fig. 1Cb) compared with 1.95 ± 0.056 mm3 in control animals (Fig. 1Ca). The larger lesion size was correlated with a lack of remyelination. In 1 μm Epon sections through similar lesion regions, remyelination was apparent in saline controls (Fig. 1Cc, inset); by contrast, little evidence of remyelination was apparent in roscovitine-treated animals (Fig. 1Cd, inset). Immunohistochemistry also revealed reductions in the expression of MBP and PLP in the dorsal spinal cord LPC lesions in roscotivine-treated animals (Fig. 1Dc,Dd,Dg,Dh) compared with control (Fig. 1Da,Db,De,Df). Quantitative data of the intensity of MBP and PLP fluorescence in lesioned dorsal spinal cord confirmed the reduction of MBP and PLP (Fig. 1E). These data suggest that inhibiting Cdks (including Cdk5) activity during the initial phase of myelin repair compromises recovery. Treatment with roscovitine may inhibit multiple Cdks in multiple cell types in the lesion area, although only a slight decrease in the number of Olig2/Ki67 double-labeled cells was seen after roscovitine treatment (data not shown), which may reflect the inhibition of Cdk2 (Caillava et al., 2011). To specifically assess the role of neural cell Cdks in myelin repair, a remyelinating slice culture model was used.
Roscovitine treatment inhibits remyelination in cerebellar slice culture
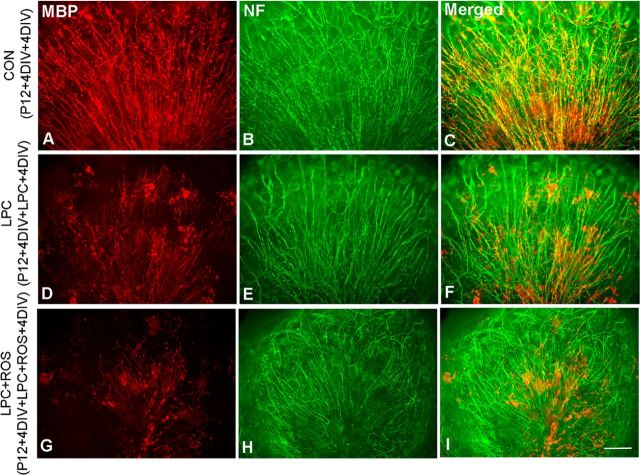
Treatment of myelinated cerebellar slices with LPC results in demyelination that repair spontaneously once LPC is removed (Birgbauer et al., 2004; Zhang et al., 2011). To assess whether this recovery is dependent on Cdk signaling, mouse postnatal day 12 (P12) cerebellar slices were grown 4 d to allow myelin formation in vitro and then treated either with or without 0.1% LPC for 17 h to induce demyelination. Cultures were then grown for 4 further days in normal media to allow remyelination. Experimental slices were treated with roscovitine to inhibit Cdks after LPC exposure, and the level of myelin recovery was compared with nontreated controls. In control slice cultures not treated with either LPC or roscovitine, abundant myelinated axons were found in white matter as shown by the coregistration of myelinating processes (MBP) and neurofilament (NF) expressing axons (Fig. 2A–C). By contrast, in LPC-treated slices, whereas axons remained intact, the myelin was severely disrupted and appeared as punctate and disorganized. After a 4-day recovery period, myelin had begun to reform; and although some areas remained disrupted, remyelination was apparent throughout the slices (Fig. 2D–F). The demyelinated phenotype was more pronounced and remyelination severely limited in slices treated with roscovitine (Fig. 2G–I). Although the pattern of the axons was not dramatically altered and they largely retained their radial orientation, there was a substantial reduction in the extent of MBP expression and much of the remaining MBP after the 4-day recovery was not oriented along axons, consistent with a lack of remyelination. These data suggest that the primary target of roscovitine during remyelination is Cdk5 expressed in cells of the oligodendrocyte lineage. To directly examine the role of Cdk5 in the oligodendrocyte lineage during remyelination, it was selectively deleted from cells of the oligodendrocyte lineage using a CNP targeting approach.
Figure 2.
Inhibitory effects of roscovitine (ROS) on remyelination in LPC-induced demyelination in C57BL6 WT cerebellar slice cultures. Cerebellar slice cultures from P12 C57BL/6 mice were grown for 4 d and then treated with 0.1% LPC for 17 h to induce demyelination, followed by a 4 d recovery period in medium with or without ROS. Immunostaining of MBP (red) and neurofilament (NF, in green) in nontreated, positive control slice culture (A–C), reduced remyelination in LPC-induced demyelinating slice culture alone (D–F), and severely disturbed remyelination in LPC-lesioned slice culture-treated with ROS (G–I). Scale bar, 25 μm.
Deletion of Cdk5 in CNP+ cells results in a delay of myelin repair after LPC spinal cord lesions
To determine whether delayed myelin repair reflected loss of Cdk5 function specifically in oligodendrocytes, a conditional CNPcre/+;Cdk5fl/fl cKO (Cdk5 cKO) line was generated using Cre-Lox technology to specifically delete Cdk5 in CNP+ cells. Expression of CNP mRNA starts ∼E12-E14 in CNS (Yu et al., 1994), and CNP protein is expressed in mature OL and lasts into the adult. The Cdk5 cKO animals were born at the expected ratio and showed no significant functional deficits until 2 months of age when they exhibited a stress-induced tremor. Previous studies demonstrate that selective deletion of Cdk5 from Olig1+ cells perturbed oligodendrocyte development. To assess in detail the development of oligodendrocytes and myelination in the spinal cord of CNP cKO animals sections were labeled with antibodies to Olig2, CC1 MBP, GFAP, IBa1, and neurofilament at P14, 21 and 2 months of age. No significant differences in the spinal cord development were seen between the cKO and WT animals (Fig. 3A). Detailed analysis of the number of Olig2 and CC1+ cells at P2, 7, 14, and 21 also did not reveal significant differences between cKO and WT animals (Fig. 3B,C). Likewise, analysis of the levels of myelination and g ratios showed no significant differences between cKO and WT animals at P21 (Fig. 3D). Similar analyses on animals at 2 months of age did, however, show a slight thinning of the myelin sheaths in the cKO relative to WT littermates (Fig. 3D), suggesting that, in contrast to the Olig1 cKO animals, deletion of Cdk5 using CNP targeting does not affect development but may disrupt myelin maintenance. To assess the rate of recombination in the CNP cKOs CNPcre/+;Cdk5fl/fl; RosaYFP/+ animals, in which the Cdk5 gene was conditionally deleted in CNP+ cells and marked with YFP reporter alle were generated. In the spinal cords of both CNPcre/+;Cdk5+/+; RosaYFP/+ WT and CNPcre/+;Cdk5fl/fl; RosaYFP/+ cKO animals ∼70% of CC1+ cells were double-labeled with YFP, suggesting that the recombination rate is similar and >70% in both WT and Cdk5 cKO animals (Fig. 4Aa,Ab). The level of Cdk5 was reduced by ∼40% in cKO animals with residual expression likely to be in neurons. To assess the effect of deletion of Cdk5 in the oligodendrocyte lineage on remyelination, a local LPC lesion was generated in the dorsal spinal cord of WT and Cdk5 cKO animals at ∼2 months of age and the rate of repair assessed. The extent of the lesions in Cdk5 cKO was significantly larger after 3, 7, 14, and 21 dpl (Fig. 4Bb,Bd,Bf,Bh) compared with lesions in WT animals (Fig. 4Ba,Bc,Be,Bg). In both genotypes, lesion size peaked ∼7 dpl.
Figure 3.
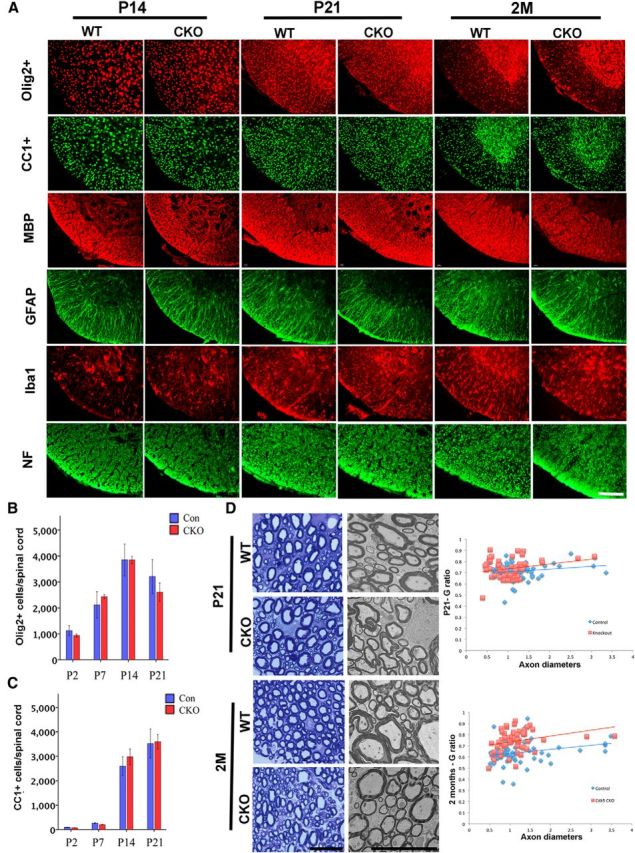
Characterization of oligodendrocyte and myelin development in CNP-Cre; Cdk5 cKO spinal cord. A, There are no significantly differences in the developing (P14 and P21) and adult spinal cords (2 months old) of WT and Cdk5 cKO in the numbers of Olig2+, CC1+ cells, GFAP+, Iba1+, and levels of neurofilament. However, by the age of 2 months, Cdk5 cKO showed reduction of MBP expression compared with control animal, B, C, Quantification of cell number and myelin formation. D, Ultrastructural analysis confirmed reduced myelin in the spinal cord of adult Cdk5 cKO at 2 months of age but not at postnatal day 21. Relative G ratios are shown in D. Scale bar, 25 μm.
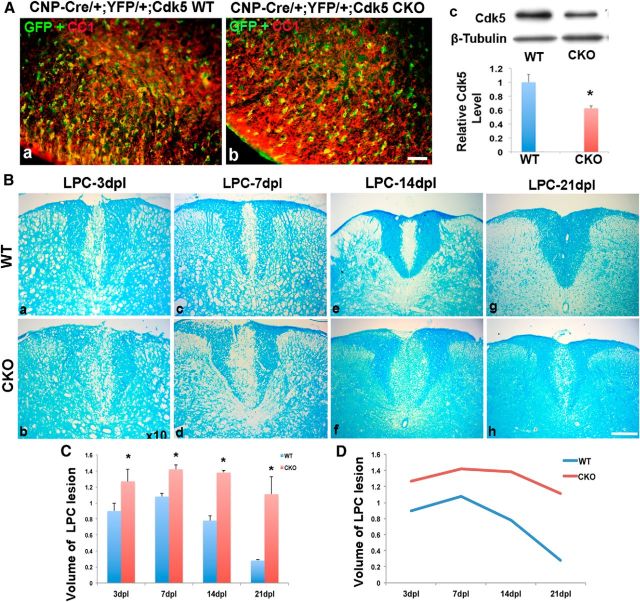
Figure 4.
Delayed myelin repair in LPC-lesioned spinal cord of Cdk5 cKO mice. A, Deletion of Cdk5 in CNP+ cells and marked with YFP reporter to define the Cre recombination rate. Similar ∼70% recombination rate showed in CC1+ cells (red) were double-labeled with YFP (GFP, green) in both CNPcre/+;Cdk5+/+; RosaYFP/+ WT (Aa) and CNPcre/+;Cdk5fl/fl; RosaYFP cKO (Ab) animals. Ac, Western blot analyses. B, Luxol fast blue staining showed larger lesioned spinal cord volumes in Cdk5 cKO mice (Bb, Bd, Bf, Bh) compared with WT mice (Ba, Bc, Be, Bg) at 3, 7, 14, and 21 dpl. C, D, Quantitative analysis of the volume of lesioned spinal cord in Cdk5 cKO mice and WT mice (C), and the progress of remyelination at 3, 7, 14, and 21 dpl. Values are mean ± SEM (n = 3). *p < 0.05. Scale bar, 25 μm.
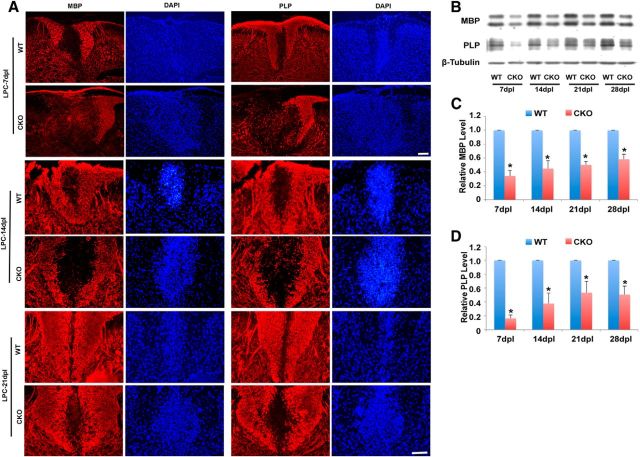
At 21 dpl, lesions in WT mice showed extensive repair (Fig. 4Bg), whereas a prominent demyelinating lesion remained at the injection site in Cdk5 cKO animals (Fig. 4Bh). Analysis of total lesion volume confirmed significantly larger lesions in Cdk5 cKO compared with WT animals at all time points (Fig. 4B,C). Comparison of the temporal rate of lesion formation and repair showed that, although the initial lesion volume was larger in Cdk5 cKO animals possibly as a result of greater susceptibility to LPC, it increased over the first 7 d in parallel with controls. By contrast, the rate of repair was markedly slower in Cdk5 cKO animals, particularly at longer postlesion periods, suggesting that later stages of myelin repair are predominantly impacted in the Cdk5 cKO animals (Fig. 4D). Consistent with this hypothesis, there was decreased expression of MBP and PLP in LPC lesions of Cdk5 cKO that increased slowly and was ∼50% of that seen in controls, even at 28 d postlesion (Fig. 5A–D). These data suggest that loss of Cdk5 in adult oligodendrocyte lineage cells results in delayed repair of demyelinating insults.
Figure 5.
Decreased expression of MBP and PLP protein in LPC-induced lesions in Cdk5 cKO animals compared with WT mice. A, Immunofluorescent staining of MBP and PLP in LPC-lesioned dorsal spinal cord of Cdk5 cKO mice and WT mice at 7 dpl (top), 14 dpl (middle), and 21 dpl (bottom). B, Western blot analysis of MBP and PLP protein expression in LPC-lesioned areas of Cdk5 cKO and WT at 7, 14, 21, and 28 dpl. C, D, Quantification of relative protein expression level of MBP and PLP in LPC-induced lesion areas of Cdk5 cKO mice and WT mice from B. Values are mean ± SEM (n = 3). *p < 0.05. Scale bar, 25 μm.
Impaired remyelination in CNPCre/+;Cdk5fl/fl cKO
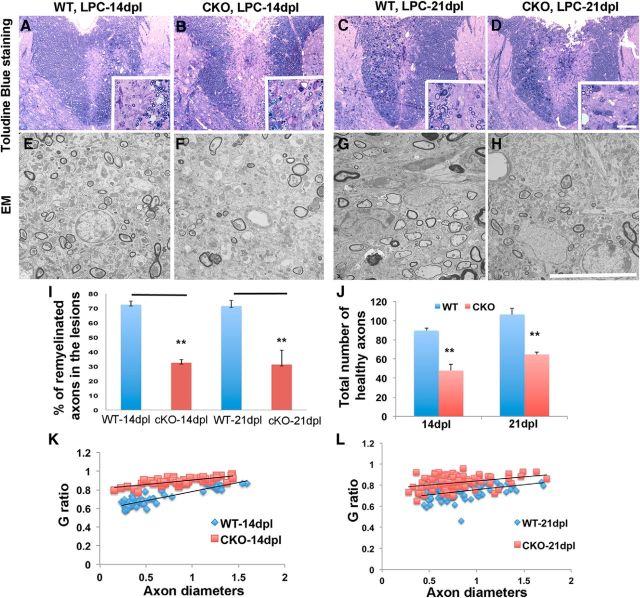
To determine whether the larger lesion sizes in Cdk5 cKO animals after LPC lesions reflected a failure of remyelination, the extent of myelin repair was assessed at 14 and 21 dpl. Analysis of 1 μm toluidine blue-stained sections confirmed larger lesions in Cdk5-cKO animals at 14 and 21 dpl and suggested reductions in the extent of remyelination compared with WT animals (Fig. 6A–D). Ultrastructural analyses revealed a significant reduction in the number of myelinated axons in LPC-lesioned Cdk5 cKO at 14 and 21 dpl (Fig. 6F,H) compared with match WT controls (Fig. 6E,G). For example, the proportion of myelinated axons was reduced from 72.5 ± 2.4% in WT animals to 32.6 ± 2.29% in Cdk5 cKO after 14 dpl and from 71.5% in WT to only 31.4% in Cdk5 cKO animals at 21 dpl (Fig. 6I). The total number of healthy unmyelinated axons was also reduced in the cKO lesions, which may reflect a more pronounced response to the LPC lesion. Analyses of G ratios between WT and Cdk5 cKO revealed consistent findings of reduction in the thickness of myelin sheaths in LPC-lesioned Cdk5 cKO compared with WT at both 14 and 21 dpl (Fig. 6K,I).
Figure 6.
Absence of Cdk5 in oligodendrocyte lineages impairs remyelination in Cdk5 cKO mice. Reduction of myelin repair and remyelinated axons in LPC-lesioned spinal cord of Cdk5 cKO mice at 14 and 21 dpl shown by toluidine blue staining (B, D) and EM analysis (F, H) (insets, higher magnificent) compared with WT control animals (A, C; E, G). I, Percentages of remyelinated axons were significantly reduced in Cdk5 cKO mice at 14 and 21 dpl. J, Total number of axons was significantly decreased in lesioned spinal cord of Cdk5 cKO mice at 14 and 21 dpl. K, L, G-ratio analysis revealed the reduction of myelin thickness in LPC-lesioned Cdk5 cKO mice compared with WT mice at 14 and 21 dpl. Values are mean ± SEM. *p < 0.05. **p < 0.01. Scale bars: A–D, 25 μm; E–H, 10 μm.
Reduction of mature CC1+ cells, but increased Olig2+ cells, in LPC-induced lesion in Cdk5-CNP cKO
The delay in myelin repair may reflect either a failure of recruitment of OPC to the lesion site or a failure of OPCs to differentiate into myelinating oligodendrocytes and effect myelin repair. To distinguish between these possibilities, we compared recruitment of oligodendrocyte lineage cells between WT and Cdk5 cKO at different postlesion intervals. The total number of recruited Olig2+ cells to LPC-induced lesions was significantly higher in Cdk5 cKO (Fig. 7D,J,P) compared with WT (Fig. 7A,G,M). The increase in density of Olig2+ cells in Cdk5 cKO lesions was evident at 3 dpl; it increased at 7 dpl and was maintained at least until 14 dpl. This increase suggests that the recruitment of OPCs into the lesion is not inhibited by the absence of Cdk5 but may either be increased or the levels of cell differentiation, proliferation, or death are altered. The differentiation of OPCs was reduced in Cdk5 cKO lesion as shown by a significant reduction in the density of mature oligodendrocytes detected by CC1 (Fig. 7E,K,Q) compared with WT (Fig. 7B,H,N) at all time points assayed. The reduction in CC1+ cells was more pronounced during the first week postlesion when virtually no increase was detected. During the second week postlesion, the density of CC1+ cells slightly increased but remained <70% that of WT (at 14 dpl CC1+ cells: WT 122 ± 5.9 vs cKO 84 ± 5.93). The selective reduction in density of CC1+ oligodendrocytes in cKO-lesioned animals suggests that the maturation of oligodendrocytes was affected by Cdk5 loss, and the reduction of mature oligodendrocytes in lesions likely results in the defect in remyelination in spinal cord of Cdk5 cKO. One possible explanation for the increase in Olig2+ cells in the Cdk5 cKO lesions at 7 dpl is that the absence of Cdk5 results in enhanced OPC proliferation; however, comparison of the levels of proliferation between WT and cKO-lesioned animals revealed no significant changes in the proportion of Olig2+ cells labeled with antibodies to Ki67 at any postlesion interval examined (Fig. 8A–C,G–I) and Cdk5 cKO (Fig. 8D–F,J,L,M). Likewise, only a slight increase in the levels of cell death were seen by TUNEL between cKO and WT animals within the lesion.
Figure 7.
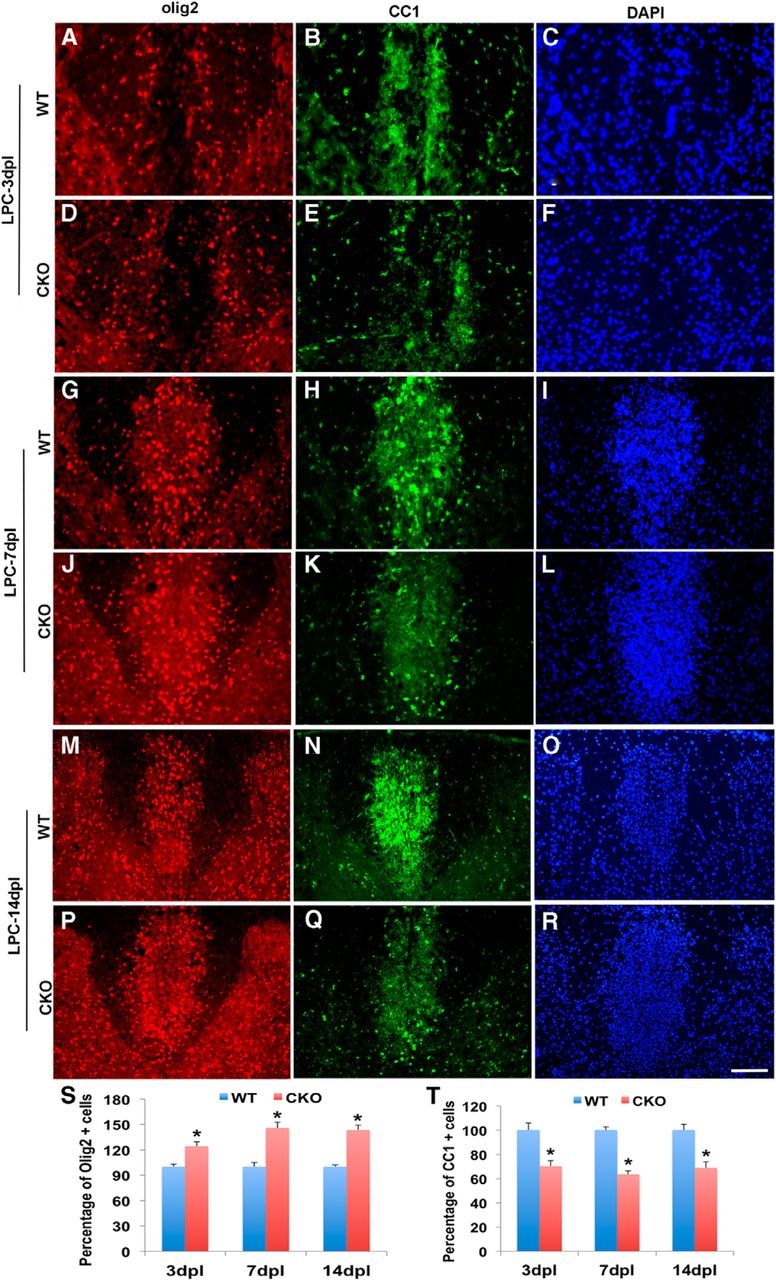
Reduction of mature CC1+ cells but increased Olig2+ cells in LPC-induced lesions in Cdk5 cKO. A–R, Double immunofluorescent staining of Olig2+ (red) and CC1 (green) in LPC-induced dorsal spinal cord lesion of Cdk5 cKO and WT mice at 3, 7, and 14 dpl using DAPI (blue) as nuclei count staining. Quantification of percentages of Olig2+ cells (S) and CC1+ cells (T) in the lesioned areas of Cdk5 cKO compared with WT. Quantification data showed the percentage of CC1+ cells in LPC-induced lesion areas in Cdk5 cKO mice and WT mice. Values are mean ± SEM. *p < 0.05. Scale bar, 25 μm.
Figure 8.
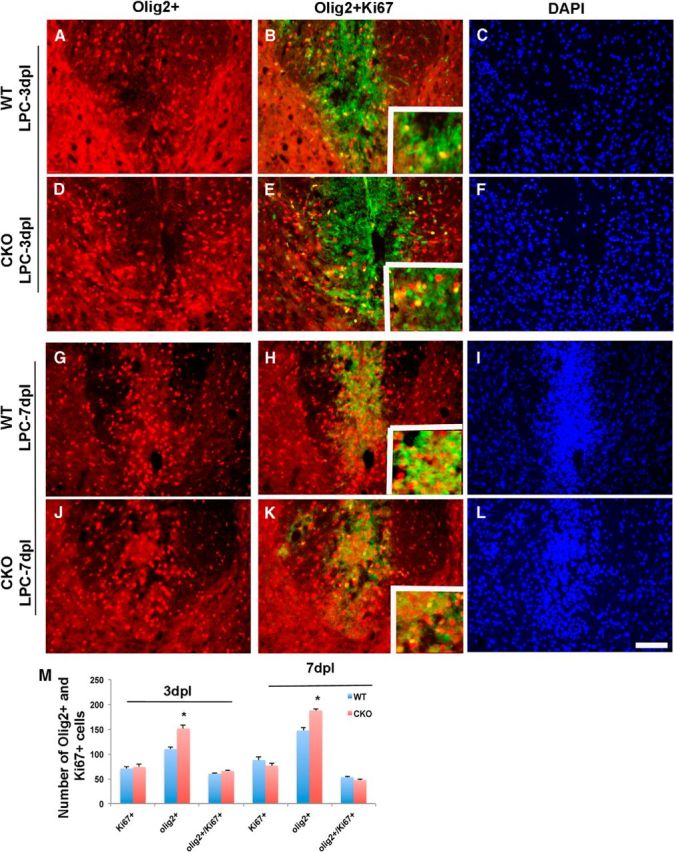
The proliferation of OPCs is not significantly different between Cdk5 cKO and WT animals after an LPC lesion. Double immunofluorescent staining of Olig2+ (red) and Ki67+ (green) in LPC dorsal spinal cord lesions of Cdk5 cKO (D–F, J–L) and WT animals (A–C, G–I) at 3 and 7 dpl. DAPI (blue) was used as nuclei count staining. M, Quantification of total number of Olig2+ cells, Ki67+ cells, and doubled-labeled Olig2+/Ki67+ cells (see insets) in LPC-lesioned area of Cdk5 cKO and WT mice. Values are mean ± SEM. *p < 0.05, statistically significant. Scale bar, 25 μm.
Enhanced levels of microglia in LPC induced lesion in Cdk5-CNP cKO
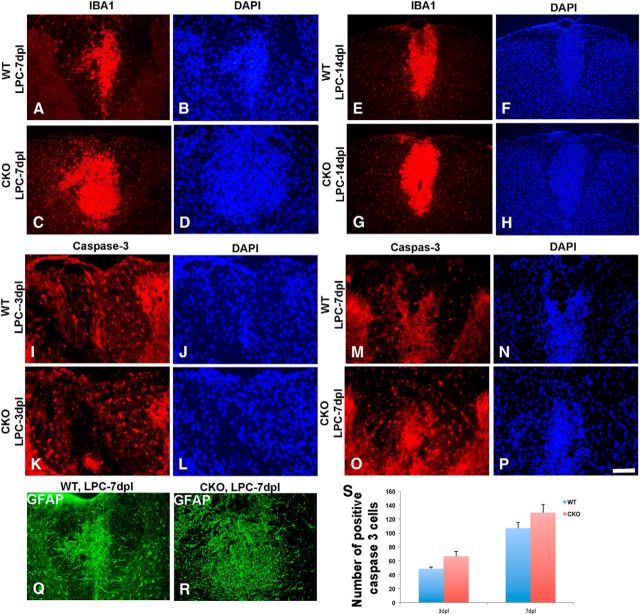
The role of inflammation in myelin repair is complex with evidence for both positive and negative effects of microglia on myelin repair. To determine whether there were changes in microglial infiltration in cKO lesions, sections were labeled with Iba1. Lesions in Cdk5 cKO animals demonstrated an elevated level of inflammation marked by activated Iba1+ cells and increased GFAP+ cells compared with WT at 7 dpl (Fig. 9A–D,Q,R), 14 dpl (Fig. 9E–H), and 21 dpl (data not shown). Although local inflammation was detected in lesioned WT animals, the level of inflammation was markedly increased in Cdk5 cKO animals with abundant foamy macrophages detected in the lesion. This increased inflammation was associated with slight but not statistically significant increase in the number of positive caspase-3+ cells in Cdk5 cKO compared with WT animals at 3 and 7 dpl, suggesting an increase in cell death (Fig. 9I–L,M–P). These findings suggest that lack of Cdk5 in oligogendrocytes may contribute to enhance vulnerable to inflammation and apoptotic cell death in demyelinating environment that would further disrupt the myelin repair process.
Figure 9.
Enhanced numbers of microglia and increased astrogliosis but modest increase in cell death detected in LPC-induced lesions in Cdk5 cKO mice compared with WT. Immunofluorescent staining of IBA1 (red) in LPC-lesioned Cdk5 cKO (C, D) and WT (A, B) mice at 7 dpl and in LPC-lesioned Cdk5 cKO (G, H) and WT (E, F) mice at 14 dpl. No significant difference in total number of immunostained activated Caspase-3 (red) between LPC-lesioned Cdk5 cKO (K, L) and WT mice (I, J) at 3 dpl and between LPC-lesioned Cdk5 cKO (O, P) and WT mice (M, N) at 7 dpl. DAPI (blue) was used as nuclei count staining. Increased level of astrogliosis marked by immunostained GFAP+ cells (green) in LPC-lesioned Cdk5 cKO (Q) and WT mice at 7 dpl (R). S, Quantification analysis showed the total number of activated caspase-3+ cells. Values are mean ± SEM. *p < 0.05, statistically significant. Scale bar, 25 μm.
Cdk5 modulation of remyelination influences remyelination through regulating Akt and Gsk-3β, but not ERK, signaling pathways
Myelination and remyelination are dependent on a number of intracellular signaling pathways, including Akt and Gsk-3β (Flores et al., 2008; Narayanan et al., 2009; Galimberti et al., 2011). Although promotion of the PI3k/Akt/mTOR pathway enhances myelination and remyelination in the CNS (Flores et al., 2008; Narayanan et al., 2009; Kumar et al., 2013), upregulation of Gsk-3β appears to inhibit myelin formation (Azim and Butt, 2011). To determine whether signaling through either pathway was affected by the absence of Cdk5 during myelin repair, total protein was extracted from micro-dissected lesions at various days postlesion and assayed by Western blot. The total levels of Akt and mTOR were similar in Cdk5 cKO and WT-lesioned animals, whereas the levels of phosphorylated Akt and mTOR were significantly reduced in the LPC-induced lesions of Cdk5 cKO compared with controls throughout the entire period of myelin repair (Fig. 10A,C). The activity of Gsk-3β is negatively regulated through phosphorylation on Serine 9, and analysis of the levels of Gsk-3β showed no significant difference in the total protein levels but a dramatic reduction in the levels of phosphorylated Gsk-3β (Ser 9) in lesioned Cdk5 cKO (Fig. 10B), suggesting enhanced inhibition of remyelination in the absence of Cdk5. Not all signaling pathways were reduced. Analysis of the ERK signaling pathway showed no difference between WT and Cdk5 cKO animals (Fig. 10D). To determine whether activation of the AKT pathway in the absence of Cdk5 was sufficient to stimulate remyelination, the AKT activator (SC79) was delivered to an LPC lesion in cKO animals 4 dpl. At 14 dpl, lesions in which AKT was activated were substantially smaller and had enhanced repair compared with saline-treated lesions. The enhanced repair was reflected in small lesion in LFB-stained section (Fig. 10Eb) as well as increased expression of MBP and Flouromyelin (Fig. 10Ed,Ef).
Figure 10.
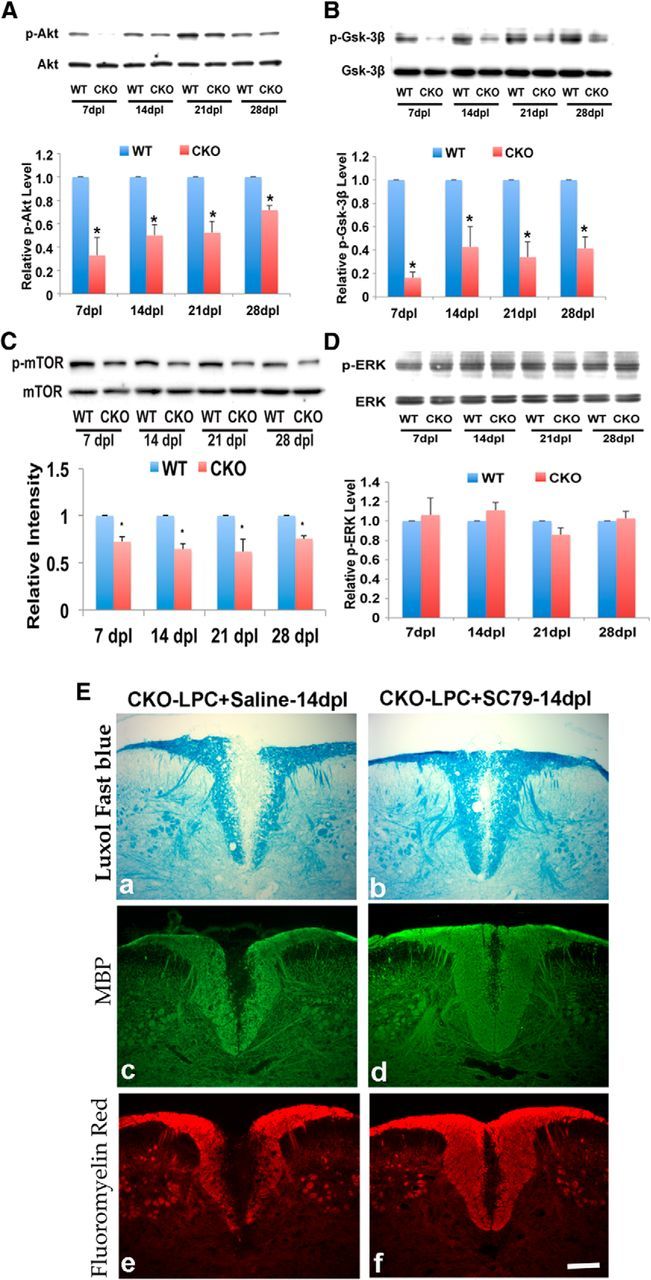
Cdk5 modulates myelin repair through regulation of Akt/mTOR and Gsk-3β, but not ERK, signaling pathways. Western blot analysis showed decreased phosphorylation levels of Akt/mTOR (A, C) and decreased phosphorylated Gsk-3β (Ser9) (B) in lesioned Cdk5 cKO compared with WT mice at 7, 14, 21, and 28 dpl. No changes in ERK (D). Activation of the AKT/mTOR pathway enhances myelin repair in Cdk5 cKO, suggesting that AKT/mTOR is downstream of Cdk5 (E). Bar, 25 μm. Values are mean ± SEM (n = 3). *p < 0.05, statistically significant.
Together, these data provide a mechanism by which Cdk5 regulates remyelination through both positively and negatively influencing pathways of Akt and Gsk-3β in adult CNS.
Discussion
In the vertebrate CNS, Cdk5 has been implicated in regulating cell morphology, migration, and differentiation during development (Tsai et al., 1993; Gilmore et al., 1998; Yang et al., 2013). Here we show that, in the adult CNS, Cdk5 is functionally important for timely myelin repair. After a dorsal spinal cord LPC-induced lesion, local levels of Cdk5 rapidly increased, suggesting that Cdk5 has an important role in demyelination or remyelination. Consistent with this hypothesis, local inhibition of Cdk5 through targeted delivery of the inhibitor roscovitine resulted in larger lesions and impaired remyelination. A similar lack of remyelination followed inhibition of Cdk5 in slice cultures of cerebellum-treated with LPC, suggesting that CNS expression of Cdk5 is important for repair. To demonstrate a specific role for oligodendrocyte lineage cell expression of Cdk5 in myelin repair, a conditional knock-out was generated using CNP-Cre targeting. Unlike the olig1 conditional knock-out used previously (Yang et al., 2013), CNP animals displayed very limited developmental phenotype and were functionally indistinguishable from WT littermates for the first 2 months of life, even though the levels of recombination in oligodendrocyte lineage cells was >70%. This difference may reflect either specific cell targeting or timing of Cdk5 deletion. The CNP Cdk5 cKO animals did, however, show slightly thinner myelin at 2 months of age, suggesting that CNP-modulated expression of Cdk5, although not critical for developmental myelination, may be important for myelin maintenance. By contrast, the rate of myelin repair in response to a spinal cord LPC lesion was significantly compromised in the Cdk5 cKO animals compared with WT or CNP-Cre controls. Several aspects contributed to reduced repair. Analysis of overall lesion size demonstrated ∼20% larger initial lesions that increased in size in proportion to control lesions. The rate of repair as defined by lesion volume was, however, significantly different in the Cdk5-cKO animals compared with control. Initial repair between 7 dpl and 14 dpl was slower in both groups; but whereas the rate of repair accelerated in WT animals between 14 and 21 dpl, it remained slow in Cdk5 cKO animals. These data suggest that later stages of myelin repair were preferentially compromised in the absence of Cdk5 than earlier stages. The larger overall lesion size during repair in the Cdk5 cKO animals was correlated with reductions in the temporal recovery of MBP and PLP expression and a significant reduction in the number of remyelinated axons.
The lack of Cdk5 in cells of the oligodendrocyte lineage did not affect OPC recruitment or generation in response to demyelinating lesions. Previous studies have suggested that the lack of Cdk5 in developing OPCs influences their process outgrowth through the phosphorylation of paxillin and its association with the focal adhesion kinase (Miyamoto et al., 2007). In addition, Cdk5 has been reported to modulate OPC migration (Miyamoto et al., 2008). The increased numbers of Olig2+ cells in the Cdk5 cKO lesions combined with similar levels of cell proliferation between the WT and cKO animals suggest that the influx of OPCs into adult LPC lesions is not compromised by the lack of Cdk5 and could reflect a difference between developmental and adult OPCs. Alternatively, it may be that CNP targeting does not sufficiently deplete Cdk5 from OPCs early enough to inhibit their migration into the lesion, even though during development it is known to be expressed from E12 onward (Yu et al., 1994). The observation that Cdk5 cKO lesions contain increased numbers of OPCs, but reduced numbers of mature oligodendrocytes is, however, consistent with studies on developmental OPCs, which suggest the lack of Cdk5 significantly inhibits their differentiation (Yang et al., 2013) as well as recent studies suggesting that, in oligodendrocytes, p39 is the primary activator for Cdk5 and is essential for OL differentiation and myelin repair (Bankston et al., 2013).
Delayed myelin repair seen in the absence of Cdk5 is correlated with reductions in the levels of phosphorylated Akt and Gsk-3β but not ERKs. These data suggest that Cdk5 selectively mediates Akt and Gsk-3β signaling during myelin repair, whereas phosphorylation of Akt promotes signaling through the Akt /mTOR pathway that is known to promote the generation of CNS myelin, and phosphorylation of Gsk-3β promotes Notch 1 signaling pathway, which inhibits OPC differentiation and myelination (Azim and Butt, 2011; Wahl et al., 2014; Lebrun-Julien et al., 2014). Several studies have implicated both signaling pathways in the development of oligodendrocytes, myelination, and remyelination (Bibollet-Bahena and Almazan, 2009; Narayanan et al., 2009; Guardiola-Diaz et al., 2012; Kumar et al., 2013). Furthermore, increasing Akt activity in oligodendrocytes was sufficient to reverse the hypomyelination in BACE1−/− animals that showed hypomyelination and impaired remyelination (Hu et al., 2013). Although signaling through the ERK pathway has been shown to regulate the thickness of myelin sheaths around individual axons, there is less evidence that this pathway is involved in the early development or maturation of oligodendrocytes (Fyffe-Maricich et al., 2013); thus, it is not surprising that this pathway is not influenced by Cdk5, which appears to be a primary integrator of signals during the transition of OPCs to mature oligodendrocytes. The current data provide strong evidence that Cdk5 acts as an integrated mediator in regulating a number of proteins and signaling pathways related in OL development, myelination, and remyelination. Although the molecular details and biological outcomes are cell type specific, the concept that Cdk5 modulates P13K/Akt in the oligodendrocyte lineage is consistent with studies of neuronal development. In neurons, it has been shown that Cdk5-mediated PI3K/Akt pathway signaling confers neuron resistance to apoptosis (Wang et al., 2006); in addition, Cdk5 is involved in neuregulin-dependent activation of PI3K/Akt activity mediating neuronal survival (Li et al., 2003). Likewise, Cdk5 has been implicated in mediating signaling by Gsk-3β through phosphorylation of Axin that contributes to axon formation during cortical development (Fang et al., 2011). In the oligodendrocyte lineage, previous studies have shown that Gsk-3β negatively regulates oligodendrocytes differentiation and myelination in vivo (Azim and Butt, 2011) and that inhibitors of Gsk-3β could promote remyelination in CNS and peripheral nervous system (Azim and Butt, 2011). One potential pathway is through signaling of the neuregulin receptors. It has been shown that Cdk5/P35 interacts with the receptor ErbB of neuregulin in neurons (Fu et al., 2005), and neuregulin signaling has been reported to play critical roles in OPC differentiation and remyelination (Sussman et al., 2005; Gao et al., 2006; Gauthier et al., 2013). Thus, we propose that Cdk5 is a crucial integrator of cellular signaling after demyelination and essential for timely remyelination.
The larger, slower recovering demyelinated lesions that developed in Cdk5 cKO animals were associated with a significant increase in the activated microglia as characterized by Iba1+ labeling and an upregulation of reactive astrogliosis. Both cell types are highly responsive to disturbances in tissue homeostasis and in response to injury act as a source for factors that may be either positive or negative for myelin repair. The association between inflammation and myelin repair is complex. In studies on MS tissue, some animal models suggest that an inflammatory response is beneficial in promoting repair (Foote and Blakemore, 2005; Setzu et al., 2006; Skripuletz et al., 2013), although significant data indicate that inflammation is not only detrimental to repair but may actually drive demyelination. It is possible that the increased levels of microglial activation seen in the cKO-lesioned animals are contributing directly to the lack of repair. Although Cdk5 has been shown in modulation of neuroinflammation through TGF-beta1, IL-1, and induced cytosolic PLA2-mediated lysophosphatidylcholine production trigger neurodegeneration (Sundaram et al., 2012; Lyman et al., 2014), it is unlikely that the lack of Cdk5 in oligodendrocytes directly regulates microglial activation. It seems more likely that the lack of rapid repair resulting from impaired oligodendrocyte differentiation provides a continuous signal that drives microglial activation. This hypothesis is supported by the finding that in both WT and cKO animals the number of Iba1+ cells increases between day 3 and 7 as the lesion expands and then subsequently decreases. Whether this inflammatory response represents aborted repair or contributes to the lack of lesion recovery is currently unclear.
The effects of Cdk5 on inhibition of remyelination may be generalizable to human demyelinating conditions. In models of EAE immune-mediated disease, Cdk5 activity as well as its activators p25 and p35 were upregulated while proteomic analysis of chronic active plaques from MS tissue (Han et al., 2008) suggests a significant increase in the expression of Cdk5. Clearly, Cdk5 has the potential to influence the behavior of a range of different cell types involved in immune-mediated demyelination, including the activation of T cells (Pareek et al., 2010). The current data strongly suggest that myelin repair is compromised by the absence of Cdk5 in cell of the oligodendrocyte lineage and, as such, may represent a potential therapeutic target for adult demyelinating diseases.
Footnotes
This work was supported by the National Institutes of Health Grant RO1NS077942 to R.H.M. and Y.Y. and Myelin Repair Foundation to R.H.M.
The authors declare no competing financial interests.
References
- Aparicio E, Mathieu P, Pereira Luppi M, Almeira Gubiani MF, Adamo AM. The notch signaling pathway: its role in focal CNS demyelination and apotransferrin-induced remyelination. J Neurochem. 2013;127:819–836. doi: 10.1111/jnc.12440. [DOI] [PubMed] [Google Scholar]
- Azim K, Butt AM. GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia. 2011;59:540–553. doi: 10.1002/glia.21122. [DOI] [PubMed] [Google Scholar]
- Bankston AN, Li W, Zhang H, Ku L, Liu G, Papa F, Zhao L, Bibb JA, Cambi F, Tiwari-Woodruff SK, Feng Y. p39, the primary activator for cyclin-dependent kinase 5 (Cdk5) in oligodendroglia, is essential for oligodendroglia differentiation and myelin repair. J Biol Chem. 2013;288:18047–18057. doi: 10.1074/jbc.M113.453688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Rao TS, Webb M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J Neurosci Res. 2004;78:157–166. doi: 10.1002/jnr.20248. [DOI] [PubMed] [Google Scholar]
- Caillava C, Vandenbosch R, Jablonska B, Deboux C, Spigoni G, Gallo V, Malgrange B, Baron-Van Evercooren A. Cdk2 loss accelerates precursor differentiation and remyelination in the adult central nervous system. J Cell Biol. 2011;193:397–407. doi: 10.1083/jcb.201004146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- Fang WQ, Ip JP, Li R, Ng YP, Lin SC, Chen Y, Fu AK, Ip NY. Cdk5-mediated phosphorylation of Axin directs axon formation during cerebral cortex development. J Neurosci. 2011;31:13613–13624. doi: 10.1523/JNEUROSCI.3120-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128:528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Fu AK, Ip FC, Fu WY, Cheung J, Wang JH, Yung WH, Ip NY. Aberrant motor axon projection, acetylcholine receptor clustering, and neurotransmission in cyclin-dependent kinase 5 null mice. Proc Natl Acad Sci U S A. 2005;102:15224–15229. doi: 10.1073/pnas.0507678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe-Maricich SL, Schott A, Karl M, Krasno J, Miller RH. Signaling through ERK1/2 controls myelin thickness during myelin repair in the adult central nervous system. J Neurosci. 2013;33:18402–18408. doi: 10.1523/JNEUROSCI.2381-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Macmurray J, Scalabrini D, Fenoglio C, De Riz M, Comi C, Comings D, Cortini F, Villa C, Serpente M, Cantoni C, Ridolfi E, Fardipoor MH, Leone M, Monaco F, Bresolin N, Scarpini E. GSK3beta genetic variability in patients with Multiple Sclerosis. Neurosci Lett. 2011;497:46–48. doi: 10.1016/j.neulet.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Gao L, Macklin W, Gerson J, Miller RH. Intrinsic and extrinsic inhibition of oligodendrocyte development by rat retina. Dev Biol. 2006;290:277–286. doi: 10.1016/j.ydbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Gauthier MK, Kosciuczyk K, Tapley L, Karimi-Abdolrezaee S. Dysregulation of the neuregulin-1-ErbB network modulates endogenous oligodendrocyte differentiation and preservation after spinal cord injury. Eur J Neurosci. 2013;38:2693–2715. doi: 10.1111/ejn.12268. [DOI] [PubMed] [Google Scholar]
- Ghasemlou N, Jeong SY, Lacroix S, David S. T cells contribute to lysophosphatidylcholine-induced macrophage activation and demyelination in the CNS. Glia. 2007;55:294–302. doi: 10.1002/glia.20449. [DOI] [PubMed] [Google Scholar]
- Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia. 2012;60:476–486. doi: 10.1002/glia.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Tsai LH. Cyclin-dependent kinase 5 and neuronal migration in the neocortex. Neurosignals. 2003;12:173–179. doi: 10.1159/000074618. [DOI] [PubMed] [Google Scholar]
- Gupta A, Sanada K, Miyamoto DT, Rovelstad S, Nadarajah B, Pearlman AL, Brunstrom J, Tsai LH. Layering defect in p35 deficiency is linked to improper neuronal-glial interaction in radial migration. Nat Neurosci. 2003;6:1284–1291. doi: 10.1038/nn1151. [DOI] [PubMed] [Google Scholar]
- Han MH, Hwang SI, Roy DB, Lundgren DH, Price JV, Ousman SS, Fernald GH, Gerlitz B, Robinson WH, Baranzini SE, Grinnell BW, Raine CS, Sobel RA, Han DK, Steinman L. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- Hu X, Schlanger R, He W, Macklin WB, Yan R. Reversing hypomyelination in BACE1-null mice with Akt-DD overexpression. FASEB J. 2013;27:1868–1873. doi: 10.1096/fj.12-224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JK, Franklin RJ. Regenerative medicine in multiple sclerosis: identifying pharmacological targets of adult neural stem cell differentiation. Neurochem Int. 2011;59:329–332. doi: 10.1016/j.neuint.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A, Chambon P, Ffrench-Constant C, Franklin RJ. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain. 1997;120:27–37. doi: 10.1093/brain/120.1.27. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Skoff RP, Sprinkle TJ. Differential expression of galactocerebroside, myelin basic protein, and 2′,3′-cyclic nucleotide 3′-phosphohydrolase during development of oligodendrocytes in vitro. J Neurosci Res. 1988;21:249–259. doi: 10.1002/jnr.490210217. [DOI] [PubMed] [Google Scholar]
- Kumar S, Patel R, Moore S, Crawford DK, Suwanna N, Mangiardi M, Tiwari-Woodruff SK. Estrogen receptor beta ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol Dis. 2013;56:131–144. doi: 10.1016/j.nbd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Bachmann L, Norrmen C, Trotzmuller M, Kofeler H, Ruegg MA, Hall MN, Suter U. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J Neurosci. 2014;34:8432–8448. doi: 10.1523/JNEUROSCI.1105-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Li BS, Ma W, Jaffe H, Zheng Y, Takahashi S, Zhang L, Kulkarni AB, Pant HC. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J Biol Chem. 2003;278:35702–35709. doi: 10.1074/jbc.M302004200. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Merkler D. Effects of commissural de- and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp Neurol. 2006;202:217–224. doi: 10.1016/j.expneurol.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Mi S, Sandrock A, Miller RH. LINGO-1 and its role in CNS repair. Int J Biochem Cell Biol. 2008;40:1971–1978. doi: 10.1016/j.biocel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Chan JR, Okada A, Tomooka Y, Hisanaga S, Tanoue A. Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J Cell Sci. 2007;120:4355–4366. doi: 10.1242/jcs.018218. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A. Cdk5 phosphorylation of WAVE2 regulates oligodendrocyte precursor cell migration through nonreceptor tyrosine kinase Fyn. J Neurosci. 2008;28:8326–8337. doi: 10.1523/JNEUROSCI.1482-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29:6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek TK, Lam E, Zheng X, Askew D, Kulkarni AB, Chance MR, Huang AY, Cooke KR, Letterio JJ. Cyclin-dependent kinase 5 activity is required for T cell activation and induction of experimental autoimmune encephalomyelitis. J Exp Med. 2010;207:2507–2519. doi: 10.1084/jem.20100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:24057–24064. doi: 10.1074/jbc.273.37.24057. [DOI] [PubMed] [Google Scholar]
- Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Brück W, Lucchinetti C, Lassmann H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- Setzu A, Lathia JD, Zhao C, Wells K, Rao MS, Ffrench-Constant C, Franklin RJ. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, Berger K, Kipp M, Baumgärtner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain. 2013;136:147–167. doi: 10.1093/brain/aws262. [DOI] [PubMed] [Google Scholar]
- Sundaram JR, Chan ES, Poore CP, Pareek TK, Cheong WF, Shui G, Tang N, Low CM, Wenk MR, Kesavapany S. Cdk5/p25-induced cytosolic PLA2-mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci. 2012;32:1020–1034. doi: 10.1523/JNEUROSCI.5177-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman CR, Vartanian T, Miller RH. The ErbB4 neuregulin receptor mediates suppression of oligodendrocyte maturation. J Neurosci. 2005;25:5757–5762. doi: 10.1523/JNEUROSCI.4748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Wang CX, Song JH, Song DK, Yong VW, Shuaib A, Hao C. Cyclin-dependent kinase-5 prevents neuronal apoptosis through ERK-mediated upregulation of Bcl-2. Cell Death Differ. 2006;13:1203–1212. doi: 10.1038/sj.cdd.4401804. [DOI] [PubMed] [Google Scholar]
- Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL. Mammalian target of rapamycin promotes oligodendrocyte differentiation, initiation and extent of CNS myelination. J Neurosci. 2014;34:4453–4465. doi: 10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang H, Zhang J, Luo F, Herrup K, Bibb JA, Lu R, Miller RH. Cyclin-dependent kinase 5 is required for the normal development of oligodendrocytes and myelin formation. Dev Biol. 2013;378:94–106. doi: 10.1016/j.ydbio.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WP, Collarini EJ, Pringle NP, Richardson WD. Embryonic expression of myelin genes: evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron. 1994;12:1353–1362. doi: 10.1016/0896-6273(94)90450-2. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jarjour AA, Boyd A, Williams A. Central nervous system remyelination in culture: a tool for multiple sclerosis research. Exp Neurol. 2011;230:138–148. doi: 10.1016/j.expneurol.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]