Abstract
Under conditions of feeding at will and normal light-dark alternation, rats consume the major portion of their daily food intake during the dark period and the circadian peak of plasma corticosteroid concentrations and of body temperature levels occurs just prior to or subsequent to the time of light-dark transition. Both light-dark transition and time of food presentation have been implicated as “Zeitgebers” in determining the phase of these two circadian rhythms.
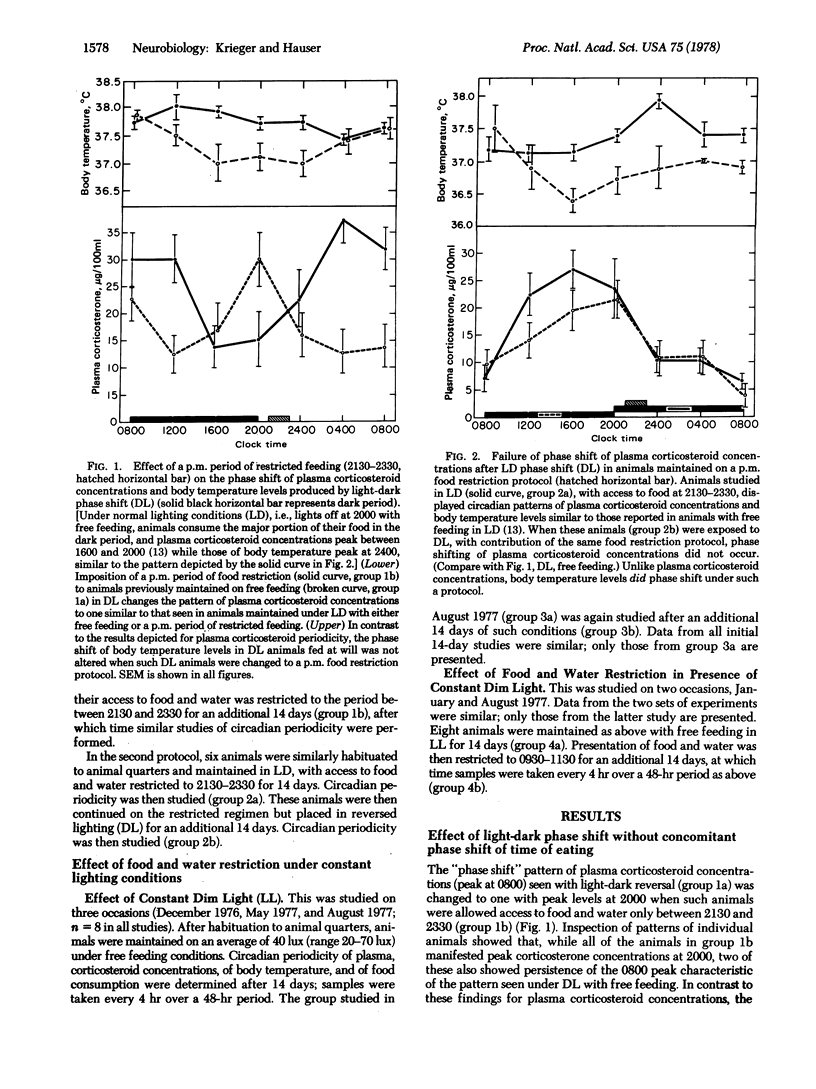
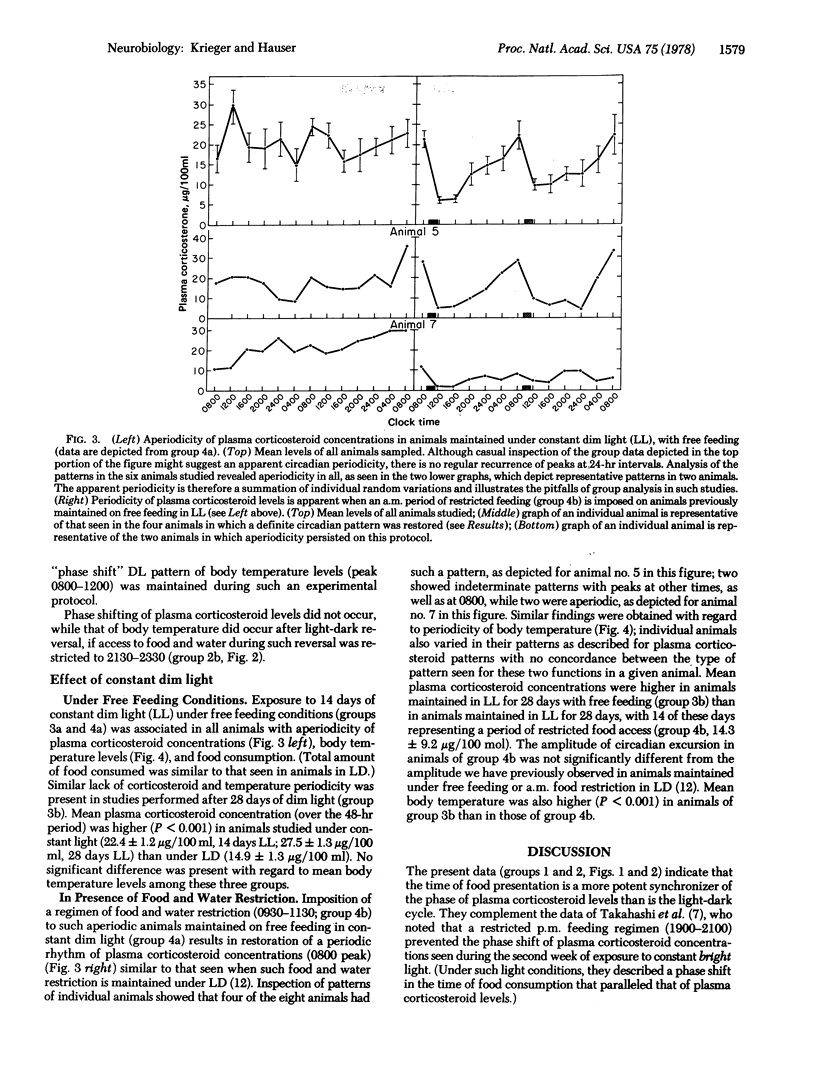
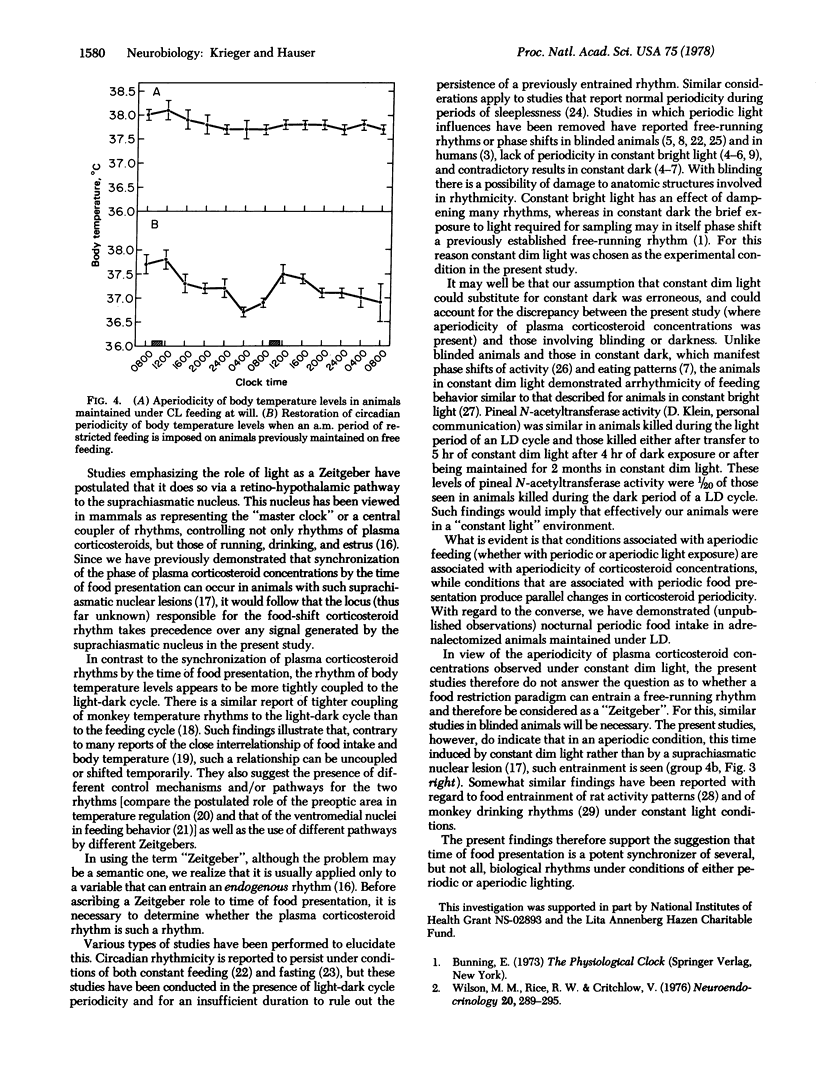
The present data indicate the following: (i) The time of food presentation appears to be a more potent synchronizer of the phase of plasma corticosteroid levels than is the light-dark cycle. This has been demonstrated in rats under conditions in which light-dark phase shift has been dissociated from a concomitant shift of time of eating. In contrast, under such conditions, the rhythm of body temperature appears to be more tightly coupled to the light-dark cycle. This illustrates that the time of food ingestion and the peak of body temperature rhythms can be uncoupled and that the phasing effects of restricted food ingestion on corticosteroid rhythms does not extend to body temperature rhythms. It also suggests the presence of different control mechanisms and/or pathways for corticosteroid and body temperature rhythms as well as the use of different pathways by different Zeitgebers. (ii) Rats maintained in constant dim light with free access to food exhibit aperiodic feeding behavior; plasma corticosteroid concentrations and body temperature levels are also aperiodic. Imposition of a restricted period of food access under such constant light conditions is associated with the appearance of a circadian periodicity of both plasma corticosteroid concentrations and body temperature levels, with peaks, respectively, just before and after the time of food presentation. This represents an additional example of food entrainment of previously aperiodic functions, similar to the food entrainment we have described in animals rendered aperiodic by lesions of the suprachiasmatic nucleus.
Keywords: temperature, phase shift
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSSON B., LARSSON B. Influence of local temperature changes in the preoptic area and rostral hypothalamus on the regulation of food and water intake. Acta Physiol Scand. 1961 May;52:75–89. doi: 10.1111/j.1748-1716.1961.tb02203.x. [DOI] [PubMed] [Google Scholar]
- Cheifetz P., Gaffud N., Dingman J. F. Effects of bilateral adrenalectomy and continuous light on the circadian rhythm of corticotropin in female rats. Endocrinology. 1968 Jun;82(6):1117–1124. doi: 10.1210/endo-82-6-1117. [DOI] [PubMed] [Google Scholar]
- Critchlow V., Liebelt R. A., Bar-Sela M., Mountcastle W., Lipscomb H. S. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963 Nov;205(5):807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Edmonds S. C. Food and light as entrainers of circadian running activity in the rat. Physiol Behav. 1977 May;18(5):915–919. doi: 10.1016/0031-9384(77)90201-3. [DOI] [PubMed] [Google Scholar]
- Johnson J. T., Levine S. Influence of water deprivation on adrenocortical rhythms. Neuroendocrinology. 1973;11(5):268–273. doi: 10.1159/000122139. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Allen W., Rizzo F., Krieger H. P. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab. 1971 Feb;32(2):266–284. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- Krieger D. T. Effect of ocular enucleation and altered lighting regimens at various ages on the circadian periodicity of plasma corticosteroid levels in the rat. Endocrinology. 1973 Nov;93(5):1077–1091. doi: 10.1210/endo-93-5-1077. [DOI] [PubMed] [Google Scholar]
- Krieger D. T. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974 Nov;95(5):1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Hauser H., Krey L. C. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977 Jul 22;197(4301):398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Marti H., Studer H., Dettwiler W., Rohner R. Persistence of a physiological circadian rhythm of plasma-free 11-hydroxycorticosteroid levels in totally fasting obese subjects. Experientia. 1969 Mar 15;25(3):320–321. doi: 10.1007/BF02034421. [DOI] [PubMed] [Google Scholar]
- Moore-Ede M. C., Sulzman F. M. The physiological basis of circadian timekeeping in primates. Physiologist. 1977 Jun;20(3):17–25. [PubMed] [Google Scholar]
- Moore R. Y., Eichler V. B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972 Jul 13;42(1):201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morimoto Y., Oishi T., Arisue K., Ogawa Z., Tanaka F. Circadian rhythm of plasma corticosteroid in adult female rats: chronological shifts in abnormal lighting regimens and connection with oestrous cycle. Acta Endocrinol (Copenh) 1975 Nov;80(3):527–541. doi: 10.1530/acta.0.0800527. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- Nelson W., Scheving L., Halberg F. Circadian rhythms in mice fed a single daily meal at different stages of lighting regimen. J Nutr. 1975 Feb;105(2):171–184. doi: 10.1093/jn/105.2.171. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Island D. P. Light synchronization of the circadian rhythm in plasma cortisol (17-OHCS) concentration in man. J Clin Endocrinol Metab. 1969 Apr;29(4):479–486. doi: 10.1210/jcem-29-4-479. [DOI] [PubMed] [Google Scholar]
- Poland R. E., Rubin R. T., Clark B. R., Gouin P. R. Circadian patterns of urine 17-OHC and VMA excretion during sleep deprivation. Dis Nerv Syst. 1972 Jul;33(7):456–458. [PubMed] [Google Scholar]
- Rusak B., Zucker I. Biological rhythms and animal behavior. Annu Rev Psychol. 1975;26:137–171. doi: 10.1146/annurev.ps.26.020175.001033. [DOI] [PubMed] [Google Scholar]
- Scheving L. E., Pauly J. E. Effect of light on corticosterone levels in plasma of rats. Am J Physiol. 1966 May;210(5):1112–1117. doi: 10.1152/ajplegacy.1966.210.5.1112. [DOI] [PubMed] [Google Scholar]
- Sulzman F. M., Fuller C. A., Moore-Ede M. C. Feeding time synchronizes primate circadian rhythms. Physiol Behav. 1977 May;18(5):775–779. doi: 10.1016/0031-9384(77)90182-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Inoue K., Takahashi Y. Parallel shift in circadian rhythms of adrenocortical activity and food intake in blinded and intact rats exposed to continuous illumination. Endocrinology. 1977 Apr;100(4):1097–1107. doi: 10.1210/endo-100-4-1097. [DOI] [PubMed] [Google Scholar]
- Wilson M. M., Rice R. W., Critchlow V. Evidence for a free-running circadian rhythm in pituitary-adrenal function in blinded adult female rats. Neuroendocrinology. 1976;20(4):289–295. doi: 10.1159/000122495. [DOI] [PubMed] [Google Scholar]



