Abstract
The aim of this study was to compare the prevalence of diagnosed neurodevelopmental disorders in children exposed, in utero, to different antiepileptic drug (AED) treatments.
A prospective cohort of women with epilepsy and a control group of women without epilepsy were recruited from antenatal clinics. The children of this cohort were followed longitudinally until six years of age (n=415). Diagnosis of a neurodevelopmental disorder was made independently of the research team.
Multiple logistic regression analysis revealed an increase in risk of neurodevelopmental disorders in children exposed to monotherapy sodium valproate (6/50, 12.0%; aOR 6.05, 95%CI 1.65–24.53; p=0.007) and in those exposed to polytherapy with sodium valproate (3/20, 15.0%; aOR 9.97, 95%CI 1.82–49.40; p=0.005) compared to control children (4/214; 1.87%). Autistic spectrum disorder was the most frequent diagnosis. No significant increase was found amongst children exposed to carbamazepine (1/50) or lamotrigine (2/30).
An accumulation of evidence demonstrates that the risks associated with prenatal sodium valproate exposure include an increased prevalence of neurodevelopmental disorders. Whether such disorders are discrete or represent the severe end of a continuum of altered neurodevelopmental functioning requires further investigation. Replication and extension of this research is required to investigate the mechanism(s) underpinning the relationship. Finally, the increased likelihood of neurodevelopmental disorders should be communicated to women for whom sodium valproate is a treatment option.
Keywords: Epilepsy, Pregnancy, Neurodevelopment, Antiepileptic drugs, Autistic spectrum disorder
INTRODUCTION
Prenatal exposure is associated for the majority of antiepileptic drugs (AEDs), with an increased risk of major congenital malformations in a dose dependent manner (1). There is also evidence demonstrating that prenatal exposure to sodium valproate (VPA) is associated with reduced cognitive functioning in the exposed child (2–4). This has increased concern about the longer term influences of prenatal exposure to AEDs.
Neurodevelopmental disorders, such as autistic spectrum disorders (ASD), attention deficit hyperactivity disorder (ADHD) and dyspraxia, are reportedly more prevalent following prenatal exposure to a number of teratogenic substances (5–10). The question remains as to whether prenatal exposure to AEDs is associated with an increased likelihood of neurodevelopmental disorders. Altered neuronal development is regarded as a pathological cause of ASD (11) and AEDs have been shown to alter neuronal development (12,13), in a manner that is consistent with autistic behaviours in rats and mice (12, 14–16). Additionally, there is evidence from case series (17–20) and retrospective human studies (2, 21–23) that neurodevelopmental disorders are more prevalent in children exposed to VPA. Adab and colleagues (2) observed five cases of neurodevelopmental disorder in children exposed to VPA monotherapy (5/41;12.1%) in a retrospective cohort. This increase was not found in the groups exposed to carbamazepine (CBZ) or phenytoin (PHT). A systematic review of children with a history of prenatal exposure to AEDs revealed that 8.9% of children exposed to VPA and 2.5% of children exposed to CBZ met the diagnostic criteria for ASD (23), although no adjustment for potential confounding variables was undertaken. High rates of neurodevelopmental disorders have also been reported in children diagnosed with Fetal Valproate Syndrome (21). This is a condition characterised by a pattern of major and minor congenital malformations, dysmorphic features and cognitive deficits (24, 25), and is likely to represent the severely effected end of a spectrum of difficulties associated with prenatal exposure to VPA. Preliminary findings regarding the prevalence of ASD within this cohort have previously been published (26), and highlight an increased risk of neurodevelopmental disorders following VPA exposure. Further, Cohen and colleagues (27) documented significantly higher maternal ratings of inattention, hyperactivity and reduced social skills in children prenatally exposed to VPA at three years of age.
An association between prenatal CBZ exposure and ASD has been reported in one study (23), which is not consistent with another (2). There have been no reports of a significant association between other AEDs and neurodevelopmental disorders to date.
Prospective observational studies utilising a control group, provide the most reliable evidence to test this possible association, as randomised controlled trials to establish teratogenic risk, are thought to be unethical. The final results of this 11 year prospective study are reported here.
METHODOLOGY
A prospective study of the children born to women with epilepsy (WWE) was initiated in 2000, aiming to document physical health and cognitive development. Between 2000 and 2004, 628 pregnant women were recruited from antenatal clinics in the North West of England. Over 99% of the cohort were Caucasian. Twenty pregnancies were excluded due to fetal or child death, or on the basis that the children or mothers had conditions likely to influence neurodevelopmental outcome (including chromosomal disorders and hydrocephalus). Eighty women (29 WWE and 51 controls) were never accessible to the research team, even by telephone and were excluded. Therefore the total number of eligible pregnancies between 2000 and 2004 was 528 (243 WWE and 285 controls). Of those exposed to AEDs in utero 59 were exposed to CBZ, 59 to VPA, 36 to lamotrigine (LTG), 14 to other monotherapy treatments and 41 to polytherapy. Thirty four children were born to women with epilepsy who were not taking medication during their pregnancy.
At recruitment each woman provided information relating to education, occupation, and lifestyle issues such as smoking and alcohol use during pregnancy. Maternal health and pregnancy information was recorded from maternal interview and checked against medical records. An epilepsy specialist (G.M.) confirmed seizure type, syndrome diagnosis (symptomatic/cryptogenic focal, idiopathic generalised epilepsy (IGE) or not classifiable), current seizure frequency as well as AED type and dose. Treatment was classed as polytherapy if a second AED (including a benzodiazepine) had been prescribed even for a short period. Seizure frequency was ascertained from the patient and, where possible a family member. The study methodology has been detailed in the earlier paper describing the immediate pregnancy outcomes (28).
Children from this cohort were assessed at one, three and six years of age to monitor their physical and cognitive development (4,28). Thirty nine percent of children born to WWE were also enrolled into a US/UK study (3, 27, 29,30). This collaborative study has reported on infant behaviour in infants at three years of age (27) which included earlier age data on 21% (n=86) of children reported here. Structured interviews with the parents were conducted by a trained research assistant or authors M.B, R.B, R.S or J.C-S, blinded as to whether the mother had epilepsy and whether the child had been exposed to medication during pregnancy. It was recorded whether the child had been ill, attended hospital, or whether a professional had been consulted regarding the health, development, behaviour or educational progress of the child. Information was specifically collected on ASD, ADHD and dyspraxia due to their increased prevalence in an earlier retrospective cohort (2). Indication that a child had seen a specialist pertaining to a developmental difficulty or neurodevelopmental disorder was documented and followed up with the diagnosing health professional, family doctor or school nurse. The researchers played no clinical role in the treatment of the WWE or in the diagnosis of the child. Children were referred to specialist services following contact with the research team if there were significant concerns about development. In one case a referral was made following concerns regarding speech quality, which led to a diagnosis of ASD. The referring researcher in this case was blind to the exposure type. Outcome at six years of age was the end point of this longitudinal study and the presence of a confirmed diagnosis of a neurodevelopmental disorder (ASD, ADHD or dyspraxia) at the time of the six-year assessment is reported here.
Data analyses were conducted in two stages, by author S.B.L using SPSS 17, and by author C.C under the supervision of M.G.F using R version 2.12.1. Multiple logistic regression analysis was applied to explore the likelihood of neurodevelopmental disorder of the offspring of WWE exposed to AED treatments (CBZ, LTG, VPA, other monotherapy and polytherapy) compared to controls and to identify the demographic and clinical variables associated with the diagnosis of a neurodevelopmental disorder. Due to the relatively low frequency of diagnosis this was only possible for the overall category of neurodevelopmental disorder category and not for the individual types (ASD, ADHD or dyspraxia). A similar analysis was applied to compare the likelihood of neurodevelopmental disorder across maternal epilepsy types. A preliminary exploratory data analysis was used to identify candidate variables for consideration in the logistic model by individually exploring their association with the occurrence of neurodevelopmental disorder. The variables included seizures during pregnancy, maternal IQ, maternal age, socio-economic status, alcohol or nicotine exposure, gender and gestational age at birth. Paternal date of birth was missing in over 25% of cases and was therefore not included.
Ethical approval for this cohort study was awarded by the Northwest Regional Ethics Committee and research approval was obtained from individual participating hospitals. All families provided informed consent to participate in the study. Preliminary findings regarding the prevalence of ASD within this cohort have previously been reported (26).
RESULTS
Of the 528 children who actively participated in the longitudinal follow up, 415 (214 controls and 201 children born to WWE) were assessed at the main outcome age of six years (78.6%). The majority of missing cases were lost to follow up with less than 5% formally withdrawing or indicating that they were emigrating. Significant differences were found between the demographics of those assessed and those not, with lower maternal IQ (P=0.01), socio-economic status (P=0.01) and maternal age (P=0.02) associated with non-completion. Further, mothers whose child participated in the six year assessments were less likely to have smoked during the pregnancy (P=0.04).
The demographics of the cohort completing assessment at six years of age is displayed in Table 1. WWE were marginally younger than the control women (28 vs 29 years, P=0.002) and had a lower FSIQ as measured by the National Adult Reading Test (P<0.001). Significantly more control women reported drinking alcohol during the pregnancy (30.8% vs 17.9%, P=0.004), but significantly more WWE reported smoking (27.9% vs 15.9, P=0.008). There was no significant difference in the gestational age of the child at birth (P=0.5) or in the distribution of gender between the groups of children born to WWE and controls (P=0.3).
Table 1.
Cohort demographics in the Liverpool and Manchester Neurodevelopment Group prospective study
| Numbers enrolled 2000–2004 (n)đ |
Outcome known (n) |
% retained |
Seizures during pregnancy (%) |
Maternal epilepsy type |
Mean maternal age (years) |
Alcohol (%) |
Nicotine (%) |
Socioeconomic status (% professional) |
Gender (% male) |
Mean child age (mths) |
Mean gestational age at birth (weeks) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Convulsive | Non Convulsive |
IGE (%) |
F (%) |
UC (%) |
|||||||||||
| Controls○ | 285 | 214 | 75% | - | - | - | - | - | 29 | 30.8 | 16.4 | 40.7 | 51.9 | 74 | 40 |
| WWE◆ | 243 | 201 | 83% | 33.5 | 14.3 | 32.0 | 54.2 | 13.8 | 28 | 17.9 | 27.9 | 31.0 | 57.1 | 74 | 39 |
| CBZ | 59 | 50 | 85% | 19.6 | 19.6 | 9.8 | 80.4 | 9.8 | 30 | 17.6 | 23.5 | 41.2 | 49.0 | 74 | 39 |
| LTG | 36 | 30 | 83% | 40.0 | 10.0 | 23.3 | 56.7 | 20.0 | 28 | 10.0 | 16.7 | 40.0 | 56.7 | 74 | 40 |
| Other | 14 | 14 | 100% | 35.7 | 21.4 | 28.6 | 71.4 | 0.0 | 30 | 28.6 | 50.0 | 28.6 | 57.1 | 74 | 40 |
| VPA | 59 | 50 | 85% | 41.2 | 11.8 | 66.7 | 21.6 | 11.8 | 27 | 17.6 | 29.4 | 23.5 | 68.6 | 74 | 39 |
| Poly VPA | 30 | 20 | 67% | 50.0 | 10 | 35.0 | 55.0 | 10.0 | 26 | 10.5 | 26.3 | 25.0 | 50.0 | 75 | 39 |
| Poly Other | 11 | 11 | 100% | 81.8 | 9.1 | 0 | 81.8 | 18.2 | 29 | 27.3 | 18.2 | 18.2 | 54.5 | 74 | 39 |
| No Med | 34 | 26 | 76* | 4.0 | 12.0 | 32.0 | 40.0 | 28.0 | 26 | 25.0 | 37.5 | 32.0 | 72.0 | 75 | 39 |
Figures are inclusive of children recruited between 2000 and 2004 who attended at least one appointment or completed one phone visit with investigators (actively enrolled).
2 sets of dizygotic twins included.
5 sets of twins – 3 exposed to monotherapy VPA (1 dizygotic pair and one unconfirmed) and 2 exposed to monotherapy CBZ (1 dizygotic pair). Alcohol – any level of consumption during pregnancy (including prior to conception). Nicotine – including prior to knowledge of conception. WWE – women with epilepsy, IGE- idiopathic generalised epilepsy, F- focal, UC- unclassified.
Nineteen children had a diagnosis of a neurodevelopmental disorder at the six year assessment (Table 2). Twelve were diagnosed with ASD; one of which was also diagnosed with ADHD. Three children had ADHD in isolation. The four remaining children had a diagnosis of dyspraxia. Of the 19 with a diagnosis of neurodevelopmental disorders, physical malformations were noted in three cases (16%) all of whom were prenatally exposed to AEDs (Table 2).
Table 2.
Individual cases of neurodevelopmental disorder alongside demographic information
| Diagnosis | Gender | AED | Dose (mg daily) |
Maternal Epilepsy Type |
Seizures | Congenital Malformation |
Maternal age |
Paternal age |
Siblings with ASD■ |
Alcohol | Nicotine | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autistic Spectrum Disorders | ||||||||||||
| 1 | Aspergersđ | M | VPA | 1000 | IGE | 1TC | Multiple minor | 33 | 39 | None | Yes | No |
| 2 | Autism đ | M | VPA | 600 | F | 1 PS+SG | None | 27 | 29 | 1sibling (VPA exposed) | No | No |
| 3 | Autismđ | M | VPA | 1,500 | F | 2PS+SG | None | 22 | 30 | None | No | Yes |
| 4 | Pervasive Developmental Disorderđ | M | VPA | 2500 | IGE | 2TC | None | 23 | 29 | None | Yes | Yes |
| 5 | Autism đ | F | VPA+LTG | 2000+100 | F | 2TC | None | 26 | 37 | None | No | No |
| 6 | Aspergersđ | M | LTG | 500 | F | 1PS+SG | None | 30 | 31 | None | No | No |
| 7 | Aspergers & ADHD | F | VGB | 4000 | F | 2TC | None | 19 | Not known | None | No | Yes |
| 8 | Autismđ | M | PHT | 300 | F | 0 | None | 35 | Not known | None | Yes | Not known |
| 9 | Autismđ | M | Control | N/A | N/A | N/A | None | 32 | Not known | None | Yes | No |
| 10 | Aspergersđ | M | Control | N/A | N/A | N/A | None | 31 | 32 | None | No | No |
| 11 | Aspergersđ | F | Control | N/A | N/A | N/A | None | 32 | 31 | 1 sibling | No | No |
| 12 | Autism | M | Control | N/A | N/A | N/A | 37 | None | No | Yes | ||
| ADHD | ||||||||||||
| 13 | ADHD | M | VPA+LTG | 2000+50 | IGE | 0 | 1 major | 27 | 40 | 2 Siblings (VPA exposed) | No | No |
| 14 | ADHD○ | F | CBZ | 1200 | F | 0 | None | 24 | 31 | None | No | No |
| 15 | ADHD | M | VPA+LTG | 1500+25 | F | 2TC | None | 18 | Not known | None | Yes | No |
| Dyspraxia | ||||||||||||
| 16 | Dyspraxia | M | LTG | 100 | F | 2 TC | None | 22 | Not known | None | No | No |
| 17 | Dyspraxia | M | VPA | 600 | IGE | 0 | None | 26 | 30 | None | No | No |
| 18 | Dyspraxia | M | CBZ+LTG | 600+50 | UC | 2TC | Isolated minor | 24 | Not known | None | No | No |
| 19 | Dyspraxia | M | VPA | 1000 | UC | 2TC | None | 40 | Not known | None | No | No |
Case was included in the preliminary report from this cohort (26).
Child also has idiopathic generalised epilepsy. IGE- Idiopathic generalised epilepsy, F- focal, UC – unclassified, PS – partial seizures, TC- tonic clonic seizures. Alcohol includes intake prior to knowledge of conception. Nicotine – smoking even prior to knowledge of conception.
ASD was the only reported diagnosed disorder in siblings.
Diagnosis by group
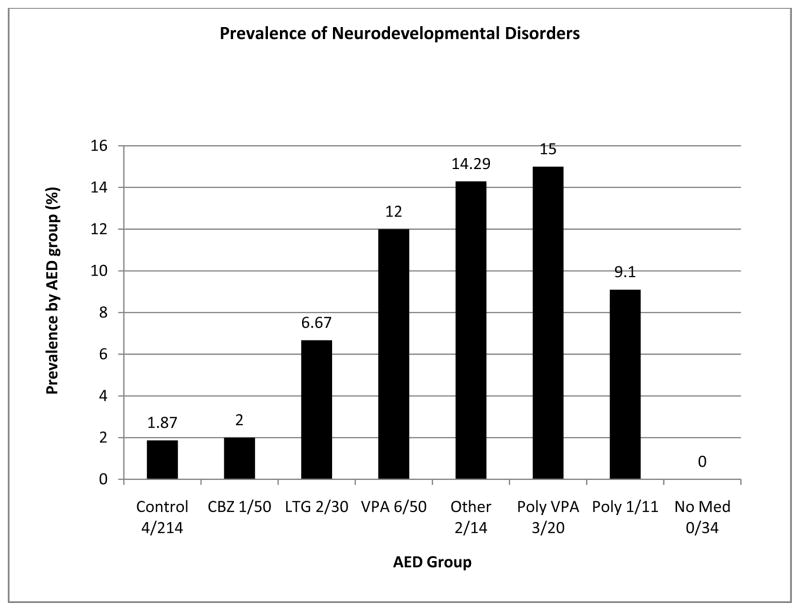
Neurodevelopmental disorders were more frequently reported in the children of WWE (15/201; 7.46%) than in the control group (4/214; 1.87%). Prevalence of neurodevelopmental disorders differed across the AED groups (Figure 1). Children exposed to VPA showed a high prevalence of neurodevelopmental disorders (6/50: 12.0% for VPA monotherapy and 3/20: 15.0% for VPA polytherapy). Prevalence of neurodevelopmental disorder differed for the other exposures. The small ‘other monotherapy group’, which comprised of a number of different AEDs had a prevalence of 14.29% (2/14), with one case prenatally exposed to PHT and the other vigabatrin (VGB). A single case of ADHD was found in the group of children exposed to CBZ (1/50: 2.0%). This child also had IGE with active seizures. For the LTG exposed group there were two cases with a diagnosis (2/30: 6.67%), one child with a diagnosis of Aspergers syndrome and another of dyspraxia. There were no reported cases of neurodevelopmental disorder in the children born to untreated WWE. A single case of dyspraxia was found in the group of children exposed to polytherapy without VPA (1/11: 9.1%). Finally, four control children (4/214: 1.87%) were diagnosed with a neurodevelopmental disorder, which was ASD in all cases.
Figure 1.
Prevalence of neurodevelopmental disorder by group.
Multiple logistic regression analysis revealed that children born to WWE exposed to VPA monotherapy and VPA polytherapy were, respectively, six times (aOR 6.05, 95% CI 1.65–24.53; P=0.007) and ten times (aOR 9.97, 95% CI 1.82–49.40; P=0.005) more likely to be diagnosed with a neurodevelopmental disorder than controls(Table 3).
Table 3.
Crude and adjusted odds ratios for AED type and maternal epilepsy type
| Group | Total | NDDs | No NDDs | Incidence Rate (%) | Unadjusted Odds Ratio | Adjusted Odds Ratio*(95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Control | 214 | 4 | 210 | 1.87 | Reference Group | Reference Group | - |
| No Medication | 26 | 0 | 26 | 0.00 | - | - | - |
| Valproate | 50 | 6 | 44 | 12.00 | 7.16 | 6.05 (1.65, 24.53) | 0.007 |
| CBZ | 50 | 1 | 49 | 2.00 | 1.07 | 1.09 (0.06, 7.39) | 0.9 |
| LTG | 30 | 2 | 28 | 6.67 | 3.75 | 4.06 (0.55, 22.20) | 0.1 |
| Other Monotherapy | 14 | 2 | 12 | 14.29 | 8.75 | 8.17 (1.09, 49.40) | 0.02 |
| Polytherapy with VPA | 20 | 3 | 17 | 15.00 | 9.26 | 9.97 (1.82, 49.40) | 0.005 |
| Other Polytherapy | 11 | 1 | 10 | 9.09 | 5.25 | 4.95 (0.25, 40.45) | 0.2 |
|
| |||||||
| Focal | 108 | 9 | 99 | 8.33 | 4.77 | 4.76 (1.42, 15.94) | 0.01 |
| Idiopathic Generalised | 65 | 4 | 61 | 6.15 | 3.44 | 3.15 (0.76, 13.09) | 0.1 |
| Unclassified | 28 | 2 | 26 | 7.14 | 4.04 | 3.74 (0.65, 21.67) | 0.1 |
separate regression models were created for AED and maternal epilepsy type regressions due to the limited numbers of cases per drug by maternal epilepsy type.
The ‘other monotherapy group’ showed also a significantly higher risk compared to controls (aOR 8.17, 95% CI 1.1–49.4; P=0.02) but, similarly to what is observed for the VPA polytherapy group, the low sample sizes in these two groups are linked to wide confidence intervals and large standard errors of the corresponding aORs (Table 3). For the other AED groups, the risk of neurodevelopmental disorder was not statistically significant when compared to the control group (CBZ, LTG and Polytherapy without VPA).
When the VPA exposed groups were split by dose the prevalence of NDD appeared to increase with dose (Table 4). A logistic regression analysis, unadjusted for other factors due to the low number of cases per dose and VPA exposure groups, showed a positive association between the prevalence of neurodevelopmental disorder and the preconceptual dose of VPA in monotherapy but this failed to attain significance (P = 0.1).
Table 4.
Prevalence of neurodevelopmental disorders by dose of sodium valproate
| Dose of Valproate mg/dailya | Valproate combinedb (n=70) | Valproate monotherapy (n=50) | Valproate polytherapy (n=20) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Yes | No | % | Yes | No | % | Yes | No | % | |
| 2000–3000 | 2 | 2 | 50 | 0 | 1 | 0 | 2 | 1 | 66.7 |
| 1000–1900 | 5 | 28 | 15.2 | 4 | 22 | 15.4 | 1 | 6 | 14.3 |
| 100–900 | 2 | 31 | 6.5 | 2 | 21 | 8.7 | 0 | 10 | 0 |
Preconception dose
Monotherapy and polytherapy combined
The prevalence of neurodevelopmental disorder was similar across maternal epilepsy type: focal 8.33%; IGE 6.15% and unclassified type 7.14% (Table 3). A significant aOR was demonstrated for maternal focal epilepsy (aOR 4.76, 95% CI 1.42–15.94, P=0.01) in comparison to the control group, but the aOR remained similar to that for IGE and unclassified type (Table 3). Differences due to epilepsy type are unavoidably confounded with differences due to AED treatment. After exclusion of all cases exposed to VPA there were five cases of NDD in the children of 86 women with focal epilepsy. The corresponding figures for IGE were 0/25 and for unclassified epilepsy 1/22. There were no cases of neurodevelopmental disorder in the children of women with untreated epilepsy.
In terms of demographic influences, boys tend to be three times more likely than girls to be diagnosed with a neurodevelopmental disorder (aOR 3.2, 95% CI 1.0–10.1; P=0.05), but no significant association was found for maternal age, maternal IQ, gestational age at birth, seizure exposure or any other demographic variable (data not shown).
Discussion
The prevalence of ASD in the control group (1.87%) was comparable to population rates reported for the UK (1%)(31), suggesting that the control group was representative. Furthermore the association of neurodevelopmental disorder and male gender is consistent with a large existing body of evidence (32). No diagnosis of dyspraxia or ADHD was reported in the control group, which may be related to the relatively young age of this cohort and the average age of diagnosis for these conditions in the UK, 5–11 years of age for ASD and 7 years of age for ADHD (33–35). No cases of neurodevelopmental disorder were reported in the children of women with untreated epilepsy. This is likely linked to the small size of the group (n=26), as all groups displayed similar mean child age.
This is the first prospective report regarding prevalence, and replication and extension is required. A six or 10 times increased prevalence of neurodevelopmental disorders is reported here for children with a history of prenatal VPA exposure respectively for monotherapy and polytherapy exposure. The most common neurodevelopmental disorder at six years of age for VPA exposed children was ASD. The increased prevalence of ASD within this group is consistent with previous retrospective clinical research (21–23) and reports from animal studies (14–16).
Consistent with previous research, a dose effect for VPA was indicated but such an association failed to reach statistical significance for monotherapy VPA, possibly due to limited power. Analysis of monotherapy and polytherapy VPA combined was not undertaken due to the potential bias. Further research is required to confirm a VPA dose dependent effect. The demonstration of dose dependency strengthens the case against a suspected teratogen (36) and dose effects have been reported for both rates of malformations and impaired cognitive abilities following VPA exposure (1–4,37).
In earlier publications from this cohort increased risk of major congenital malformations and reduced early cognitive ability were documented (4,28). Further investigation is required into whether an increased prevalence of neurodevelopmental disorders are discretely diagnosable conditions or whether they represent the severe end of a continuum of altered neurodevelopmental functioning following VPA exposure. Previous research has documented that prenatal exposure to VPA is associated with verbal, social and attentional difficulties (2, 4, 27, 30), and it is proposed here the raised prevalence of neurodevelopmental disorders is due to severe difficulties with language, social and attentional abilities, which meet a threshold level for diagnosis of a neurodevelopmental disorder. The majority of children received a diagnosis between three and five years of age. Of interest, global or specific developmental difficulties were noted under the age of two years in 6/7 VPA (monotherapy or polytherapy) exposed infants previously assessed. Such an observation demonstrates that the early developmental trajectory of these children is altered and surveillance through the early childhood years is necessary. Deficits in neurodevelopment of this nature can have a large impact on the child and their families, and present increased costs to society through increased health and educational support.
The use of VPA was associated with maternal IGE (66.7%), an expected association due to its efficacy in this epilepsy syndrome (38). Therefore the effects of one could not be viewed in isolation of the other in the logistic regression analysis. However, no significant aOR was found for IGE. Maternal focal epilepsy and not IGE, was associated with an increased rate of neurodevelopmental disorders in the offspring. Of the cases in offspring born to women with focal epilepsy 4/9 were treated with VPA in either monotherapy or polytherapy form, with the remaining cases exposed to other monotherapy or polytherapy AEDs.
The suggestion that VPA exposure is associated with an increased prevalence of neurodevelopmental disorders, which is independent of the maternal epilepsy type is consistent with the results of animal work, where there is no maternal epilepsy influence (12, 14–16). Studies are now required into the mechanism by which VPA is associated with this increased risk of neurodevelopmental disorders, considering that environmental (in utero) and genetic influences may not be mutually exclusive (11,16).
This study does not replicate the finding of Rasalam et al (23) in relation to CBZ, as there was only a single case of ADHD amongst the CBZ-exposed children. Single cases of neurodevelopmental disorder were also noted following exposure to PHT and VGB, and two cases exposed to LTG. Larger studies including wider dose ranges for individual AEDs are called for in order to provide reliable evidence in regards to AED treatment other than VPA exposure and potential occurrence of neurodevelopmental disorders.
A major strength of this study is its prospective design. The enrolment of mothers during pregnancy reduces the bias associated with knowledge of outcome and selective recall of health information. The utilisation of a control group representative of the general population provides risk information relevant to the effects of AEDs not only in epilepsy but also in other conditions (e.g. mood disorders and pain). Ascertainment of diagnosis independently of the research team, through routine clinical practice, where other causes are likely to have been considered and excluded (e.g. chromosomal syndromes) helps to ensure objectivity. Additional strengths of this study include its: (i)follow up into middle childhood; (ii) inclusion of multiple AED groups; (iii) blinding of researchers to exposure type and epilepsy type and (iv) control for a number of influential confounding variables.
A potential weakness was the setting of the final study outcome at six years of age which, as discussed above, has implications for prevalence of diagnosis. It is possible that reassessment at an older age would reveal an even higher prevalence of neurodevelopmental disorder. Furthermore, only 79% of those actively enrolled, completed the assessment at six years. It could be suggested that families of a child with difficulties would be more likely to bring their child for assessment. This however is unlikely to account for a selective rise in prevalence for the VPA exposed only, as the dropout rate did not differ significantly across AED groups.
Although the within data correlation from twin pairs was not taken into account in the analysis, it is felt to be justified on the relative infrequency and the lack of association with neurodevelopmental disorder (only a single case of ASD). Further limitations are (i) low sample size of disorder cases (n=19) were involved in the analysis, (ii) due to the low number of cases, the individual types of neurodevelopmental disorder (ASD, ADHD and dyspraxia) were not investigated through regression analysis, (iii) the limitations imposed by the relationship between maternal epilepsy and type of prescribed medication (e.g. VPA and IGE and CBZ and focal epilepsy), discussed above. Further a binary outcome (presence or absence of a disorder) was taken as a measure of prevalence. Questionnaires completed by parents and formal systematic clinical observations could provide graded information on children experiencing mild-moderate levels of difficulty and should be considered in future work.
CONCLUSIONS
Consideration of the results here, in the context of already published work, suggests that the risks associated with VPA treatment during pregnancy include neurodevelopmental disorders. The extent to which such conditions are discrete or are part of the wider neurobehavioural effects of prenatal exposure to VPA requires further investigation. If VPA is the treatment of choice, women should be provided with as much information as possible to enable them to make an informed decision. This should take place prior to conception as the evidence suggests that the neuropathology of ASD develops early in gestation (39,40). Further, these findings have implications for the care of children with a history of prenatal exposure to AEDs. Children exposed to AEDs in utero, particularly VPA, should be monitored closely during early childhood to allow for early intervention, diagnosis and support, should it be required.
Acknowledgments
The authors would like to acknowledge the families for their participation in this longitudinal study and also Dr Kimford Meador for his comments on the manuscript and ongoing collaboration as part of the NEAD study.
Funding
Supported by grants from Epilepsy Research UK RB219738 (National Lottery Charities Board) and through two educational grants from Sanofi Aventis pharmaceutical company. The research group was also in receipt of funding from the National Institute of Health (NIH grant #NS038455) as part of the NEAD study, these funds were not directly utilised for the collection of data included in this paper, however the study group was supported more generally by such funding.
Footnotes
Competing interests
Dr Bromley has received honorarium from Sanofi-Aventis for presenting to their Advisory Panel on two occasions. Dr Bromley has also provided expert testimony regarding fetal exposure to antiepileptic drugs. Travel support for two conferences has also been received from UCB Pharma and she has worked on projects supported by Educational grants from Sanofi Aventis and UCB Pharma.
Professor Mawer has provided expert testimony regarding fetal exposure to antiepileptic drugs.
Ms Briggs has no disclosures.
Dr Cheyne has no disclosures.
Professor Jill Clayton-Smith has provided expert testimony regarding fetal exposure to antiepileptic drugs and has received honorarium from GSK.
Dr García-Fiñana has no disclosures.
Dr Kneen has no disclosures.
Dr Lucas has no disclosures.
Dr Shallcross has received honorarium from UCB pharma for a lecture and also travel support from UCB pharma for conference attendance.
Professor Baker has received educational grants for this research from Sanofi-Aventis and has also received an educational grant from UCB Pharma for work not reported here. Professor Baker has also provided expert testimony regarding fetal exposure to antiepileptic drugs and has received honorarium from UCB pharma, GSK and Sanofi Aventis for lectures given.
References
- 1.Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurology. 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 2.Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–1583. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meador K, Baker G, Browning N, et al. Cognitive Function at 3 Years of Age After Fetal Exposure to Antiepileptic Drugs. The New England Journal of Medicine. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromley RL, Mawer G, Love J, et al. Early cognitive development in children born to women with epilepsy: A prospective report. Epilepsia. 2010 Jul 14; doi: 10.1111/j.1528-1167.2010.02668.x. [DOI] [PubMed] [Google Scholar]
- 5.Stromland K, Nordin V, Miller M, Akerstrom B, Gillberg C. Autism in Thalidomide Embryopathy: A Population Study. Developmental Medicine and Child Neurology. 1994;36:351–356. doi: 10.1111/j.1469-8749.1994.tb11856.x. [DOI] [PubMed] [Google Scholar]
- 6.Knopik V, Heath A, Jacob T, et al. Matneral Alcohol Use Disorder and Offspring ADHD: Disentangling Genetic and Environmental Effects using Children- Of -Twins Design. Psychological Medicine. 2006;36:1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- 7.Linnet KM, Dalsgaard S, Obel C, et al. Maternal Lifestyle Factors in Pregnancy Risk of Attention Deficit Hyperactivity Disorders and Associated behaviours: Review of the Current Evidence. American Journal of Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 8.Nanson J. Autism in Fetal Alcohol Syndrome: A Report of Six Cases. Alcoholism:Clinical and Experimental Research. 1992;16:558–565. doi: 10.1111/j.1530-0277.1992.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 9.Aronson M, Hagberg B, Gillberg C. Attention Deficits and Autistic Spectrum Problems in Children Exposed to Alcohol During Gestation: A Follow-Up Study. Developmental Medicine and Child Neurology. 1997;39:583–587. doi: 10.1111/j.1469-8749.1997.tb07493.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis E, Fennoy I, Laraque D, Kanem N, Brown G, Mitchell J. Autism and Developmental Abnormalities in Children with Perinatal Cocaine Exposure. Journal of The National Medical Association. 1992;84:315–319. [PMC free article] [PubMed] [Google Scholar]
- 11.Persico A, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental cues. Trends in Neuroscience. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki K, Narita N, Narita M. Maternal Administration of Thalidomide or Valproic Acid Causes Abnormal Serotonergic Neurons in the the Offspring: Implications for Pathogenesis of Autism. International Journal of Developmental Neuroscience. 2005;23:287–297. doi: 10.1016/j.ijdevneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomidou C. Prenatal effects of antiepileptic drugs. Epilepsy Currents. 2010;10:42–46. doi: 10.1111/j.1535-7511.2009.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingram JL, Peckham SM, Tisdale B, Rodier PM. Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicology and Teratology. 2000;22:319–324. doi: 10.1016/s0892-0362(99)00083-5. [DOI] [PubMed] [Google Scholar]
- 15.Arndt T, Stodgell C, Rodier P. The Teratology of Autism. International Journal of Developmental Neuroscience. 2005;23:189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Rodier PM, Hyman S. Early Environmental Factors in Autism. Mental Retardation and Developmental Disabilities. 1998;4:121–128. [Google Scholar]
- 17.Christianson A, Chesler N, Kromberg J. Fetal Valproate Syndrome: Clinical and Neurodevelopmental Features in Two Sibling Pairs. Developmental Medicine and Child Neurology. 1994;36:357–369. doi: 10.1111/j.1469-8749.1994.tb11858.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol. 2001;43:202–206. [PubMed] [Google Scholar]
- 19.Williams P, Hersh J. A Male with Fetal Valproate Syndrome and Autism. Developmental Medicine and Child Neurology. 1997;39:632–634. doi: 10.1111/j.1469-8749.1997.tb07500.x. [DOI] [PubMed] [Google Scholar]
- 20.Bescoby-Chambers N, Forster P, Bates G. Foetal valproate syndrome and autism: additional evidence of an association. Developmental Medicine and Child Neurology. 2001;43:847–848. doi: 10.1017/s0012162201211542. [DOI] [PubMed] [Google Scholar]
- 21.Moore SJ, Turnpenny PD, Quinn A, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genetics. 2000;37:489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean JCS, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genetics. 2002;39:251–259. doi: 10.1136/jmg.39.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasalam AD, Hailey H, Williams JH, et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Developmental Medicine and Child Neurology. 2005;47:551–555. doi: 10.1017/s0012162205001076. [DOI] [PubMed] [Google Scholar]
- 24.Ardinger H, Atkin J, Blackston R, et al. Verficiation of the Fetal Valproate Syndrome Phenotype. Amercian Journal of Medical Genetics. 1988;29:171–185. doi: 10.1002/ajmg.1320290123. [DOI] [PubMed] [Google Scholar]
- 25.Clayton-Smith J, Donnai D. Fetal Valproate Syndrome. Journal of Medical Genetics. 1995;32:724–727. doi: 10.1136/jmg.32.9.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bromley R, Mawer G, Clayton-Smith J, Baker GA On Behalf of the Liverpool and Manchester Neurodevelopment Group. Autism Spectrum Disorders Following In Utero Exposure To Antiepileptic Drugs. Neurology. 2008;71:1923–1924. doi: 10.1212/01.wnl.0000339399.64213.1a. [DOI] [PubMed] [Google Scholar]
- 27.Cohen MJ, Meador KJ, Browning N, Baker GA, Clayton-Smith J, Kalayjian LA, et al. Fetal antiepileptic drug exposure: Motor, adaptive, and emotional/behavioral functioning at age 3 years. Epilepsy and Behavior. 2011;22(2):240–246. doi: 10.1016/j.yebeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mawer G, Briggs M, Baker GA, et al. Pregnancy With Epilepsy: Obstetric and Neonatal Outcome of a Controlled Study. Seizure. 2010;19:112–119. doi: 10.1016/j.seizure.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meador KJ, Baker GA, Finnel RH, et al. In Utero Antiepileptic Drug Exposure: Fetal Death and Malformations. Neurology. 2006;67:407–412. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meador KJ, Baker GA, Browning N, et al. Fetal Antiepileptic Drug Exposure and Verbal vs Non-Verbal Abilities at Age 3. Brain. 2011;134:396–404. doi: 10.1093/brain/awq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutter M. Incidence of Autism Spectrum Disorders: Changes Over Time and Their Meaning. Acta Paediatrica. 2005;94:2–15. doi: 10.1111/j.1651-2227.2005.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 32.Durkin M, Maenner M, Meaney F, et al. Socioeconomic inequality in the prevalence of autism spectrum disorder: evidence from a U.S. cross-sectional study. PLos ONE. 2010;5:e11551. doi: 10.1371/journal.pone.0011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selikowitz M. ADHD. Oxford: Oxford University Press; 2004. [Google Scholar]
- 34.Howlin P, Asgharian A. The diagnosis of autism and Asperger syndrome: findings from a survey of 770 families. Developmental Medicine and Child Neurology. 1999;41:834–839. doi: 10.1017/s0012162299001656. [DOI] [PubMed] [Google Scholar]
- 35.Holowenko H, Pashute K. ADHD in schools: a survey of prevalence and coherence across a local UK population. Educational Psychology in Practice. 2000;16:181–190. [Google Scholar]
- 36.Brent R. Environmental Causes of Human Congenital Malformations: The Pediatrician’s Role in Dealing with these Complex Clinical Problems Caused by a Multiplicity of Environmental and Genetic Factors. Pediatrics. 2004;113:957–968. [PubMed] [Google Scholar]
- 37.Vajda FJ, O’Brien TJ, Hitchcock A, et al. Critical Relationship Between Sodium Valproate Dose and Human Teratogenicity: Results of the Australian Register of Anti-Epileptic Drugs in Pregnancy. Journal of Clinical Neuroscience. 2004;11:854–858. doi: 10.1016/j.jocn.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Marson T, Al-Kharusi A, Alwaidh M, et al. The SANAD Study of Effectiveness of Valproate, Lamotrigine or Topiramate for Generalised and Unclassified Epilepsy: An Unblinded Randomised Controlled Trial. Lancet. 2007;369:1016–1026. doi: 10.1016/S0140-6736(07)60461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodier PM, Bryson S, Welsh J. Minor Physical Anomalies and Physical Measurements in Autism:Data from Nova Scotia. Teratology. 1997;55:319–325. doi: 10.1002/(SICI)1096-9926(199705)55:5<319::AID-TERA4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 40.Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological Origin for Autism: Developmental Abnormalities of the Cranial Nerve Motor Nuclei. The Journal of Comparative Neurology. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]