Synopsis
Anemia of inflammation (AI, also called anemia of chronic disease) is a common, typically normocytic normochromic anemia that is caused by an underlying inflammatory disease. It is diagnosed when serum iron concentrations are low despite adequate iron stores, as evidenced by serum ferritin that is not low. In the setting of inflammation, AI may be difficult to differentiate from iron deficiency anemia, and the two conditions may coexist. In AI, erythropoiesis is iron-restricted by hepcidin-mediated hypoferremia and erythrocyte production is suppressed by cytokines acting on erythroid progenitors. Decreased erythropoiesis is unable to compensate for shortened erythrocyte lifespan caused by enhanced erythrophagocytosis by cytokine-activated macrophages. Treatment should focus on the underlying disease. If this is not feasible and the anemia limits the quality of life or the performance of daily activities, a combination of erythropoiesis-stimulating agents and intravenous iron may be effective but should be attempted only after careful consideration of risk and benefit. Recent advances in molecular understanding of AI are stimulating the development of new pathophysiologically targeted experimental therapies.
Keywords: anemia of chronic disease, hepcidin, ferroportin, cytokines, erythropoiesis-stimulating agents
Clinical Presentation
Mild to moderate anemia (hemoglobin rarely less than 8 g/dl)
Occurring in a setting of infection, inflammatory disease or malignancy
Low serum iron
Systemic iron stores not depleted
Definitions
Anemia of inflammation (AI, formerly also called anemia of chronic disease or anemia of chronic disorders) is usually a mild to moderately severe anemia (hemoglobin rarely lower than 8 g/dL) that develops in the setting of infection, inflammatory disease or malignancy1. The defining biochemical features of AI include low serum iron despite adequate systemic iron stores. The concentration of serum transferrin is also decreased during chronic inflammation but this is a lagging indicator because of the long half-life of transferrin (about 8 days) compared to iron (about 1.5 hours)2. The erythrocytes are usually of normal size and have normal hemoglobin content but are reduced in number (normocytic, normochromic anemia). In some cases, particularly if the inflammatory disease is longstanding, the red cells can be mildly decreased in size and hemoglobin content.
Related conditions
Anemia of critical illness presents with a similar pattern of findings but develops within days in patients who are hospitalized in intensive care units with infections, sepsis or other inflammatory conditions3. Anemia of critical illness may be exacerbated by frequent diagnostic phlebotomies or increased gastrointestinal blood loss as is common in such settings. Anemia of aging4 is a chronic anemia similar to AI but often occurring in the elderly without a specific diagnosis of a predisposing underlying disease. The prevalence of this anemia increases with age, and detailed studies often detect evidence of inflammation, including increased serum C-reactive protein or other biomarkers of inflammation. Anemia of chronic kidney disease is commonly attributed to erythropoietin deficiency but accumulating evidence favors a more complex pathogenesis with a large component of AI whose exacerbations may be manifested as “erythropoietin resistance”5.
Diagnosis
The traditional gold standard for the diagnosis of AI was anemia with hypoferremia or with low transferrin saturation, despite the presence of Prussian-blue-stainable iron in bone marrow macrophages. The main confounding diagnostic entity that also presents with anemia and hypoferremia is iron deficiency anemia where there is no stainable iron in the marrow macrophages. This gold standard has been challenged not only because of the invasive nature of the marrow sampling procedure but also because of findings that bone marrow iron readings are qualitative and not always consistent between evaluators and in multiple specimens6;7 and that iron therapy may cause marrow iron deposition in a poorly bioavailable form which cannot be used by iron-deficient patients8. The marrow iron stain has largely been replaced by serum ferritin determinations. Low serum ferritin (less than 15 ng/ml for general population with some labs using age and gender-specific norms) is highly specific for iron deficiency9 (genetic deficiency of L-ferritin is an extremely rare exception10) and effectively rules out AI. AI is diagnosed when anemia and hypoferremia are accompanied by serum ferritin that is not low. Serum ferritin is increased by inflammation, in part reflecting direct inflammatory regulation of ferroportin synthesis11;12 and in part because serum ferritin originates in macrophages where its synthesis is increased by iron sequestration13 that takes place during inflammation. Iron deficiency is presumed to coexist with AI when ferritin is insufficiently elevated for the intensity of inflammation. Serum ferritin is also increased by tissue injury, especially to the liver.
Diagnostic challenges
The determination of what constitutes “inappropriately low” ferritin may be difficult in practice because even patients with very high serum ferritins may respond to intravenous iron therapy by increasing hemoglobin14. In principle, the limitations of serum ferritin could be circumvented by assaying additional markers of iron deficiency less affected by inflammation, most prominently soluble transferrin receptor15-17. However, the relevant assays have not been standardized, the added value of such studies has not yet been convincingly demonstrated18 and none have been widely adopted. When the anemia is clinically significant and a component of iron deficiency is suspected in a patient with AI, it may be reasonable to perform a therapeutic trial of intravenous iron. Current intravenous iron preparations are quite safe, but the very rare reactions to their administration and the possibility of exacerbating an existing or occult infectious process should be included in the risk-benefit analysis19.
Prevalence
Detailed statistics about the prevalence of AI are not available. It is estimated that the aging of the population and the high prevalence of chronic infections and inflammatory disorders worldwide combine to make AI the second most common cause of anemia worldwide, after iron deficiency. The order may eventually reverse as iron deficiency anemia is more effectively treated or prevented by dietary iron supplementation and by public health measures that curb intestinal parasitic infections.
Pathophysiology
Mildly shortened erythrocyte survival (increased destruction)
Hypoferremia, iron-restricted erythropoiesis from cytokine-stimulated hepcidin increase
Suppression of erythropoiesis by direct effects of cytokines on the marrow
Variable effects of inflammation on erythropoietin production, renal excretion of hepcidin
Overview of the causative factors
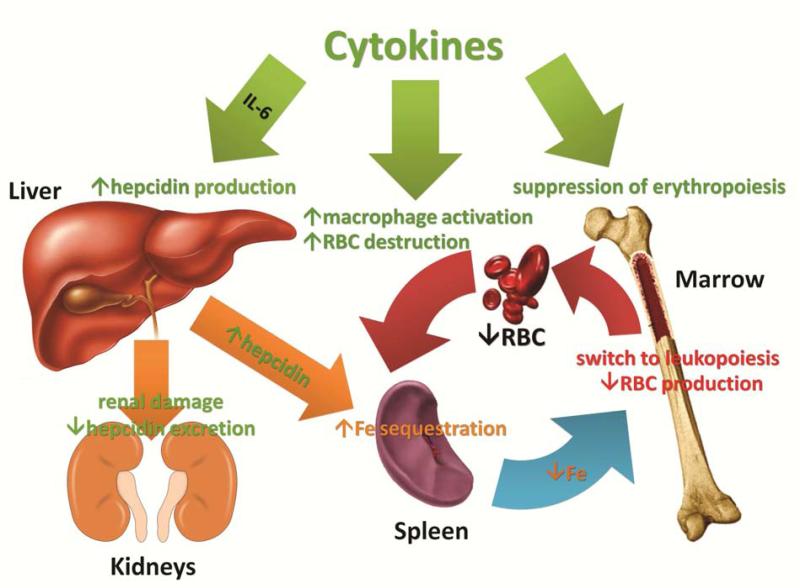
Despite more than fifty years of investigation, our understanding of the pathophysiology of AI is incomplete. Already the earliest studies of AI indicated that the disorder is a consequence of a mild decrease in erythrocyte survival combined with impaired production of erythrocytes1;20. The increased destruction of erythrocytes is predominantly attributable to macrophage activation by inflammatory cytokines but other hemolytic mechanisms may contribute in specific inflammatory diseases. The suppression of erythrocyte production has two major components, iron restriction and direct cytokine effects on erythropoietic progenitors. These effects combine to limit the erythropoietic response to erythropoietin which becomes insufficient to compensate for the increased destruction of erythrocytes. In some situations, the production of erythropoietin may also be decreased, perhaps due to cytokine effects on the renal cells that produce the hormone. In severe inflammation, or when the primary pathology involves the kidneys, decreased renal excretion of hepcidin contributes to hepcidin accumulation and iron restriction21. The complex pathogenesis of AI is summarized in Figure 1 and discussed further.
Figure 1.
The pathogenesis of AI is mediated by inflammatory cytokines and hepcidin, acting together to suppress erythropoiesis and shorten erythrocyte survival in blood. The effects of cytokines are denoted in light green, hepcidin effects are depicted in orange, and combined effects in red.
Erythrocyte destruction
Experiments with transfused erythrocytes showed that erythrocytes from AI patients and from normal controls survived longer in healthy recipients that in patients with AI20. The shortened survival of erythrocytes in AI has been attributed to macrophage activation by inflammatory cytokines that causes the macrophages to ingest and destroy erythrocytes prematurely. Anemia and excessive erythrophagocytosis are prominent features of macrophage activation syndromes, especially those associated with systemic juvenile rheumatoid arthritis22. Here, treatment targeting IL-1 or IL-6 is proving effective, suggesting an important (although possibly indirect) role of these cytokines in the pathogenesis of excessive erythrophagocytosis. In mouse models, multiple cytokines, including interferon-γ and IL-4, have been implicated in activating macrophages for erythrophagocytosis23;24. With the exception of fulminant hemophagocytic states, which are fortunately rare, erythrophagocytosis in AI is only mildly increased and could be readily compensated if the production of erythrocytes was not also impaired1;20.
Hypoferremia
A recent review of mechanisms governing iron homeostasis is provided elsewhere25. Briefly, plasma iron concentrations are under homeostatic control of the hepatic iron-regulatory hormone hepcidin26, and are normally maintained in the 10-30 μM range. Hepcidin acts by regulating the iron delivery to plasma from macrophages that recycle senescent erythrocytes, from duodenal enterocytes that absorb dietary iron and from hepatocytes involved in iron storage. The molecular target of hepcidin is the sole known cellular iron exporter ferroportin27, expressed on cell membranes in tissues that deliver iron to plasma. The binding of hepcidin to ferroportin causes ferroportin endocytosis and its subsequent proteolysis in lysosomes. The loss of ferroportin from cell membranes causes a proportional reduction of iron export to plasma. The production of hepcidin by hepatocytes is in turn regulated by plasma and hepatic iron concentrations and inflammatory cytokines, chiefly IL-628;29. Inflammatory stimuli administered to humans30 or experimental animals elicit a decrease in serum iron concentration within a few hours. The response is dependent on inflammation-induced increase in plasma concentrations of hepcidin31. Increased hepcidin degrades cellular ferroportin, traps iron in macrophages, hepatocytes and intestinal enterocytes so that less iron is delivered to plasma transferrin. The plasma iron compartment is then rapidly depleted of iron through continuing iron uptake by erythroid precursors.
Increased hepcidin causes an iron-restricted anemia even in the absence of inflammation
An experiment of nature, the syndrome of iron-refractory iron deficiency anemia (IRIDA)32, provides an important insight into the role of hepcidin in the regulation of erythropoiesis and as a pathogenic component of AI. The otherwise healthy children with IRIDA suffer from a severely microcytic, hypochromic anemia and hypoferremia that respond poorly to treatment with oral iron and incompletely even to treatment with IV iron33;34. Guided by a mouse model of this condition35, the pathogenesis of IRIDA is now partially understood36. Most of the patients with IRIDA have homozygous or compound heterozygous mutations in the gene encoding the transmembrane serine protease TMPRSS6 (also called matriptase-2), leading to serum hepcidin levels that are high, or inappropriately elevated considering that the patients are iron deficient. Hepcidin-mediated block in duodenal iron absorption is likely responsible for the ineffectiveness of oral iron therapy and hepcidin-induced retention of iron in macrophages reduces the response to IV iron replacement therapy. Importantly, IRIDA patients continue to have microcytosis and hypochromia even after iron therapy, indicating that hemoglobin synthesis is impaired more than the production of erythrocytes. This is in contrast to AI which is usually a normochromic normocytic anemia, indicating that in AI the impairment of hemoglobin synthesis is roughly balanced by decreased production of erythrocytes. Thus, direct suppression of erythrocyte production by inflammatory cytokines in AI may “compensate” for the effect of hypoferremia on hemoglobin synthesis, generating fewer erythrocytes but with normal size and hemoglobin content.
Suppression of erythropoiesis by inflammation
Inflammatory cytokines, including TNFα, IL-1 and interferon-γ, have been reported to suppress erythropoiesis in vitro 37-41 as well as in mouse models24;42. Detailed understanding of the mechanisms involved has been hindered by the complexity of cytokine effects and the ability of each cytokine to regulate the production of many other cytokines41. Nevertheless, several new and promising concepts about the effects of cytokines on erythropoiesis have recently emerged. Libregts at al24 developed a mouse model where overproduction of interferon-γ leads to the development of a mild-to-moderate normocytic normochromic anemia. The model manifests a 50% decrease in erythrocyte survival attributable to interferon-γ-mediated activation of macrophages in the splenic red pulp. The model also shows suppression of erythrocyte production affecting the erythroblast stages and the earliest erythroid-committed precursor BFU-e (burst-forming unit-erythrocyte) but not proerythroblasts and CFU-e (colony-forming unit-erythrocyte). Importantly, myeloid CFU-G/M (colony-forming unit-granulocyte/macrophage) colonies were increased. Microarray analysis of erythroblasts indicated that interferon-γ promotes the transcription of PU.1 and its target genes in an IRF-1-dependent manner but does not affect GATA-1 or its targets. PU.1 and GATA-1 antagonize each other's activity so the increase in PU.1 would be expected to promote myelopoiesis at the expense of erythropoiesis. During infections with viruses or intracellular pathogens known to induce interferon-γ, this mechanism may assure sufficient production of monocytes and macrophages, at the expense of temporary impairment of erythropoiesis. Whether other inflammatory cytokines utilize a similar or different mechanism remains to be determined.
Hepcidin-induced hypoferremia and interferon-γ synergize to suppress erythropoiesis
Richardson et al.43 examined how inflammatory cytokines and hypoferremia interact to affect erythropoiesis during AI. Using in vitro culture of human CD34+ primary progenitors, they documented that hypoferremia (transferrin saturation ≤ 15%) potentiates the suppressive effects of TNF-α and interferon-γ on erythropoiesis. Surprisingly, erythropoietic suppression could be reversed by the addition of the Krebs cycle intermediate isocitrate, a product of the enzyme aconitase which also functions as a cellular iron sensor. Isocitrate injections also reversed AI in a rat model of autoimmune arthritis induced by injection of streptococcal peptidoglycan– polysaccharide. The authors present evidence that hypoferremia activates PU.1 via a protein kinase C pathway, synergizing with the effect of interferon-γ. Isocitrate, acting on aconitase reverses the effect of hypoferremia on PU.1 and relieves the suppression of erythropoiesis. It remains to be seen if these effects are important in other animal models and in human subjects.
Animal models of AI show partial dependence on hepcidin
A new mouse model of AI was generated by a single intraperitoneal injection of heat-killed Brucella abortus44. Like human AI, this model showed multifactorial pathogenesis including iron restriction from increased hepcidin, transient suppression of erythropoiesis and shortened erythrocyte lifespan. Mice developed severe anemia with mild microcytosis and mild hypochromia, a Hb nadir at 14 days and partial recovery by 28 days 45;46. After an early increase in inflammatory markers and hepcidin, the mice manifested hypoferremia despite iron accumulation in the liver. Erythropoiesis was suppressed between days 1 and 7, and erythrocyte destruction was increased as evidenced by shortened RBC lifespan and rare schistocytes on blood smears. Erythropoietic recovery began after 14 days but was iron-restricted, as documented by increased erythrocyte zinc protoporphyrin. In mice with ablated hepcidin-1 gene, anemia was milder, not iron-restricted and with faster recovery, supporting the role of hepcidin in the development of AI.
In the same mouse model of AI, the therapeutic administration of anti-hepcidin monoclonal antibodies decreased the severity of anemia44;47. Moreover, resistance to exogenous erythropoietin doses observed in this model was relieved by coadministration of the antibodies with erythropoietin. In the rat model of autoimmune arthritis induced by injection of streptococcal peptidoglycan–polysaccharide, suppressing hepcidin production by administration of the dorsomorphin derivative LDN-193189 or soluble hemojuvelin-Fc fusion protein, two agents that interfere with bone morphogenetic protein receptor signaling, also ameliorated anemia48.
Treatment of AI
Treat the underlying disease
Treat anemia specifically only if severe or limits activities of daily living
Erythrocyte transfusion for acute symptoms
Erythropoiesis-stimulating agents (ESAs) with or without IV iron (off label treatment)
Experimental therapies under development include new ESAs, anti-cytokine drugs and agents targeting the hepcidin-ferroportin pathway
Current therapy
AI is a secondary manifestation of inflammatory disorders, and treating the underlying disease will correct the anemia. Such treatment is not always possible. Direct treatment of anemia should be considered only if it is impairing the patient's performance, quality of life or recovery from underlying illness. Inflammatory diseases sufficiently severe to cause AI may also cause fatigue or malaise through cytokine-dependent mechanisms, so these symptoms need not be caused by anemia. Potential therapies for AI include erythrocyte transfusions usually reserved for severe and acutely symptomatic anemia, and erythropoiesis-stimulating agents (ESAs: erythropoietin and its derivatives, mimics or inducers, as they become available) with or without intravenous iron supplementation. AI is not a specifically-approved indication for the use of ESAs but should be considered as an alternative to chronic erythrocyte transfusion. The use of ESAs in AI is based on a small number of anecdotal reports49-53 that reported improvement of anemia, and similarities between AI and anemia of chronic kidney disease (CKD), the main indication for ESAs. In CKD, IV iron supplementation potentiates the effect of erythropoietin and its derivatives54, and it has been reported that IV iron may have a similar activity in AI53.
Experimental therapy
Experimental treatments of AI target cytokines or the hepcidinferroportin axis and its various regulators (Table 1). Most of these agents have proven effective in animal models and several are undergoing human trials. Anti-IL-6 agents and other anti-cytokine drugs that indirectly lower IL-6 levels are already approved for the treatment of severe inflammatory diseases. Some of these agents may prove to be very effective for the treatment of AI in other settings, reflecting the important role of IL-6 in its pathogenesis. Because AI impacts symptoms and quality of life but has not been demonstrated to affect survival from the underlying disease, drugs specifically targeted for AI must not only demonstrate efficacy but also must be well-tolerated and free of serious side effects.
Table 1.
Experimental therapeutics for the treatment of AI (not including ESAs)
| Agent or activity | Target | Chemistry | Development status | Key published results |
|---|---|---|---|---|
| tocilizumab | IL-6 receptor | humanized monoclonal antibody | approved to treat rheumatoid arthritis and juvenile rheumatoid arthritis (Genentech, Roche, Chugai) | tocilizumab rapidly reduced hepcidin levels and improved anemia in patients with Castleman's syndrome55 or rheumatoid arthritis56;57 |
| siltuximab | IL-6 | chimeric mouse-human monoclonal antibody | submitted for FDA approval for multifocal Castleman's disease (Janssen) | siltuximab lowered hepcidin and improved anemia in patients with renal cell carcinoma58 |
| hepcidin binders | hepcidin peptide | monoclonal antibody | preclinical to phase 1 (Lilly) | |
| anticalins | preclinical (Pieris) | PRS-080 increased serum iron in monkeys59 | ||
| spiegelmers | phase 2* (Noxxon) | NOX-H94 alleviated IL-6-induced anemia in a primate model60 | ||
| inhibitors of hepcidin production | inhibit signaling by bone morphogenetic protein receptor type I | the kinase site of bone morphogenetic receptor I | preclinical | LDN-193189 improved anemia in the mouse model of turpentine-induced AI61 |
| neutralize bone morphogenetic proteins | soluble hemojuvelin-Fc fusion protein | phase 2a* (Ferrumax) | hemojuvelin-Fc fusion protein alleviated anemia in a rat model of arthritis48 elicited by Group A Streptococcal Peptidoglycan-Polysaccharide | |
| heparin derivatives | preclinical | heparin reduced hepcidin expression62 in mice and serum hepcidin concentrations in patients62 | ||
| inactivate hepcidin mRNA | antisense oligonucleotides (ASO) | preclinical (Xenon/ISIS) | anti-mouse hepcidin ASO improved anemia in a turpentine model of AI in mice63 | |
| inactivate transferrin receptor 2 mRNA | siRNA oligonucleotides | preclinical (Alnylam) | anti-TfR2 siRNA alleviated AI in rodent models64 | |
| ferroportin blockers | hepcidin binding site on ferroportin | monoclonal antibody | phase 1* (Lilly) |
Clinicaltrials.gov
Key Points.
Anemia of inflammation results from hepcidin-induced hypoferremia combined with cytokine-mediated suppression of erythropoiesis and decreased lifespan of erythrocytes
Treatment of the cause of inflammation improves the anemia
Treatment with erythropoiesis-stimulating agents and/or intravenous iron is rarely necessary
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cartwright GE, Lee GR. The anaemia of chronic disorders. Br J Haematol. 1971;21(2):147–152. doi: 10.1111/j.1365-2141.1971.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 2.KATZ JH. Iron and protein kinetics studied by means of doubly labeled human crystalline transferrin. J Clin Invest. 1961:402143–2152. doi: 10.1172/JCI104440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corwin HL, Krantz SB. Anemia of the critically ill: “acute” anemia of chronic disease. Crit Care Med. 2000;28(8):3098–3099. doi: 10.1097/00003246-200008000-00079. [DOI] [PubMed] [Google Scholar]
- 4.Beghe C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. The American Journal of Medicine. 2004;116(7, Supplement 1):3–10. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Elliott J, Mishler D, Agarwal R. Hyporesponsiveness to Erythropoietin: Causes and Management. Advances in Chronic Kidney Disease. 2009;16(2):94–100. doi: 10.1053/j.ackd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Barron BA, Hoyer JD, Tefferi A. A bone marrow report of absent stainable iron is not diagnostic of iron deficiency. Ann Hematol. 2001;80(3):166–169. doi: 10.1007/s002770000261. [DOI] [PubMed] [Google Scholar]
- 7.Krause JR, Brubaker D, Kaplan S. Comparison of stainable iron in aspirated and needle-biopsy specimens of bone marrow. Am J Clin Pathol. 1979;72(1):68–70. doi: 10.1093/ajcp/72.1.68. [DOI] [PubMed] [Google Scholar]
- 8.Thomason RW, Lavelle J, Nelson D, Lin K, Uherova P. Parenteral iron therapy is associated with a characteristic pattern of iron staining on bone marrow aspirate smears. Am J Clin Pathol. 2007;128(4):590–593. doi: 10.1309/WEDK7D1P7YPT8G0F. [DOI] [PubMed] [Google Scholar]
- 9.Ferraro S, Mozzi R, Panteghini M. Revaluating serum ferritin as a marker of body iron stores in the traceability era. Clin Chem Lab Med. 2012;50(11):1911–1916. doi: 10.1515/cclm-2012-0129. [DOI] [PubMed] [Google Scholar]
- 10.Cozzi A, Santambrogio P, Privitera D, Broccoli V, Rotundo LI, Garavaglia B, et al. Human L-ferritin deficiency is characterized by idiopathic generalized seizures and atypical restless leg syndrome. J Exp Med. 2013;210(9):1779–1791. doi: 10.1084/jem.20130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran TN, Eubanks SK, Schaffer KJ, Zhou CY, Linder MC. Secretion of ferritin by rat hepatoma cells and its regulation by inflammatory cytokines and iron. Blood. 1997;90(12):4979–4986. [PubMed] [Google Scholar]
- 12.Thomson AM, Cahill CM, Cho HH, Kassachau KD, Epis MR, Bridges KR, et al. The Acute Box cis-Element in Human Heavy Ferritin mRNA 5′-Untranslated Region Is a Unique Translation Enhancer That Binds Poly(C)-binding Proteins. J Biol Chem. 2005;280(34):30032–30045. doi: 10.1074/jbc.M502951200. [DOI] [PubMed] [Google Scholar]
- 13.Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116(9):1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- 14.Cazzola M, Ponchio L, de Benedetti F, Ravelli A, Rosti V, Beguin Y, et al. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood. 1996;87(11):4824–4830. [PubMed] [Google Scholar]
- 15.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3363. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 16.Pettersson T, Kivivuori SM, Siimes MA. Is serum transferrin receptor useful for detecting iron-deficiency in anaemic patients with chronic inflammatory diseases? Br J Rheumatol. 1994;33(8):740–744. doi: 10.1093/rheumatology/33.8.740. [DOI] [PubMed] [Google Scholar]
- 17.Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood. 1990;75(9):1870–1876. [PubMed] [Google Scholar]
- 18.Infusino I, Braga F, Dolci A, Panteghini M. Soluble Transferrin Receptor (sTfR) and sTfR/log Ferritin Index for the Diagnosis of Iron-Deficiency Anemia A Meta-Analysis. American Journal of Clinical Pathology. 2012;138(5):642–649. doi: 10.1309/AJCP16NTXZLZFAIB. [DOI] [PubMed] [Google Scholar]
- 19.Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013:347f4822. doi: 10.1136/bmj.f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freireich EJ, Ross JF, BAYLES TB, EMERSON CP, FINCH SC. Radioactive iron metabolism and erythrocyte survival studies of the mechanism of the anemia associated with rheumatoid arthritis. J Clin Invest. 1957;36(7):1043–1058. doi: 10.1172/JCI103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troutt JS, Butterfield AM, Konrad RJ. Hepcidin-25 concentrations are markedly increased in patients with chronic kidney disease and are inversely correlated with estimated glomerular filtration rates. J Clin Lab Anal. 2013;27(6):504–510. doi: 10.1002/jcla.21634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correll CK, Binstadt BA. Advances in the pathogenesis and treatment of systemic juvenile idiopathic arthritis. Pediatric Res. 2013 doi: 10.1038/pr.2013.187. [DOI] [PubMed] [Google Scholar]
- 23.Milner JD, Orekov T, Ward JM, Cheng L, Torres-Velez F, Junttila I, et al. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 2010;116(14):2476–2483. doi: 10.1182/blood-2009-11-255174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libregts SF, Gutierrez L, de Bruin AM, Wensveen FM, Papadopoulos P, van IW, et al. Chronic IFN-gamma production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118(9):2578–2588. doi: 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- 25.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93(4):1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 26.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 28.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez R, Jung CL, Gabayan V, Deng JC, Ganz T, Nemeth E, et al. HEPCIDIN INDUCTION BY PATHOGENS AND PATHOGEN-DERIVED MOLECULES IS STRONGLY DEPENDENT ON INTERLEUKIN-6. Infect Immun. 2013 doi: 10.1128/IAI.00983-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemna E, Pickkers P, Nemeth E, van der HH, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106(5):1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 31.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finberg KE. Iron-refractory iron deficiency anemia. Semin Hematol. 2009;46(4):378–386. doi: 10.1053/j.seminhematol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Camaschella C, Poggiali E. Inherited disorders of iron metabolism. Curr Opin Pediatr. 2011;23(1):14–20. doi: 10.1097/MOP.0b013e3283425591. [DOI] [PubMed] [Google Scholar]
- 35.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Means RT, Jr., Krantz SB. Inhibition of human erythroid colony-forming units by tumor necrosis factor requires beta interferon. J Clin Invest. 1993;91(2):416–419. doi: 10.1172/JCI116216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Means RT, Jr., Dessypris EN, Krantz SB. Inhibition of human erythroid colony-forming units by interleukin-1 is mediated by gamma interferon. J Cell Physiol. 1992;150(1):59–64. doi: 10.1002/jcp.1041500109. [DOI] [PubMed] [Google Scholar]
- 39.Means RT, Jr., Krantz SB. Inhibition of human erythroid colony-forming units by gamma interferon can be corrected by recombinant human erythropoietin. Blood. 1991;78(10):2564–2567. [PubMed] [Google Scholar]
- 40.Broxmeyer HE, Williams DE, Lu L, Cooper S, Anderson SL, Beyer GS, et al. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma. J Immunol. 1986;136(12):4487–4495. [PubMed] [Google Scholar]
- 41.Felli N, Pedini F, Zeuner A, Petrucci E, Testa U, Conticello C, et al. Multiple Members of the TNF Superfamily Contribute to IFN-[.gamma]Mediated Inhibition of Erythropoiesis. J Immunol. 2005;175(3):1464–1472. doi: 10.4049/jimmunol.175.3.1464. [DOI] [PubMed] [Google Scholar]
- 42.Johnson RA, Waddelow TA, Caro J, Oliff A, Roodman GD. Chronic exposure to tumor necrosis factor in vivo preferentially inhibits erythropoiesis in nude mice. Blood. 1989;74(1):130–138. [PubMed] [Google Scholar]
- 43.Richardson CL, Delehanty LL, Bullock GC, Rival CM, Tung KS, Kimpel DL, et al. Isocitrate ameliorates anemia by suppressing the erythroid iron restriction response. J Clin Invest. 2013;123(8):3614–3623. doi: 10.1172/JCI68487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115(17):3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 45.Gardenghi S, Renaud TM, Meloni A, Casu C, Crielaard BJ, Bystrom LM, et al. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood. 2014;123(8):1137–1145. doi: 10.1182/blood-2013-08-521625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim A, Fung E, Parikh SG, Valore EV, Gabayan V, Nemeth E, et al. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123(8):1129–1136. doi: 10.1182/blood-2013-08-521419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooke KS, Hinkle B, Salimi-Moosavi H, Foltz I, King C, Rathanaswami P, et al. A fully human anti-hepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122(17):3054–3061. doi: 10.1182/blood-2013-06-505792. [DOI] [PubMed] [Google Scholar]
- 48.Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118(18):4977–4984. doi: 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato Y, Takagi C, Tanaka J, Masaki Y, Furuya H. Effect of daily subcutaneous administration of recombinant erythropoietin on chronic anemia in rheumatoid arthritis. Intern Med. 1994;33(4):193–197. doi: 10.2169/internalmedicine.33.193. [DOI] [PubMed] [Google Scholar]
- 50.Peeters HR, Jongen-Lavrencic M, Bakker CH, Vreugdenhil G, Breedveld FC, Swaak AJ. Recombinant human erythropoietin improves health-related quality of life in patients with rheumatoid arthritis and anaemia of chronic disease; utility measures correlate strongly with disease activity measures. Rheumatol Int. 1999;18(5-6):201–206. doi: 10.1007/s002960050085. [DOI] [PubMed] [Google Scholar]
- 51.Peeters HR, Jongen-Lavrencic M, Vreugdenhil G, Swaak AJ. Effect of recombinant human erythropoietin on anaemia and disease activity in patients with rheumatoid arthritis and anaemia of chronic disease: a randomised placebo controlled double blind 52 weeks clinical trial. Ann Rheum Dis. 1996;55(10):739–744. doi: 10.1136/ard.55.10.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettersson T, Rosenlof K, Friman C, Mickos A, Teppo AM, Fyhrquist F. Successful treatment of the anemia of rheumatoid arthritis with subcutaneously administered recombinant human erythropoietin. Slower response in patients with more severe inflammation. Scand J Rheumatol. 1993;22(4):188–193. doi: 10.3109/03009749309099269. [DOI] [PubMed] [Google Scholar]
- 53.Arndt U, Kaltwasser JP, Gottschalk R, Hoelzer D, Moller B. Correction of iron-deficient erythropoiesis in the treatment of anemia of chronic disease with recombinant human erythropoietin. Ann Hematol. 2005;84(3):159–166. doi: 10.1007/s00277-004-0950-z. [DOI] [PubMed] [Google Scholar]
- 54.Singh A. Hemoglobin Control, ESA Resistance, and Regular Low-Dose IV Iron Therapy: A Review of the Evidence. Seminars in Dialysis. 2009;22(1):64–69. doi: 10.1111/j.1525-139X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 55.Song SN, Tomosugi N, Kawabata H, Ishikawa T, Nishikawa T, Yoshizaki K. Down-regulation of hepcidin resulting from long-term treatment with an anti-IL-6 receptor antibody (tocilizumab) improves anemia of inflammation in multicentric Castleman disease. Blood. 2010;116(18):3627–3634. doi: 10.1182/blood-2010-03-271791. [DOI] [PubMed] [Google Scholar]
- 56.Song SN, Iwahashi M, Tomosugi N, Uno K, Yamana J, Yamana S, et al. Comparative evaluation of the effects of treatment with tocilizumab and TNF-alpha inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 2013;15(5):R141. doi: 10.1186/ar4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isaacs JD, Harari O, Kobold U, Lee JS, Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther. 2013;15(6):R204. doi: 10.1186/ar4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schipperus M, Rijnbeek B, Reddy M, Qin X, Cornfield MJ. CNTO328 (Anti-IL-6 mAb) Treatment Is Associated with An Increase in Hemoglobin (Hb) and Decrease in Hepcidin Levels in Renal Cell Carcinoma (RCC) [abstract]. Blood. 2009;22(114):4045. Ref Type: Abstract. [Google Scholar]
- 59.Hohlbaum A, Gille H, Christian J, Allersdorfer A, Jaworski J, Burrows J, et al. Iron mobilization and pharmacodynic marker measurements in non-human primates following administration of PRS-080, a novel and highly specific anti-hepcidin therapeutic. American Journal of Hematology. 2013;88(5):E41. Ref Type: Abstract. [Google Scholar]
- 60.Schwoebel F, van Eijk LT, Zboralski D, Sell S, Buchner K, Maasch C, et al. The effects of the anti-hepcidin Spiegelmer NOX-H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. 2013 doi: 10.1182/blood-2012-09-456756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinbicker AU, Sachidanandan C, Vonner AJ, Yusuf RZ, Deng DY, Lai CS, et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. 2011;117(18):4915–4923. doi: 10.1182/blood-2010-10-313064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poli M, Girelli D, Campostrini N, Maccarinelli F, Finazzi D, Luscieti S, et al. Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood. 2011;117(3):997–1004. doi: 10.1182/blood-2010-06-289082. [DOI] [PubMed] [Google Scholar]
- 63.Crosby JR, Gaarde WA, Egerston J, McKay R, Sun Y, Freier S, et al. Targeting hepcidin with antisense oligonucletides improves anemia endpoints in mice. Blood. 2006;108(11, Part 1):83A–84A. [Google Scholar]
- 64.Akinc A, Chan-Daniels A, Sehgal A, Foster D, Bettencourt BR, Hettinger J, et al. Targeting the Hepcidin Pathway with RNAi Therapeutics for the Treatment of Anemia. Blood. 2011;118(21):315. [Google Scholar]