Abstract
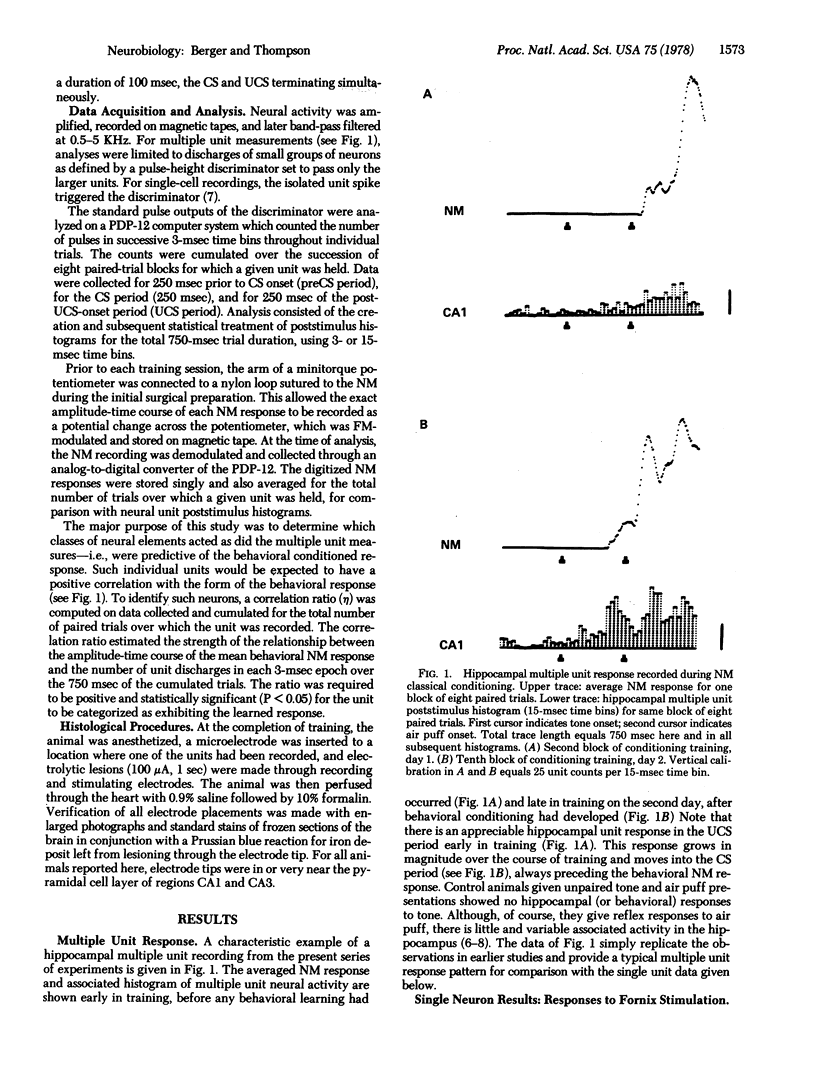
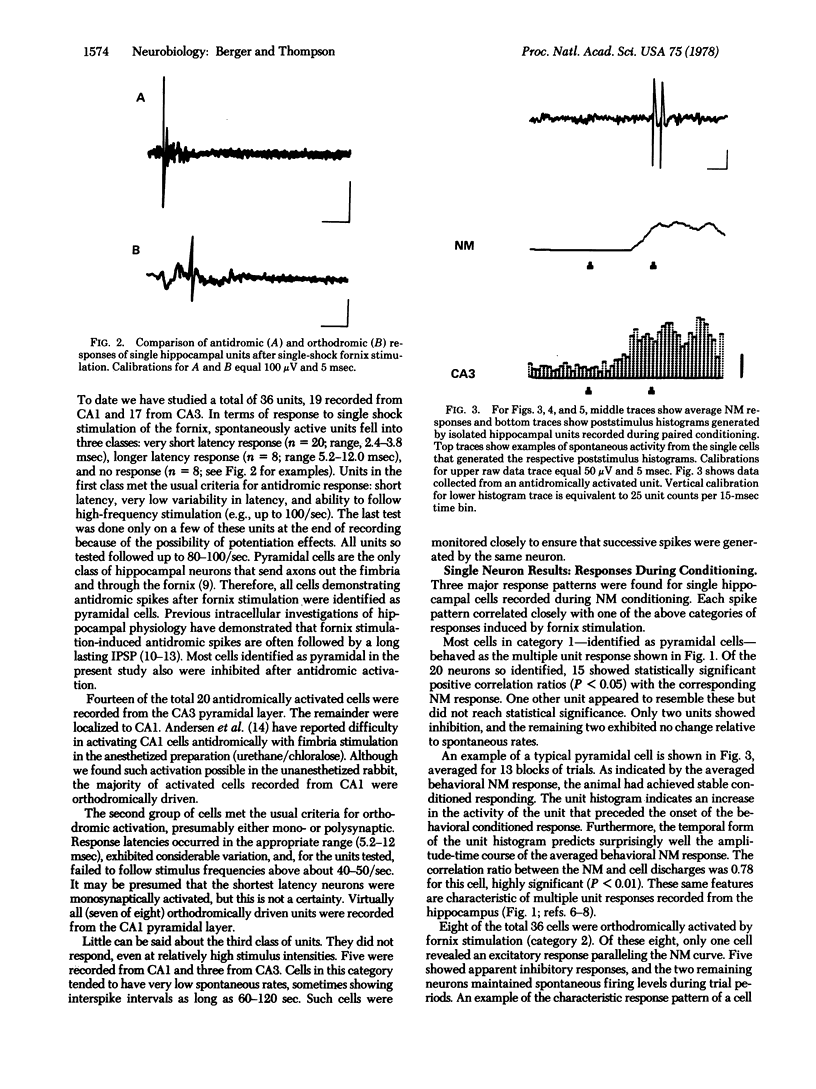
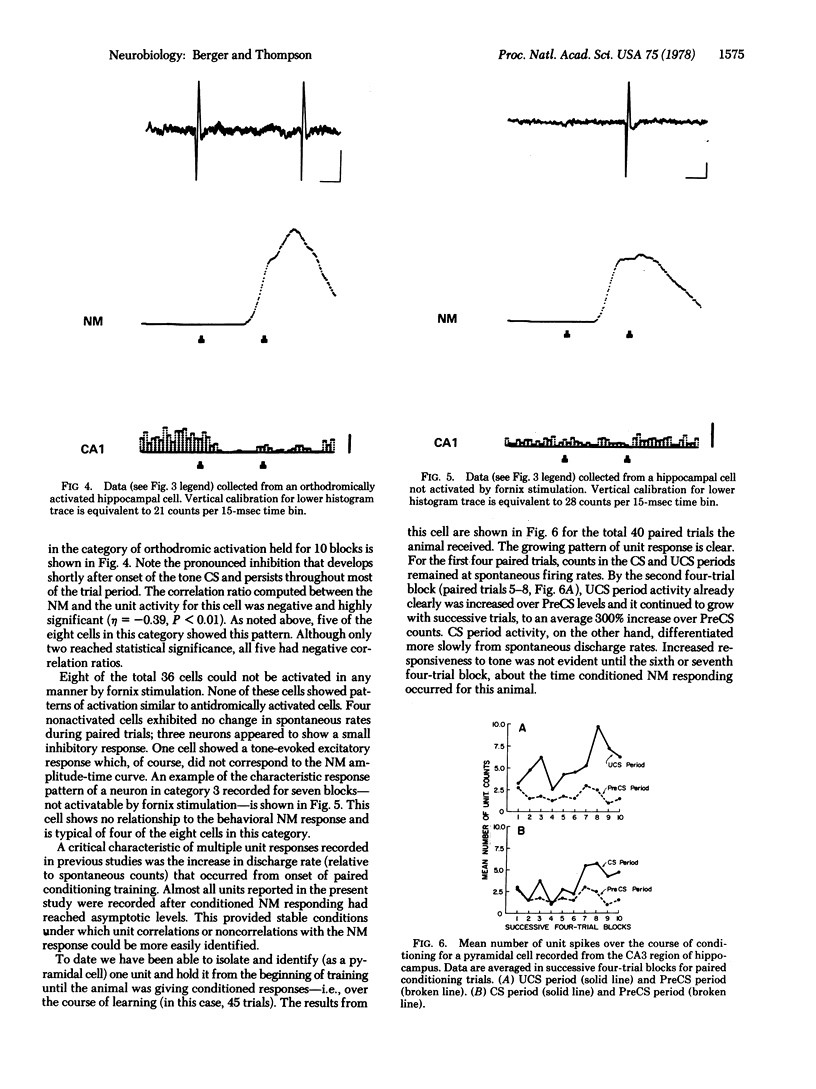
The activity of single neurons recorded from rabbit hippocampus during classical conditioning of the nictitating membrane reflex was studied. All cells were first categorized according to their responses after fornix stimulation--i.i., antidromic activation, orthodromic activation, or no activation. The majority of cells that were antidromically activated--pyramidal cells--showed a highly positive correlation between the pattern of unit discharge and the topography of the nicititating membrane response within trial periods. Units that were orthodromically driven by fornix stimulation tended to inhibit during the presentation of trial stimuli, whereas most non-activated cells maintained low spontaneous levels of activity at all times. Thus, the major output neurons of the hippocampus appear to be the neuroanatomical substrate for the large and rapidly developing neuronal plasticity induced by this classical conditioning paradigm.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. LOCATION OF POSTSYNAPTIC INHIBITORY SYNAPSES ON HIPPOCAMPAL PYRAMIDS. J Neurophysiol. 1964 Jul;27:592–607. doi: 10.1152/jn.1964.27.4.592. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., LOYNING Y. PATHWAY OF POSTSYNAPTIC INHIBITION IN THE HIPPOCAMPUS. J Neurophysiol. 1964 Jul;27:608–619. doi: 10.1152/jn.1964.27.4.608. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Teyler T. J. Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976 Jul 16;110(3):463–480. doi: 10.1016/0006-8993(76)90858-1. [DOI] [PubMed] [Google Scholar]
- Andersen P., Bland B. H., Dudar J. D. Organization of the hippocampal output. Exp Brain Res. 1973 Apr 30;17(2):152–168. doi: 10.1007/BF00235025. [DOI] [PubMed] [Google Scholar]
- Andersen P., Sundberg S. H., Sveen O., Wigström H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature. 1977 Apr 21;266(5604):736–737. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Alger B., Thompson R. F. Neuronal substrate of classical conditioning in the hippocampus. Science. 1976 Apr 30;192(4238):483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Lynch G. S., Dunwiddie T., Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977 Apr 21;266(5604):737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Olds J., Disterhoft J. F., Segal M., Kornblith C. L., Hirsh R. Learning centers of rat brain mapped by measuring latencies of conditioned unit responses. J Neurophysiol. 1972 Mar;35(2):202–219. doi: 10.1152/jn.1972.35.2.202. [DOI] [PubMed] [Google Scholar]
- SCOVILLE W. B., MILNER B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957 Feb;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. F. The search for the engram. Am Psychol. 1976 Mar;31(3):209–227. doi: 10.1037//0003-066x.31.3.209. [DOI] [PubMed] [Google Scholar]



