Abstract
Aberrant expression of microRNAs (miRNAs) plays important roles in the development and progression of pancreatic cancer (PC). Expression analysis of miR-146a in human PC tissues showed decreased expression in about 80% of samples compared to corresponding noncancerous tissue. Moreover, expression of miR-146a in eight PC cell lines, and in pancreatic tissues obtained from transgenic mouse models of K-Ras (K), Pdx1-Cre (C), K-Ras;Pdx1-Cre (KC) and K-Ras;Pdx1-Cre;INK4a/Arf (KCI), showed down-regulation of miR-146a expression in KCI mice which was in part led to over-expression of its target gene, epidermal growth factor receptor (EGFR). Treatment of PC cells with CDF, a novel synthetic compound, led to reexpression of miR-146a, resulting in the down-regulation of EGFR expression. Moreover, reexpression of miR-146a by stable transfection or treatment with CDF in vivo (xenograft animal model) resulted in decreased tumor growth which was consistent with reduced EGFR, ERK1, ERK2, and K-Ras expression. Further knock-down of miR-146a in AsPC-1 cells led to the upregulation of EGFR expression and showed increased clonogenic growth. In addition, knockdown of EGFR by EGFR siRNA transfection of parental AsPC-1 cells and AsPC-1 cells stably transfected with pre-miR-146a resulted in decreased invasive capacity, which was further confirmed by reduced luciferase activity in cells transfected with pMIR-Luc reporter vector containing miR-146a binding site. Collectively, these results suggest that the loss of expression of miR-146a is a fundamental mechanism for over-expression of EGFR signaling and that reexpression of miR-146a by CDF treatment could be useful in designing personalized strategy for the treatment of human PC.
Keywords: EGFR, miR-146a, CDF, pancreatic cancer, xenograft mouse model
1. Introduction
Although incremental therapeutic improvements have been made in recent years, pancreatic cancer (PC) still remains the fourth leading cause of cancer-related deaths in the USA [1]. Generally, this dismal outcome is attributed to the aggressive nature of PC as well as lack of early-stage symptoms and screening methods. Hence, there is a dire need for the development of new diagnostic biomarkers and innovative therapeutic strategies for improving the overall survival outcome of PC patients. An early stage blood test as a potential diagnostic marker for PC would have tremendous impact on treatment options, and thus fewer cancer-related deaths; however, such marker is not yet available. Emerging evidence suggest that aberrant expression of microRNAs (miRNAs) is vital in the development of various types of cancer, including PC. These are small, stable, endogenous non-coding RNAs that characteristically function as negative regulators of expression of protein-coding genes, and they have been detected in human plasma, fresh-frozen and formalin-fixed paraffin-embedded (FFPE) tissues, and thus they could serve as early diagnostic markers [2, 3].
Several miRNAs such as miR-21, miR-155, and miR-221 have been reported to be elevated in PC tissues, suggesting the oncogenic role of those miRNAs [2, 4]. Likewise, numerous miRNAs has been found to have reduced expression including miR-146a in many cancers including PC, and thus they function as tumor suppressor [5–8]. As reported in this article that we found decreased expression of miR-146a in PC tissues and PC cell lines, which is consistent with other reports. Our group has previously demonstrated that decreased expression of miR-146a was associated with increased expression of EZH2 in PC cells, which was inhibited by curcumin analog CDF treatment in part due to re-expression of miR-146a [5]. Paik et al have demonstrated that over-expression of miR-146a in extra-nodal NK/T cell lymphoma led to the down-regulation of NF-κB activity via TNF receptor-associated factor 6 (TRAF6) [8]. Similarly, Chassin et al showed that forced-expression of miR-146a could reduce the expression of postischemic interleukin 1 receptor-associated kinase (Irak1) and tissue damage in vivo [9]. Another study in a glioblastoma cell line reported that up-regulation of miR-146a could inhibit tumor growth and migration of glioma stem-like cells by down-regulating Notch-1 [7]. In contrast, miRNA profiling in thyroid cancer revealed over-expression of miR-146a along with miR-221/222 and miR-155, compared to unaffected thyroid tissue with a vivid loss of KIT transcript and protein [10]. In breast carcinomas, miR-146a silenced BRCA1 displaying its fundamental role in tumorigenesis [11]. Taken together, the above conflicting results suggest that miR-146a may differ in its roles between various types of cancers which could be accounted for differences in their target genes.
We have reported earlier that the involvement of miR-146a is associated with over-expression of EGFR and activation of NF-κB in PC cells [6]. Chen et al reported similar findings in non-small lung cancer cells (NSCLC) and it was correlated with distant metastasis in FFPE lung cancer samples [12]. A recent report suggested the involvement of EGFR and specific tumor suppressive miRNAs through phosphorylation of argonaute 2 (AGO2), indicating that modulation of miRNA biogenesis has potential in clinical setting [13]. Similarly, forced-expression of miR-146a in castration-resistant prostate cancer cells inhibited tumor growth [14]. Although numerous studies have reported the deregulation of miR-146a and EGFR expression in many cancers [7, 12, 14] including PC [4, 6], the extent of its inter-relationship and the molecular mechanism behind this biology has not been previously examined.
In the current study, we measured the expression level of commonly suppressed miR-146a in 29 PC patients and 15 normal pancreatic specimens obtained from fine-needle aspirates (FNA) preserved as FFPE cell blocks. Expression level of miR-146a was also determined in 8 well established PC cell lines and tumor specimens from transgenic mouse model, which negatively regulated EGFR expression. Furthermore, we studied the putative role of miR-146a and the expression of EGFR by transfecting miR-146a precursor (in vivo) and miR-146a inhibitor (in vitro). We found that elevated miR-146a expression in vivo leads to decreased tumor burden, which was associated with down-regulation of EGFR, ERK1, ERK2, and K-Ras expression. In addition, inhibition of EGFR by siRNA transfection in cells stably transfected with pre-miR-146a decreased cell invasion with concomitant decrease in EGFR expression. Moreover, luciferase activity was decreased in AsPC-1 cells transfected with miR-146a luciferase vector compared to the control vector, which was further decreased when treated with CDF, suggesting that our novel agent CDF can increase miR-146a and in turn down-regulates the expression of EGFR, and thus CDF could be useful for designing novel therapeutic strategies for the treatment of PC.
2. Materials and Methods
2.1. Cells Culture, Drugs and Reagents
Human PC cell lines AsPC-1, BxPC-3, COLO-357, L3.6pl, PANC-1, PANC-28, MIAPaCa-2, MIAPaCa-2-GR (gemcitabine resistant) were maintained and grown as described earlier [15] and they were chosen for this study. All cell lines were tested and authenticated as described earlier [15, 16]. CDF was synthesized as described in our earlier publications [17, 18].
2.2. Tissue Collection
Diagnostic fine needle aspirates (FNAs) from 29 patients were collected following conventional diagnostic procedure. We also collected normal appearing pancreas tissue from 15 surgical patients. For histological confirmation of cancer, hematoxylin and eosin staining was used. The institutional human investigation review board approved the study.
2.3. Mouse Model
This study was carried out with the recommendations in the guidelines for the care and use of laboratory animals of the National Institutes of Health, and the protocol was approved by Wayne State University as stated earlier [4]. The LSL-K-RasG12D strain was bred to the following strains: LSL-K-RasG12D;Pdx1-Cre, and LSL-K-RasG12D ;Pdx1-Cre; INK4a/Arf lox/lox as previously described [19, 20]. Genomic DNA from tail cuttings confirmed the presence of the oncogenic KRas, and Pdx1-Cre transgenes. Pancreata were collected from LSL-K-RasG12D (K), Pdx1-Cre (C), LSL-K-RasG12D;Pdx1-Cre (KC), LSL-K-RasG12D Pdx1-Cre;INK4a/Arf (KCI) mouse models.
2.4. RNA Isolation
RNA from human specimens was isolated using FFPE miRNeasy Kit from Qiagen. Tissue sections of 10 µm thick were placed in 1 ml xylene, and RNA was extracted according to the manufacturer's protocol. Alternately, the RNA from mouse tumor remnants and cell lines untreated and treated with CDF (200–500 nM) were isolated by Trizol (Invitrogen, Carlsbad, CA) method following manufacture’s protocol.
2.5. Real-Time Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
To examine the basal level of expression of miR-146a in human tissue samples, SYBR Green miRNA based assay with Universal cDNA Synthesis Kit (EXIQON) was used according to manufacturer’s protocol. Alternately, for transgenic mouse pancreata and mouse tumor remnants from xenograft mouse model and PC cell lines, TaqMan MicroRNA Assay kit was used (Applied Biosystems) per manufacturer’s protocol. In both cases 10 ng of RNA from each sample were reverse transcribed as described earlier [3]. For EGFR mRNA expression SYBR Green master mix (Applied Biosystems) mRNA based assay was utilized using 1 µg of RNA. All reactions were performed in triplicate using StepOnePlus Real-Time PCR (Applied Biosystems). Relative expression of miRNAs and mRNAs were analyzed using Ct method and was normalized by SNORD48, RNU48, and GAPDH expression.
2.6. Protein Extraction and Western Blot Analysis
Total protein was extracted from AsPC-1 cell lines transfected with anti-sense oligonucleotide (ASO), epidermal growth factor (EGFR) siRNA as well as from AsPC-1 and AsPC-1 stably transfected with miR-146a mouse tumor remnants from xenograft model untreated and treated with CDF and subjected to western blot analysis as described previously [21] to assess EGFR, ERK1, ERK2, and K-Ras expression. The data was normalized against β-actin expression.
2.7. Transfection of miRNA Precursor for miR-146a in vivo
Female CB17 SCID mice were purchased from Taconic Farms (Germantown, NY) and fed Lab Diet 5021 (Purina Mills, Inc., Richmond, IN). Transfection of expression plasmids with pre-miR-146a (Origene Technologies) in AsPC-1 cells were established first prior to injection in the mice following methods described earlier [22]. AsPC-1 cells were transfected with empty plasmid to which served as vector control. About two and a half million AsPC-1 cells both vector control and pre-miR-146a transfected, were injected subcutaneously bilaterally in SCID mice. Mice were randomized into three treatment groups (n = 6 per group): (1) AsPC-1 control vector; (2) pre-miR-146a transfected (3) pre-miR-146a transfected and treated with CDF (10 mg/mouse/day), intragastric once daily for two weeks. Tumor measurements were taken regularly between the three groups for 32 days. Tumor weights were also taken after euthanizing the animals on the 38th day. Both RNA and proteins were isolated from the tumor tissue for qRT-PCR and western blot analysis.
2.8. Anti-sense miR-146a Oligonucleotide Transfection
AsPC-1 cells (200,000/well) were plated in 6 well plates and incubated overnight. Cells were transfected with ASO-miR-146a (inhibitor) or scrambled control inhibitor (control) using DharmaFECT (Thermo Scientific, Pittsburgh, PA) following the manufacturer’s protocol. Cells were treated with 500 nM of CDF for 16–72 h and tested the transfection effects of miR-146a expression by qRT-PCR, and migration of cells by scratch assay and EGFR expression by western blot.
2.9. Clonogenic Assay of Transfected Cells
The effect of transfection of cells with ASO-miR-146a with and without CDF treatment was assessed by clonogenic assay after 72h of treatment. The cells were trypsinized and about 1000 viable cells were plated in 100 mm petri dishes. Cells were then incubated in a 5% CO2/5% O2 90% N2 incubator for about 10–12 days at 37°C. The colonies formed were stained with 2% crystal violet and photographed.
2.10. Scratch Assay of Transfected Cells
Scratch assay was carried out to examine the effect of transfection of ASO-miR-146a on AsPC-1 cells, and also their effect after treatment with CDF for 16h in 6 well plate as described previously [23].
2.11. EGFR siRNA Transfection
AsPC-1 cells and AsPC-1 cells transfected with miR-146a were plated in 6 well plates and incubated overnight. Cells were transfected twice with EGFR siRNA or control siRNA using DharmaFECT (Thermo-Scientific) for 72h following the manufacturer’s protocol. The transfection effects were tested for miR-146a expression by qRT-PCR, migration of cells by invasion assay and EGFR expression by western blot.
2.12. Invasion Assay
Chamber Cell invasion assay was conducted using 24-well transwell permeable supports (Corning, Lowell, MA) as described previously [24]. Cells were transfected twice either with control siRNA or EGFR siRNA for 72h. The assay was carried out and cells were stained with calcein AM (Invitrogen) and photographed as described previously [24].. The invaded cells were trypsinized and read using micro-plate reader (TECAN).
2.13. Luciferase Reporter Gene Assay
Luciferase reporter gene assay was conducted in AsPC-1 cells to determine the effect of CDF or miR-146a over-expression on miR-146a binding activity by using miR-146a-mediated luciferase reporter gene vector (Signosis, Sunnyvale, CA). The assay was carried out with about 10,000 cells per well in 96 well plate and incubated for 24–48h. The cells were transfected with either control vector or miR-146a luciferase reporter gene vector (Signosis) using ExGen 500 transfection reagent. The cells were also co-transfected with either pre-miR-146a using DharmaFECT transfection reagent (Dharmacon) according to the manufacturer’s protocol or treated with CDF. The luciferase activity was carried out using luciferase assay system (Promega) according to manufacturer’s protocol.
2.14. Statistical Methods
Comparisons of treatment outcome were tested for statistical difference by the paired t-test. Statistical significance was assumed at a p value of < 0.05.
3. Results
3.1. The expression of miR-146a was down-regulated in PC patient samples compared to normal controls
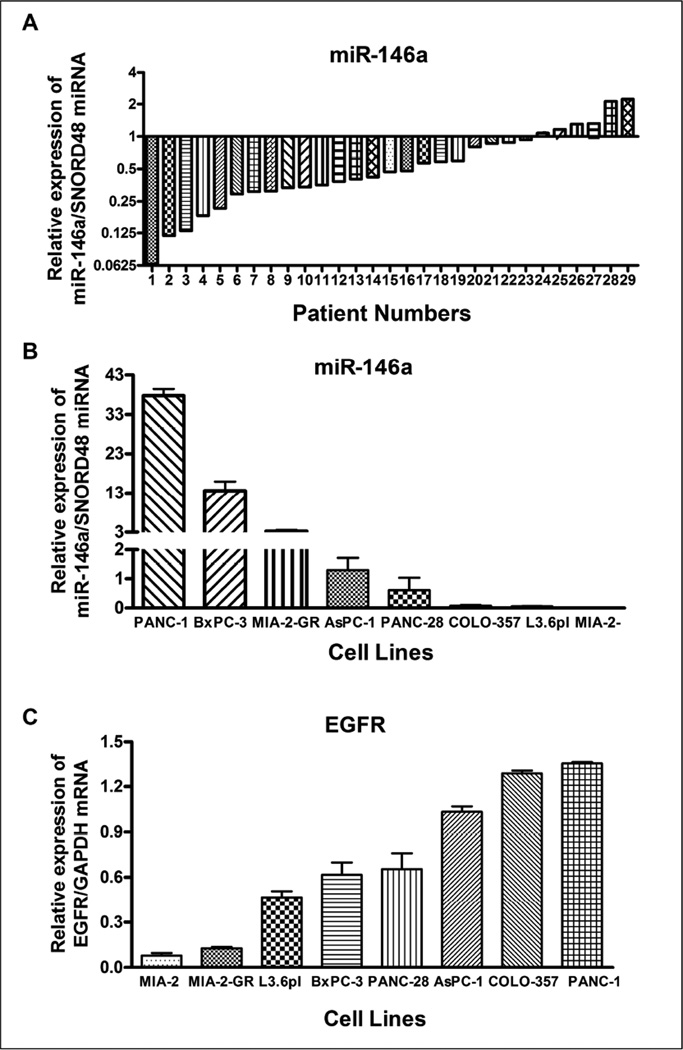
Because of the emerging role of miRNAs in PC, and evidence provided in our previous publication [2], we analyzed the expression level of miR-146a individually in 29 PC patient samples as well as in 15 normal controls. The total RNA was efficiently extracted from FNA cell blocks, and was compared across multiple samples. The expression level in controls was set at 1.0. The miRNA expression analysis of miR-146a showed significant reduction in expression in 23 patients out of a total of 29 PC patients (~80% of patients) in our study, suggesting that miR-146a is functioning as a tumor suppressor (Figure 1A).
Figure 1.
Comparative expression analysis of miR-146a in 29 FNA cell blocks from PC patients individually compared to 15 normal controls using qRT-PCR. 1.0 represents average of normal subjects (n=15). There was a significant down-regulation of miR-146a in most of the 29 PC patients compared to normal subjects (A). Basal levels of miR-146a expression (B) and EGFR expression (C) in human PC cell lines AsPC-1, BxPC-3, COLO-357, L3.6pl, MIAPaCa-2 (MIA-2), MIAPaCa-2-GR (MIA-2-GR), PANC-1 and PANC-28. The miR-146a expression was significantly lower in PC cells and was normalized using SNORD48 miRNA, whereas EGFR expression was higher and was normalized using GAPDH mRNA as assessed by qRT-PCR.
Similarly, we also examined the basal level of expression of miR-146a in eight PC cell lines. Interestingly, we found differential expression of miR-146a in cell lines in which PANC-1 and BxPC-3 showed relatively higher level of miR-146a expression, whereas the other six PC cell lines AsPC-1, COLO-357, PANC-28, L3.6pl, MIAPaCa-2, MIAPaCa-2-GR (gemcitabine resistant), showed decreased expression (Figure 1B). Decreased expression of miR-146a in cell lines was observed mostly in aggressive and drug-resistant cell lines such as AsPC-1, MIAPaCa-2, PANC-28, and MIAPaCa-2-GR. The loss of expression of miR-146a was well-correlated with higher level of EGFR expression in 6 PC cell lines, with the exception of MIAPaCa-2 and PANC-1 as shown in Figure 1C. MIAPaCa-2 cell line exhibited a lower level of both miR-146a and EGFR expression. In contrast, PANC-1 cell line showed higher levels of both miR-146a and EGFR expression.
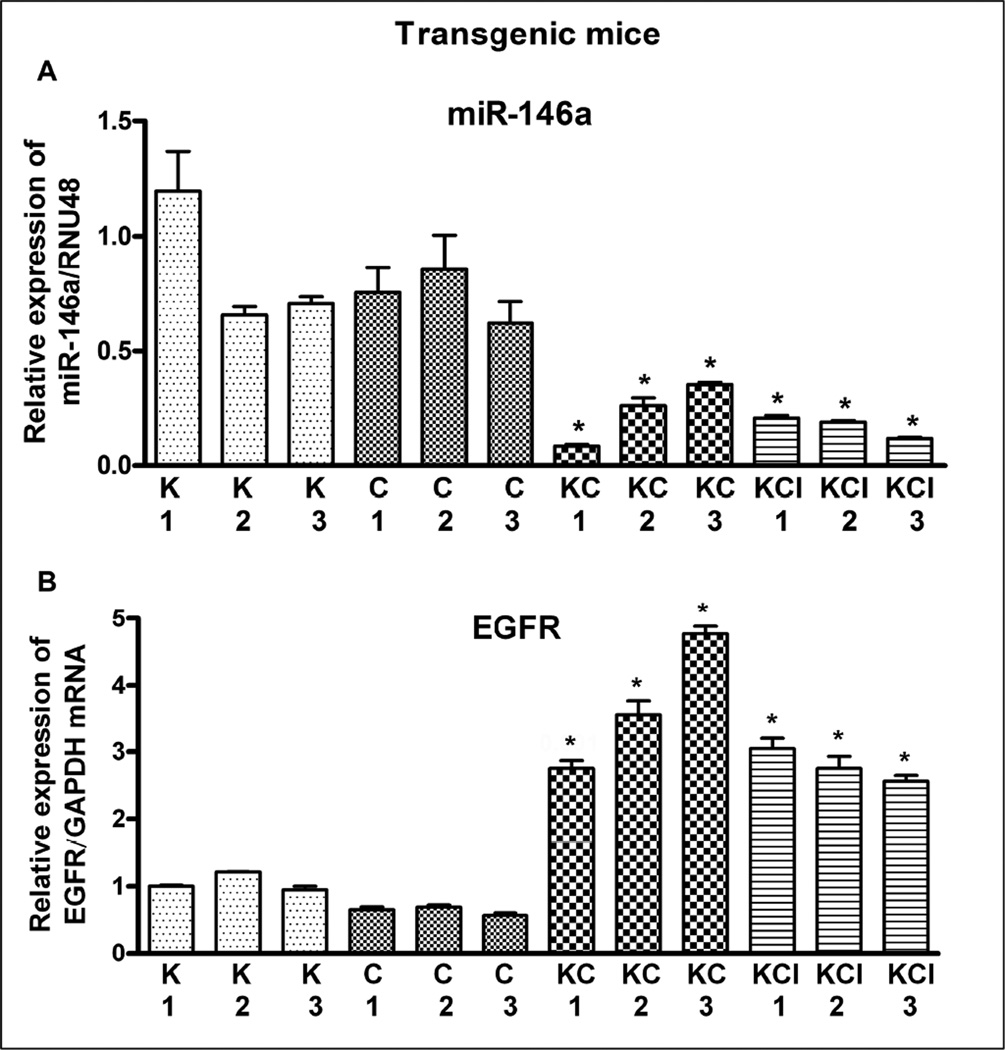
3.2. The miR-146a expression was inversely correlated with EGFR expression in transgenic mouse model
Since transgenic mice mimic human PC and has proven useful for studying tumor progression, prevention and therapy [4], we studied the inter-relationship between miR-146a and EGFR expression in pancreata of different LSL-K-RasG12D strain mice. We previously examined the protein expression of EGFR in transgenic mice tumors [4], but in this study we assessed EGFR expression at the mRNA level and tested its correlation with the expression of miR-146a. We found a significantly higher expression of EGFR even at mRNA level in transgenic mice tumors from LSL-K-RasG12D;Pdx1-Cre;Ink4a (KCI) and LSL-K-RasG12D;Pdx1-Cre (KC), compared to LSL-K-RasG12D (K) and Pdx1-Cre (C) measured by qRT-PCR. The analysis showed decreased expression of miR-146a which could contribute to over-expression of EGFR. We observed significantly lower expression of miR-146a in KC and KCI compared to K or C mouse model as shown in Figure 2A. The loss of expression of miR-146a was inversely correlated with the level of EGFR expression in KC and KCI mouse models (Figure 2B).
Figure 2.
Comparative expression of miR-146a (A) and EGFR (B) in the pancreata of K-Ras (K), Pdx1-Cre (C), K-Ras;Pdx1-Cre; (KC), and K-Ras;Pdx1-Cre;INK4a/Arf (KCI) mouse. There was a significant down-regulation in the expression of miR-146a in KC and KCI pancreata (tumors) compared to either K or C pancreata (normal pancreas). In contrast, the expression of EGFR was significantly up-regulated in KC and KCI tumors. The miR-146a expression was normalized using RNU48 miRNA and EGFR expression was normalized using GAPDH mRNA. P values represent comparison between K/C and KC/ KCI and were found to be significant (*) and less than 0.05.
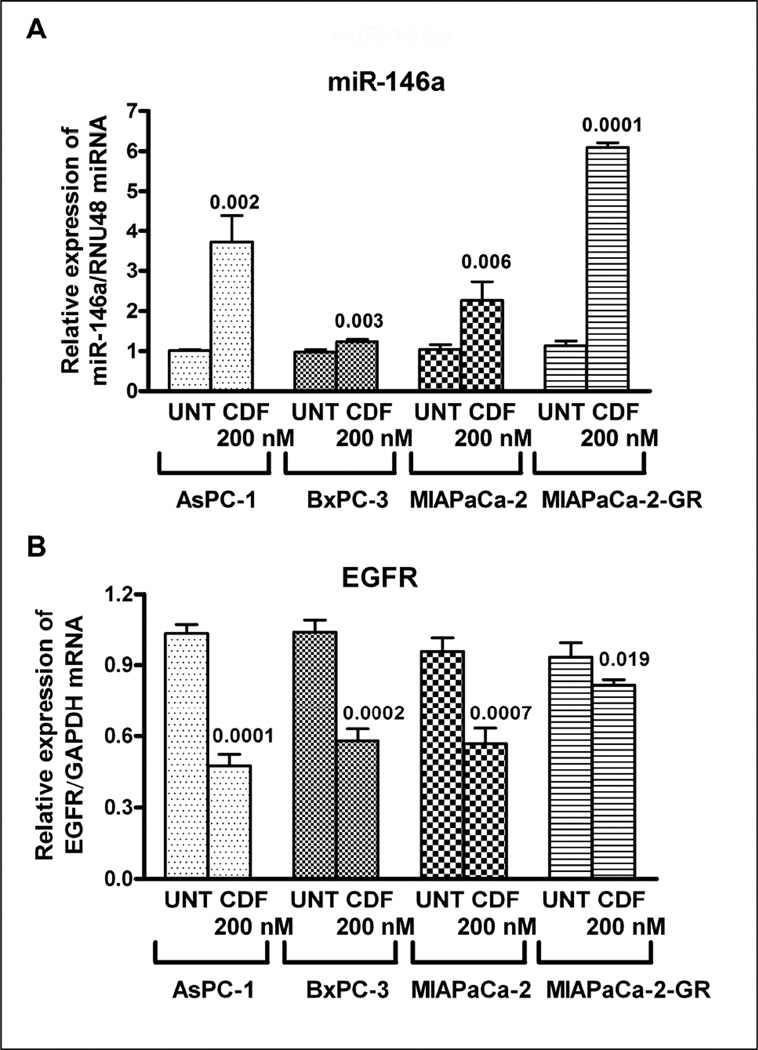
3.3. CDF treatment synergistically re-expressed miR-146a expression and inactivated EGFR expression in PC cells
The induction of miR-146a with CDF treatment as assessed by qRT-PCR confirmed the tumor suppressive role of miR-146a. We tested this hypothesis in four different PC cell lines AsPC-1, BxPC-3, MIAPaCa-2, and MIAPaCa-2-GR with 0.2 µM of CDF treatment for 72h. Indeed, we observed significant up-regulation of miR-146a expression (Figure 3A) with concomitant decreased expression of EGFR (Figure 3B) in all four cell lines treated with CDF as presented in Figure 3. From here on, we chose AsPC-1 cells for further mechanistic studies based on their medium expression level of miR-146a and EGFR. In order to recapitulate the role of miR-146a in in vivo studies, a xenograft mouse model was also chosen for our study and our results are presented below.
Figure 3.
CDF treatment significantly up-regulated miR-146a expression (A), and decreased the expression of EGFR (B) as assessed by qRT-PCR of AsPC-1, BxPC-3, MIAPaCa-2 and MIAPaCa-2-GR cells. The miR-146a expression was normalized using RNU48 miRNA and EGFR expression was normalized using GAPDH mRNA. UNT: untreated. P values, relative to individual controls, are mentioned over the bars. The relative values of the controls for each cell lines were normalized to 1.0 (unit value).
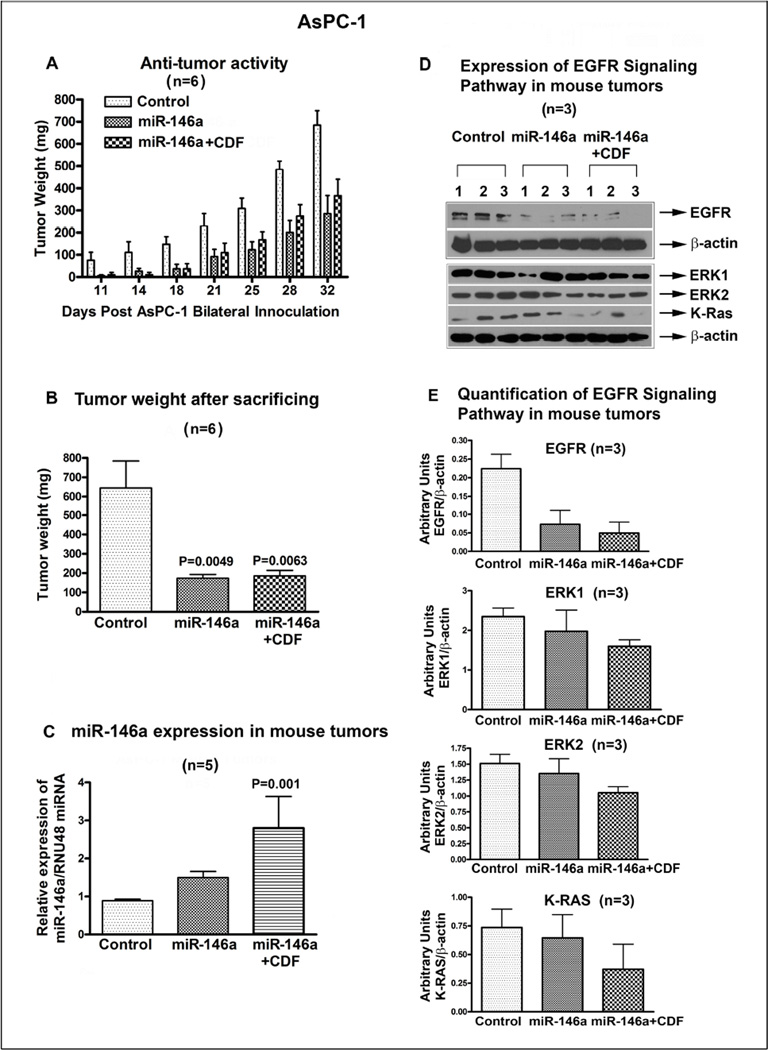
3.4. Over-expression of miR-146a inhibited tumor growth in a xenograft mouse model
In order to examine the in vivo effect of miR-146a in the development of tumor in xenograft mouse model, we stably transfected AsPC-1 cells with Pre-miR-146a prior to inoculation in mouse model. Prior to injection, we confirmed the over-expression of miR-146a by RT-PCR and subsequently we injected two and half million AsPC-1 cells (control vector transfected and Pre-miR-146a transfected cells) bilaterally in each mouse for a total of six/group. Mice were monitored on a daily basis and after the onset of tumor, the miR-146a transfected group was divided into two sub-groups one for treatment with 10 mg/mouse/day of CDF and the other without treatment. Tumor measurements were recorded twice a week (Figure 4A). The two groups with over-expression of miR-146a, but not the control group exhibited significantly reduced tumor growth; however, the inhibition of tumor growth after CDF treatment remained same as untreated Pre-miR-146a group (Figure 4B). This could be due to significant inhibition of tumor growth which had already occurred because of over-expression of miR-146a in both the groups. This data suggests that forced-expression of miR-146a led to decreased tumor weight, which could lead to increased survival. Hence targeting miR-146a in patient may serve as a better treatment option for PC.
Figure 4.
Over-expression of miR-146a using stably transfected Pre-miR-146a followed by CDF treatment in AsPC-1 cells in vivo showed decrease in tumor growth rate (A). Tumor weight was significantly reduced in pre-146a and pre-146a+CDF, compared to control, in a xenograft model (B). This was consistent with re-expression of miR-146a in miR-146a and miR-146a+CDF group (C), which was associated with decreased EGFR, ERK1, ERK2, and K-Ras expression, compared to control (D). The relative expression of EGFR, ERK1, ERK2, and K-ras proteins were quantified against β-actin (E). P values relative to controls are mentioned over the bars
3.5. Over-expression of miR-146a in vivo inhibited EGFR signaling pathways and retained miR-146a expression in tumor remnants
The expression of miR-146a in tumor remnants (n=5) was measured in all three groups as discussed above by qRT-PCR. Examination of miR-146a expression in tumor remnants from Pre-miR-146a group showed induction of miR-146a expression, which was further increased in CDF treated group as presented in Figure 4C. Although the tumor weight between untreated and CDF treated in 146a group was similar, the expression of miR-146a was enhanced in CDF treated group, suggesting that CDF can up-regulate miR-146a expression in vitro as well as in vivo. In contrast, the expression of EGFR, ERK1, ERK2, and K-Ras was reduced in both groups compared to control (n=3). Similarly, the reduction was enhanced in CDF treated group, signifying the inverse relationship between EGFR and miR-146a expression (Figure 4D). Though all the above mentioned proteins showed reduction in both the groups compared to control, the effect was more prominent with EGFR as shown in Figure 4E which represents average of the three animals per group. Taken together, the above data suggests that indeed overexpression of miR-146a both in vitro and in vivo could significantly reduce tumor burden with concomitant reduction in EGFR expression. To further investigate the involvement of miR-146a in cellular transformation and migration, we studied cellular behavior by the scratch assay and clonogenic assay, and also assessed EGFR expression in AsPC-1 cells transfected with antisense miR-146a as presented in the following section.
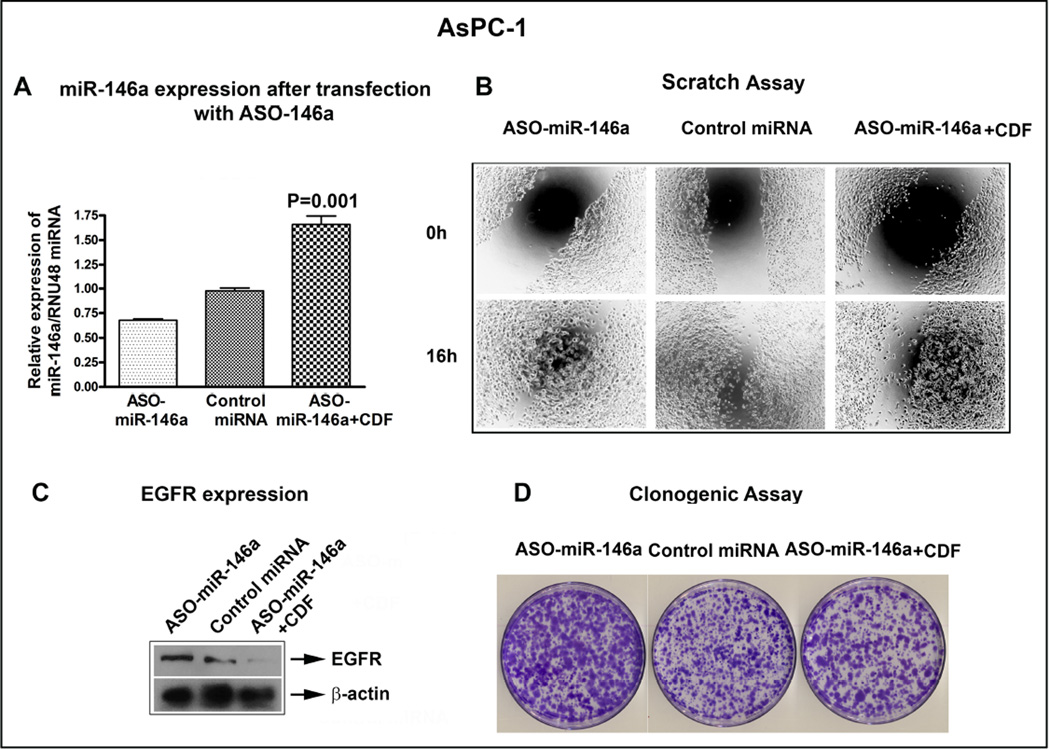
3.6. Transfection of anti-sense miR-146a in AsPC-1 cells increased EGFR expression, cell migration and colony formation
The effect of miR-146a ASO transfection on the expression of EGFR was studied in AsPC-1 cells. The cells were transfected with anti-sense miR-146a (inhibitor) followed by 0.5 µM CDF treatment for 48h. The transfected cells were used for scratch, clonogenic and for western blot assay. The transfection of ASO-miR-146a was confirmed by qRT-PCR showing reduced expression of miR-146a, which was partially rescued by CDF treatment (64% recovery) as shown in Figure 5A. Using scratch assay, we found that lowering the expression of miR-146a resulted in increased cell migration compared to scrambled control inhibitor (control) transfected cells, which again was partially rescued by CDF treatment (Figure 5B). Likewise, suppression of miR-146a resulted in over-expression of EGFR, which was reversed by CDF treatment (Figure 5C). Similarly, effect on the number of colonies formed was increased in ASO-miR-146a transfected cells which was reversed by CDF treatment (Figure 5D). These results suggest that miR-146a is a tumor suppressor in our system and that further lowering the expression of miR-146a led to increased cell migration, colony formation, and EGFR expression, and these effects were reversed by CDF treatment.
Figure 5.
Inactivation of miR-146a expression by ASO, led to reduced expression of miR-146a as assessed by qRT-PCR (A), increased cell migration by scratch assay (B), increased EGFR expression by western blot analysis (C) and increased in colony formation by clonogenic assay (D). These changes were overcome by CDF treatment. RNU48 miRNA was used to normalize miR-146a expression. Beta-actin was used as loading control for the western blot.
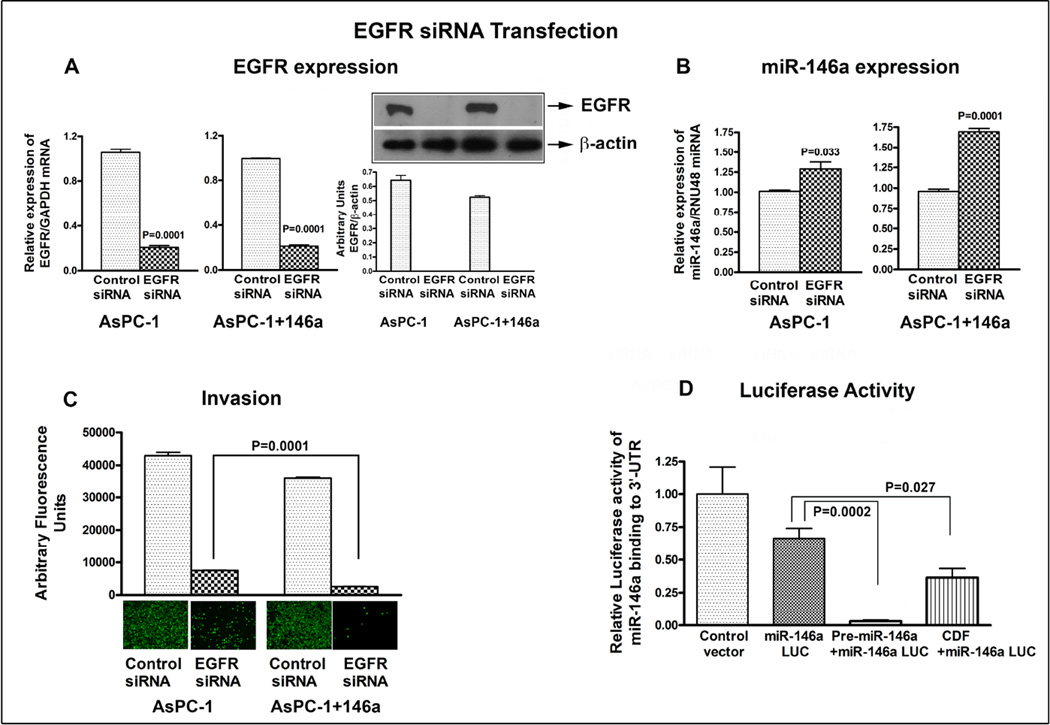
3.7. EGFR expression is regulated by miR-146a and affects cell migration
To further confirm the regulation of EGFR by miR-146a, we first transfected the AsPC-1 parental cells with Pre-miR-146a for 72h, later we transfected the parental AsPC-1 and AsPC-1+miR-146a cells with EGFR siRNA for 72h. The western blot analysis and qRT-PCR confirmed significant inhibition of EGFR by siRNA approach both at the protein and mRNA levels in AsPC-1 and as well as AsPC-1+miR-146a cells as shown in Figure.6A. The control siRNA treated cells in both AsPC-1 and AsPC-1+miR-146a cells were normalized to 1.0 (unit value). This similarity in EGFR inhibition in both cells could be due to significant inhibition of EGFR which had already occurred in AsPC-1 parental cells. The expression of miR-146a in AsPC-1+miR-146a cells treated with control siRNA showed 30% increase when compared to parental AsPC-1 cells (data not shown). In addition, the increase in expression of miR-146a in AsPC-1+miR-146a cells was much more prominent than AsPc-1 parental cells (Figure 6B); indicating down-regulation of EGFR cooperates with the gain of miR-146a expression. The control siRNA treated cells in both AsPC-1 and AsPC-1+miR-146a cells were normalized to 1.0 (unit value). This observation was further confirmed by migration assay, which showed significant reduction in migration of invasive cells upon inhibition of EGFR expression (Figure 6C). Once more, this inhibition was more prominent in AsPC-1+miR-146a cells compared to AsPC-1 cells, suggesting that EGFR not only controls the miR-146a expression but also controls the invasive behavior of cancer cells.
Figure 6.
Inactivation of EGFR expression by siRNA in AsPC-1 and AsPC-1 cells transfected with miR-146a. Decrease in EGFR expression was confirmed both at the mRNA and protein levels, respectively (A). Re-expression of miR-146a in AsPC-1+miR-146a was significantly more than AsPC-1 cells by qRT-PCR (B), inhibition of invasive cells in AsPC-1+miR-146a was more than AsPC-1 cells as assessed by invasion assay (C), and it was associated with decrease in luciferase activity observed in miR-146a luciferase vector compared to control vector as assessed by luciferase gene assay in AsPC-1 cells (D). This effect was further enhanced in cells transfected with premiR-146a and with CDF treatment. RNU48 miRNA was used to normalize miR-146a expression, and GAPDH mRNA for EGFR expression. The relative expression of EGFR and miR-146a by qRT-PCR in control siRNA treated cells were normalized to 1.0 (unit value) and were compared with their respective EGFR siRNA treated cells.
3.8. Luciferase activity was inhibited by Pre-miR-146a transfection and in cells treated with CDF
The luciferase activity was measured by luciferase assay reagent (Promega) after 48h of transfection with pMIR-Luc reporter vector containing perfect binding site for miR-146a. The AsPC-1 cells were transfected with pre-miR-146a or treated with CDF to examine the effect of over-expression of miR-146a or the treatment effect of CDF on miR-146a binding activity using miR-146a-mediated luciferase gene assay. We observed a significant decrease in luciferase activity in AsPC-1 cells transfected with miR-146a luciferase vector compared to control vector because of the endogenous level of miR-146a in AsPC-1 cells as presented in Figure 6D. Moreover, AsPC-1 cells co-transfected with pre-miR-146a significantly decreased the luciferase activity compared to the un-transfected cells, suggesting that the transfection of cells with pre-miR-146a increased the binding activity which led to a decrease in luciferase activity. These effects were also similar with CDF treatment as displayed in Figure 6D, suggesting that re-expression of miR-146a either by transfection or by treatment with our novel agent CDF can increase the binding activity that can lead to a decrease in luciferase activity. Hence CDF could be useful for designing novel therapies.
4. Discussion
The role of EGFR has been documented in a large number of epithelial tumors, including PC. The EGFR belongs to the erbB family of tyrosine kinase receptors, and it is involved in tumor growth, metastasis and disease recurrence [25, 26]. We have previously reported deregulated expression of miRNAs in PC compared to control subjects from plasma and FNA samples [2, 3]. In this study, we found decreased level of expression of miR-146a in tumors of PC patients compared to non-tumor specimens raising the possibility that the expression of miR-146a could be useful as a diagnostic marker for PC. Reduced expression of miR-146a was correlated with over-expression of EGFR, which is consistent with recent publications both in PC and in other cancers [6, 12, 14]. Based on our experimental evidence and recent publications, we found that the predicted target of miR-146a is EGFR among others, suggesting that EGFR could be an important target to inhibit by re-expression of miR-146a by novel approach as documented by our data on CDF.
EGFR is over-expressed in PC tumors compared to normal [27] and plays important roles in the progression of PC. The over-expression of EGFR is generally correlates with poor survival outcomes [28–30]. This is also in part due to loss of epithelial morphology with cancer progression and acquisition of epithelial-to-mesenchymal (EMT) transition phenotype which in turn activates the receptor tyrosine kinase signaling pathways [28]. These acquired EMT characteristics in tumor cells have been shown to be less sensitive to EGFR inhibitors such as erlotinib and gefitinib in many cancers, including PC [28]. Although EGFR inhibitors have provided significant clinical benefits, not all patients benefit from them due to acquired resistance especially because of the acquisition of EMT phenotype. Hence, identifying and implementing new targeted therapies to inhibit not only the EMT phenotype in cells but also inhibition of subsequent metastatic cascade may benefit cancer treatment outcome and will likely improve overall patient survival. Initially we started with 8 PC cell lines to measure the basal level of expression of miR-146a and EGFR. In particular, we found elevated levels of EGFR expression in most of the PC cell lines tested and in transgenic mouse tumors with concomitant reduced levels of expression of miR-146a, and this was consistent with our previous findings [6]. Based on their expression level of both miR-146a and EGFR, we subsequently chose 4 cell lines to study the effect of CDF on miR-146a and EGFR expression. Finally, we chose AsPC-1 for further mechanistic studies by transfecting miR-146a precursor in to AsPC-1 cells for in vivo studies and miR-146a inhibitor in AsPC-1 cells for in vitro studies. Nonetheless, to the best of our knowledge this is the first study to demonstrate the involvement of miR-146a and its modulation on lowering tumor burden with substantially decreased EGFR expression and the expression of its downstream genes in xenograft mouse model of PC.
As of yet, research on miRNAs in PC have focused on diagnostic tools, identification of tumor specific markers and regulators of oncogenes/tumor suppressor genes, and in predicting clinical outcomes. To understand the mechanistic role of miRNAs involved in tumor growth and metastasis, we studied the deregulation of miR-146a either by over-expression in vivo by precursors or knock-down by inhibitors and also by treatment with CDF in vitro. In the current study, treatment of cells with lower concentration of CDF (200 nM) significantly up-regulated miR-146a in all the PC cells tested. This was directly correlated with down-regulation of EGFR, suggesting the inter-relationship between miR-146a and EGFR expression, and further demonstrating that CDF could be a useful novel agent for inactivation of EGFR toward the treatment of PC especially because CDF showed no systemic toxicity in the mouse model [16].
Besides cancer, alterations in miR-146a expression have been observed in other human diseases such as inflammatory disease and innate immune response [31]. Prior studies have revealed that miR-146a performs as either an oncogene or a tumor suppressor in various cancers, [32] but there are scanty literature reporting the tumor suppressive nature of miR-146a in prostate, lung and PC [6, 12, 14]. The lower expression of miR-146a was associated with increased cell growth, colony formation and migration, suggesting that miR-146a may play a vital role in a number of key aspects of cancer development and regression [6, 12]. Yao et al reported inverse correlation of miR-146a with WASF2 protein expression, and over-expression of miR-146a inhibited migration, invasion and protein level of WASF2 in gastric cancer cell lines [32]. Similarly, lower expression of miR-146a was observed in about 84% of gastric cancer tissue samples and it was correlated with increased tumor size, poor differentiation and overall survival, suggesting the tumorigenic role of miR-146a in gastric cancer [33]. Another recent report suggested that p53-binding protein-1 inhibits cell growth partially via the suppression of NF-κB through miR-146a in both in vitro and in vivo in breast cancer [34]. In our current study, further knock-down of miR-146a with ASO-miR-146a augmented migration/invasion and colony formation and further up-regulated the expression of EGFR. Moreover, this effect was substantially overcome by CDF treatment, suggesting that CDF can aid in the up-regulation of miR-146a expression with simultaneous decrease in the expression of EGFR.
EGFR expression has been found to be detectable in more than 95% of patients with PC and its signaling has been attributed with the development of both normal epithelial cells and in tumor cell proliferation and metastasis [35, 36]. Therefore, inhibiting EGFR expression may improve treatment outcome and overall survival of PC patients. A recent report demonstrated that EGFR RNAi significantly improved cyclopamine sensitivity and decreased AKT and ERK phosphorylation in PC cells [37]. It also decreased migration and invasion capacity of cells, and also decreased the expression of mesenchymal markers such as vimentin and fibronectin, and increased the expression of epithelial marker E-cadherin, suggesting the reversal of EMT by suppression of EGFR expression in PANC-1 cells [38]. To further confirm the inter-relationship between EGFR and miR-146a, we used EGFR siRNA to knock-down EGFR expression and studied the consequence on EGFR expression and cellular behavior. Indeed, inhibition of EGFR both at the protein and mRNA level in AsPC-1 parental and AsPC-1 cells stably transfected with miR-146a, significantly inhibited invasion and up-regulated miR-146a expression. In summary, our present results suggest that increased expression of EGFR is in part due to loss of expression of miR-146a in PC. Furthermore, we have delivered experimental proof showing that targeted reexpression of miR-146a by CDF in vitro and in vivo results in decreased tumor burden and with concomitant decreased expression of EGFR. Hence, inhibition of EGFR through up-regulation of miR-146a either by synthetic precursors or by CDF treatment could serve as a novel approach for the treatment of PC especially in patients who becomes refractory to EGFR tyrosine kinase inhibotor such as erlotinib.
Acknowledgements
This work was partly funded by grants from the National Cancer Institute, NIH 5R01CA131151, 3R01CA131151-02S109, and 1R01CA132794 awarded to FHS. We also acknowledge the generous funding of Puschelberg foundation for funding initial experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
All the authors declare no competing conflict of interest.
Reference List
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Almhanna K, Chen W, Philip PA, Sarkar FH. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am. J. Transl. Res. 2010;3:28–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Ali S, Saleh H, Sethi S, Sarkar FH, Philip PA. MicroRNA profiling of diagnostic needle aspirates from patients with pancreatic cancer. Br. J. Cancer. 2012;107:1354–1360. doi: 10.1038/bjc.2012.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali S, Banerjee S, Logna F, Bao B, Philip PA, Korc M, Sarkar FH. Inactivation of Ink4a/Arf leads to deregulated expression of miRNAs in K-Ras transgenic mouse model of pancreatic cancer. J. Cell Physiol. 2012;227:3373–3380. doi: 10.1002/jcp.24036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, Sarkar FH. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Vandenboom TG, Wang Z, Kong D, Ali S, Philip PA, Sarkar FH. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei J, Bachoo R, Zhang CL. MicroRNA-146a inhibits glioma development by targeting Notch1. Mol. Cell Biol. 2011;31:3584–3592. doi: 10.1128/MCB.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik JH, Jang JY, Jeon YK, Kim WY, Kim TM, Heo DS, Kim CW. MicroRNA-146a downregulates NFkappaB activity via targeting TRAF6 and functions as a tumor suppressor having strong prognostic implications in NK/T cell lymphoma. Clin. Cancer Res. 2011;17:4761–4771. doi: 10.1158/1078-0432.CCR-11-0494. [DOI] [PubMed] [Google Scholar]
- 9.Chassin C, Hempel C, Stockinger S, Dupont A, Kubler JF, Wedemeyer J, Vandewalle A, Hornef MW. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol. Med. 2012;4:1308–1319. doi: 10.1002/emmm.201201298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Chapelle A, Jazdzewski K. MicroRNAs in thyroid cancer. J. Clin. Endocrinol. Metab. 2011;96:3326–3336. doi: 10.1210/jc.2011-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia AI, Buisson M, Bertrand P, Rimokh R, Rouleau E, Lopez BS, Lidereau R, Mikaelian I, Mazoyer S. Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol. Med. 2011;3:279–290. doi: 10.1002/emmm.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Umelo IA, Lv S, Teugels E, Fostier K, Kronenberger P, Dewaele A, Sadones J, Geers C, De GJ. miR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS. One. 2013;8:e60317. doi: 10.1371/journal.pone.0060317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, Du Y, Wang Y, Chang WC, Chen CH, Hsu JL, Wu Y, Lam YC, James BP, Liu X, Liu CG, Patel DJ, Hung MC. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature. 2013;497:383–387. doi: 10.1038/nature12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Wang N, Wang X, Tong N, Shao N, Tao J, Li P, Niu X, Feng N, Zhang L, Hua L, Wang Z, Chen M. MiR-146a suppresses tumor growth and progression by targeting EGFR pathway and in a p-ERK-dependent manner in castration-resistant prostate cancer. Prostate. 2012;72:1171–1178. doi: 10.1002/pros.22466. [DOI] [PubMed] [Google Scholar]
- 15.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–3617. doi: 10.1158/0008-5472.CAN-09-4598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Bao B, Ali S, Kong D, Sarkar SH, Wang Z, Banerjee S, Aboukameel A, Padhye S, Philip PA, Sarkar FH. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS. One. 2011;6:e17850. doi: 10.1371/journal.pone.0017850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Padhye S, Yang H, Jamadar A, Cui QC, Chavan D, Dominiak K, McKinney J, Banerjee S, Dou QP, Sarkar FH. New difluoro Knoevenagel condensates of curcumin, their Schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm. Res. 2009;26:1874–1880. doi: 10.1007/s11095-009-9900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, Li J, Dou QP, Sarkar FH. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm. Res. 2009;26:2438–2445. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carriere C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem. Biophys. Res Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali S, El-Rayes BF, Sarkar FH, Philip PA. Simultaneous targeting of the epidermal growth factor receptor and cyclooxygenase-2 pathways for pancreatic cancer therapy. Mol. Cancer Ther. 2005;4:1943–1951. doi: 10.1158/1535-7163.MCT-05-0065. [DOI] [PubMed] [Google Scholar]
- 22.Ali S, Ahmad A, Aboukameel A, Bao B, Padhye S, Philip PA, Sarkar FH. Increased Ras GTPase activity is regulated by miRNAs that can be attenuated by CDF treatment in pancreatic cancer cells. Cancer Lett. 2012;319:173–181. doi: 10.1016/j.canlet.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Soubani O, Ali AS, Logna F, Ali S, Philip PA, Sarkar FH. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis. 2012;33:1563–1571. doi: 10.1093/carcin/bgs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad A, Sarkar SH, Aboukameel A, Ali S, Biersack B, Seibt S, Li Y, Bao B, Kong D, Banerjee S, Schobert R, Padhye SB, Sarkar FH. Anticancer action of garcinol in vitro and in vivo is in part mediated through inhibition of STAT-3 signaling. Carcinogenesis. 2012;33:2450–2456. doi: 10.1093/carcin/bgs290. [DOI] [PubMed] [Google Scholar]
- 25.Pryczynicz A, Guzinska-Ustymowicz K, Czyzewska J, Kemona A. Expression of epidermal growth factors and apoptosis markers in pancreatic ductal adenocarcinoma. Folia Histochem. Cytobiol. 2009;47:667–671. doi: 10.2478/v10042-010-0008-0. [DOI] [PubMed] [Google Scholar]
- 26.Weiss GA, Rossi MR, Khushalani NI, Lo K, Gibbs JF, Bharthuar A, Cowell JK, Iyer R. Evaluation of phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) and epidermal growth factor receptor (EGFR) gene mutations in pancreaticobiliary adenocarcinoma. J. Gastrointest. Oncol. 2013;4:20–29. doi: 10.3978/j.issn.2078-6891.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muslimov GF. Role of epidermal growth factor gene in the development of pancreatic cancer and efficiency of inhibitors of this gene in the treatment of pancreatic carcinoma. Bull. Exp. Biol. Med. 2008;145:535–538. doi: 10.1007/s10517-008-0135-1. [DOI] [PubMed] [Google Scholar]
- 28.Barr S, Thomson S, Buck E, Russo S, Petti F, Sujka-Kwok I, Eyzaguirre A, Rosenfeld-Franklin M, Gibson NW, Miglarese M, Epstein D, Iwata KK, Haley JD. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin. Exp. Metastasis. 2008;25:685–693. doi: 10.1007/s10585-007-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyabayashi K, Ijichi H, Mohri D, Tada M, Yamamoto K, Asaoka Y, Ikenoue T, Tateishi K, Nakai Y, Isayama H, Morishita Y, Omata M, Moses HL, Koike K. Erlotinib prolongs survival in pancreatic cancer by blocking gemcitabine-induced MAPK signals. Cancer Res. 2013;73:2221–2234. doi: 10.1158/0008-5472.CAN-12-1453. [DOI] [PubMed] [Google Scholar]
- 30.Walters DM, Lindberg JM, Adair SJ, Newhook TE, Cowan CR, Stokes JB, Borgman CA, Stelow EB, Lowrey BT, Chopivsky ME, Gilmer TM, Parsons JT, Bauer TW. Inhibition of the growth of patient-derived pancreatic cancer xenografts with the MEK inhibitor trametinib is augmented by combined treatment with the epidermal growth factor receptor/HER2 inhibitor lapatinib. Neoplasia. 2013;15:143–155. doi: 10.1593/neo.121712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Chen XP, Li YJ. MicroRNA-146a and human disease. Scand. J. Immunol. 2010;71:227–231. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 32.Yao Q, Cao Z, Tu C, Zhao Y, Liu H, Zhang S. MicroRNA-146a acts as a metastasis suppressor in gastric cancer by targeting WASF2. Cancer Lett. 2013;335:219–224. doi: 10.1016/j.canlet.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Hou Z, Xie L, Yu L, Qian X, Liu B. MicroRNA-146a is down-regulated in gastric cancer and regulates cell proliferation and apoptosis. Med. Oncol. 2012;29:886–892. doi: 10.1007/s12032-011-9862-7. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Xu B, Moran MS, Zhao Y, Su P, Haffty BG, Shao C, Yang Q. 53BP1 functions as a tumor suppressor in breast cancer via the inhibition of NF-kappaB through miR-146a. Carcinogenesis. 2012;33:2593–2600. doi: 10.1093/carcin/bgs298. [DOI] [PubMed] [Google Scholar]
- 35.Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig. Surg. 2006;23:74–79. doi: 10.1159/000093497. [DOI] [PubMed] [Google Scholar]
- 36.Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J. Clin. Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 37.Qin CF, Hao K, Tian XD, Xie XH, Yang YM. Combined effects of EGFR and Hedgehog signaling pathway inhibition on the proliferation and apoptosis of pancreatic cancer cells. Oncol. Rep. 2012;28:519–526. doi: 10.3892/or.2012.1808. [DOI] [PubMed] [Google Scholar]
- 38.Chang ZG, Wei JM, Qin CF, Hao K, Tian XD, Xie K, Xie XH, Yang YM. Suppression of the epidermal growth factor receptor inhibits epithelial-mesenchymal transition in human pancreatic cancer PANC-1 cells. Dig. Dis. Sci. 2012;57:1181–1189. doi: 10.1007/s10620-012-2036-4. [DOI] [PubMed] [Google Scholar]