Summary
Four phytoene synthase genes and several variants were characterized, and their evolution and function in differential carotenoid accumulation in leaf, peel, and flesh of white- and red-fleshed loquats were established.
Key words: Carotenoid, function, loquat (Eriobotrya japonica), mutation, phytoene synthase, tissue-specific expression.
Abstract
Differences in carotenoid accumulation between tissues and cultivars is common in plants. White-fleshed loquat cultivars had low levels of carotenoids in the flesh, but accumulated carotenoids in peel when ripe, and the leaves accumulated similar carotenoids to those in the red-fleshed loquat cultivars. The catalytic activity and expression patterns of four phytoene synthase (PSY) genes, EjPSY1, EjPSY2A, EjPSY2B, and EjPSY3, were analysed to understand their roles in different loquat (Eriobotrya japonica Lindl.) types. EjPSY1 was responsible for carotenoid synthesis in the fruit peel but not the flesh, whereas EjPSY2A was responsible for carotenoid accumulation in flesh of ripening fruit. A mutant EjPSY2A d, with the same tissue specificity and expression level as EjPSY2A, but lacking the C-terminal region and corresponding catalytic activity, was discovered in white-fleshed varieties, explaining the lack of carotenoids in the white flesh. The catalytic role of EjPSY2B was most significant in leaves. The tissue-specific expression of EjPSY1 and EjPSY2B explained well how peel and leaf tissues can still accumulate carotenoids in white-fleshed cultivars, which have lost the functional EjPSY2A. EjPSY3 mRNA abundance was ~1000-fold less than that of other PSY mRNAs in all tissues examined. In addition, neither the normal sized transcript nor two alternatively spliced forms, EjPSY3α in LYQ and EjPSY3β in BS cultivars, encoded functional enzymes, and it is concluded that EjPSY3 plays no role in carotenoid accumulation. In addition, it was noted that recruitment of PSY genes for expression in specific tissues of different plants has occurred independently of gene structure and evolutionary origin.
Introduction
In addition to being important accessory pigments in photosynthesis (Demmig-Adams and Adams, 1992, 2002; Niyogi, 1999), carotenoids also form the basis of many flower and fruit colours that attract animals and facilitate pollination and seed dispersal (Howitt and Pogson, 2006). Dietary carotenoids are important for human health, some as essential precursors to vitamin A and others as antioxidants and anticarcinogenic agents, as well as having cardiovascular and eye disease-preventing bioactives (Krinsky et al., 2003; Clagett-Dame and Knutson, 2011; Abdel-Aal et al., 2013).
Carotenoids are synthesized by all photosynthetic organisms, mainly plants, and some non-photosynthetic bacteria, as well as certain fungi and a few species of animals (Moran and Jarvik, 2010). In view of their important roles, carotenoid biosynthesis in higher plants has been widely studied and the pathway has been elucidated in the past two decades (Hirschberg, 2001; Fraser and Bramley, 2004; DellaPenna and Pogson, 2006; Chen and Wurtzel, 2010; Ma et al., 2013; Shumskaya and Wurtzel, 2013).
Diverse mechanisms have been proposed to be responsible for the different carotenoid patterns among cultivars. In some cases, these can be explained by sequence mutation of the gene participating in the carotenoid biosynthetic pathway, either inside the open reading frames (ORFs), resulting in an increase or decrease, or even a loss of activity of the encoding enzyme, or in the promoter region, resulting in changes in the level of expression. For example, a null mutation in the B gene in old-gold (og) tomato causes the fruit to accumulate a higher ratio of lycopene to β-carotene, while mutations in the promoter of the B gene increased the level of expression and resulted in a lower ratio of lycopene to β-carotene in Beta tomato (Ronen et al., 2000). Similarly, four naturally occurring lcyE polymorphisms explained the variation in α-carotene and β-carotene biosynthetic branches and the resulting differences in provitamin A compounds in maize (Harjes et al., 2008); the carotenoid cleavage dioxygenase 4 (PpCCD4) alleles of yellow peach and white peach have arisen from different ancestral haplotypes by at least three independent mutational events (Falchi et al., 2013). Progress in plastid biology has provided other insights into mechanisms affecting carotenoid content. Carotenoid accumulation has been shown in some cases to be related to plastid biogenesis and interconversion (Egea et al., 2010), as in the high β-carotene content in the orange curd of a cauliflower mutant, which was shown to be due to the differentiation of proplastids and other non-coloured plastids into chromoplasts caused by the Or gene (Paolillo et al., 2004; Lu et al., 2006). Similarly, Kilcrease et al. (2013) demonstrated a linkage between chromoplast architecture and carotenoid composition in diverse Capsicum annuum fruit by using multiple microscopic approaches.
A sequence mutation or alteration in the mRNA level of phytoene synthase (PSY), the first committed step in the carotenoid pathway, could strongly affect plant carotenoid accumulation. Truncation of PSY1 was shown to lead to lack of carotenoid accumulation in tomato fruit (Fray and Grierson, 1993), and overexpression of PSY1 in tomato resulted in changes in pigmentation and plastid type (Fraser et al., 2007). In addition, numerous reports showed that PSY can have several family members, each, to some extent, with its own tissue-specific expression. As an example, the second phytoene synthase, PSY2, in tomato was shown to be predominantly responsible for carotenoid formation in chloroplast-containing tissues (Fraser et al., 1999). Recently, a third member of the PSY family, PSY3, was discovered from genome sequencing, namely SlPSY3 (Kachanovsky et al., 2012) and CitPSY3 (Peng et al., 2013). These third members showed fewer transcripts among all the tissues studied and their roles in carotenoid biosynthesis are largely unknown. In general, there has not been a great deal of research on the different PSY family member genes in dicot plants, probably because the model plant Arabidopsis only has one PSY gene.
Some fruits of dicot plants, such as loquat (Zhou et al., 2007; Fu et al., 2012), can accumulate carotenoids in both peel and flesh, but with considerable variation between cultivars. The white-fleshed loquat cultivars have few carotenoids in the flesh and appear ivory, but the peel is a yellow colour when ripe. In a previous study (Fu et al., 2012), it was discovered that the differential expression of carotenogenic genes was insufficient to explain the large difference in carotenoid content between the red- and white-fleshed cultivars, indicating that there may be another regulatory mechanism underlying this phenomenon. In the present study, a truncated and non-functional mutant, EjPSY2A d, was identified in the genome of white-fleshed loquat cultivars and shown to explain the low levels of carotenoids accumulated in the flesh of white-fleshed loquat. The tissue-specific expression patterns of EjPSY1 and EjPSY2B explained well how peel and leaf tissues can still accumulate carotenoids in white-fleshed cultivars, which have lost the functional EjPSY2A expressed in fruit flesh. It was speculated that EjPSY3 is functionless in loquat. In addition, it was observed that PSY expression patterns in dicot plants are independent of gene structure and evolution.
Materials and methods
Plant materials
Luoyangqing (LYQ, red-fleshed) and Baisha (BS, white-fleshed) loquats (Eriobotrya japonica Lindl.) were sampled from an orchard in Luqiao, Zhejiang, China. For analysis of different plant tissues and fruit at different developmental stages, young leaves, mature leaves, roots, stems, and petals at anthesis were collected from each cultivar. Fruit of six developmental stages, S1, fruitlet, 45 days after full bloom (DAFB); S2, immature green, 75 DAFB; S3, mature green, 95 DAFB; S4, breaker, 105 DAFB; S5, half ripe, 110 DAFB; and S6, ripe, 115 DAFB, were collected. Mature fruits and leaves of other red-fleshed (Jiajiao, Baozhu, Dameiguihongpao, Dayeyangdun, and Dawuxing) and white-fleshed (Tianzhong, Bingtangzhong, Ninghaibai, Guanyu, Baiyu, and Biqi) cultivars were picked from the Fruit Technology Extension and Service Center in Taihu, Jiangsu, China. All tissue samples were immediately frozen in liquid nitrogen and stored at –80 °C until further use. Each sample, except for that for genomic DNA extraction, consisted of three biological replicates.
Carotenoid extraction, quantification, and HPLC analysis
Carotenoids were extracted from tissues and analysed by high-performance liquid chromatography (HPLC), according to a method previously described by Xu et al. (2006).
Searching for and cloning the PSY gene family members in loquat
RNA-Seq libraries of LYQ and BS fruits were constructed (unpublished data). Nine PSY unigenes in the LYQ library and two PSY unigenes in the BS library (Supplementary Fig. S1 available at JXB online) were obtained by either searching the libraries with the name of the gene or BLASTing with the homologous sequences from the model plants Arabidopsis, rice, or tomato. Further sequence alignment analysis was carried out by the BLAST program online (http://www.ncbi.nlm.nih.gov/BLAST) and Clustal X (1.81), and these 11 unigene sequences were assembled into three PSY unigenes in LYQ and one PSY unigene in BS. The genomic database of Rosaceae species (http://www.phytozome.net/) of apple, strawberry, and peach was also used to search the PSY sequences, and degenerate primers (forward primer, 5′-CTTCCAAATGTGTTCTACAATTTC-3′; reverse primer, 5′-TGTTTTTATTATTGGGACATCAA-3′) were designed based on sequences corresponding to a highly conserved peptide in order to clone EjPSY3 from loquat.
Isolation of RNA
Total RNA was extracted from frozen powder following a previously published protocol (Fu et al., 2012). RNA integrity was electrophoretically verified with ethidium bromide staining and purity by checking that the A 260/A 280 absorption ratio was between 1.9 and 2.1. Potential contamination with DNA was eliminated by treatment with DNase I (RNase-free) (Fermentas MBI).
Full-length cDNA amplification and sequence analysis
Three PSY unigenes in LYQ and one PSY unigene in BS were amplified to obtain the full-length sequences using 5′RACE (rapid amplification of cDNA ends) and 3′RACE primers (Supplementary Table S1 at JXB online) using the SMART™ RACE cDNA amplification Kit (Clontech). Three EjPSY gene family members (EjPSY1, EjPSY2A, and EjPSY2B) were obtained from the LYQ library and one mutant EjPSY2A d was obtained from the BS library. The forward and reverse primers 5′-CTTCCAAATGTGTTCTACAATTTC-3′ and 5′-TGTTTTTATTATTGGGACATCAA-3′ were used to obtain the full-length EjPSY3 sequence. The cDNAs of the PSY genes were aligned and a phylogenetic tree constructed using MEGA (version 5.0), and the sequence alignment was used for a Neighbor–Joining (NJ) tree using default parameters in MEGA. Bootstrap analysis of the NJ tree was performed using 1000 replicates.
DNA extraction and EjPSY genomic sequence amplification
Genomic DNA was extracted from young leaves by the CTAB (cetyltrimethylammonium bromide) method as described by Huang et al. (2013). The genomic DNA sequences of EjPSY1, EjPSY2A, and EjPSY2B were cloned by genomic walking using gene-specific primers (Supplementary Table S2 at JXB online). The complete genomic sequences were obtained using primers (Supplementary Table S3 at JXB online). Another specific primer pair was designed to identify the genomic DNA sequence (Supplementary Table S4 at JXB online) of EjPSY2A and EjPSY2A d.
Real-time reverse transcription–PCR (RT–PCR)
A 3 μg aliquot of total RNA was reverse transcribed using the M-MuLV Reverse Transcriptase kit (Fermentas MBI) and oligo(dT) primers according to the manufacturer’s instructions. Gene-specific primers for EjPSY1, EjPSY2A, EjPSY2A d, EjPSY2B, EjPSY3, EjPSY3α and EjPSY3β were designed and tested for specificity (Supplementary Table S5 at JXB online). The real-time quantitative PCR was conducted in a Roche LightCycler thermocycling real-time PCR system. The expression level of actin was used to normalize the mRNA levels for each sample, with abundance expressed as a multiple of actin (Fu et al., 2012).
Plasmids and functional complementation
The pAC-85b plasmid contains all genes required for β-carotene production except for PSY and can be used for testing PSY activity (Cunningham and Gantt, 2007). The pAtPSY plasmid containing the PSY of Arabidopsis was used as a positive control in the experiment. When transformed together with pAC-85b and pAtPSY, Escherichia coli DH5α cells could accumulate the end-product β-carotene. The empty vector was constructed by a deletion of the EcoRΙ–EcoRΙ AtPSY fragment from this plasmid. pAC-85b was used for heterologous complementation to test the function of EjPSY1, EjPSY2A, EjPSY2A d, EjPSY2B, EjPSY3, EjPSY3α, and EjPSY3β. cDNAs were subcloned as in-frame translational fusions as follows: EjPSY1, EjPSY2A, EjPSY2A d, and EjPSY2B were amplified using primers (Supplementary Table S6 at JXB online) with a SalΙ site (bold) in the forward primers and an EcoRΙ site (bold) in the reverse primers; EjPSY3, EjPSY3α, and EjPSY3β were amplified using primers (Supplementary Table S6 at JXB online) with a BamHΙ site (bold) in the forward primer and a SalΙ site (bold) in the reverse primer. The above amplified cDNAs were subcloned into the corresponding sites of the digested pAtPSY plasmid, and renamed pEjPSY1, pEjPSY2A, pEjPSY2Ad, pEjPSY2B, pEjPSY3, pEjPSY3α, and pEjPSY3β, respectively.
Chemically competent pAC-85b-containing E. coli DH5α cells were prepared and transformed with pEjPSY1, pEjPSY2A, pEjPSY2Ad, pEjPSY2B, pEjPSY3, pEjPSY3α, and pEjPSY3β. A single colony was used to inoculate a 2ml Luria broth (LB) culture, supplemented with ampicillin (100 μg g–1) and chloramphenicol (50 μg g–1), grown at 37 °C and 180rpm. The overnight culture was then used to inoculate a 50ml LB culture containing the same antibiotics, which was maintained at 37 °C with shaking for 1 d, and cells were harvested by centrifugation at 5000 g for 15min. After photographing, total carotenoids were extracted from the bacterial cell pellets and analysed by HPLC by the method described above.
Results
The carotenoid accumulation patterns in the tissues of different red-fleshed and white-fleshed cultivars are grouped into two types
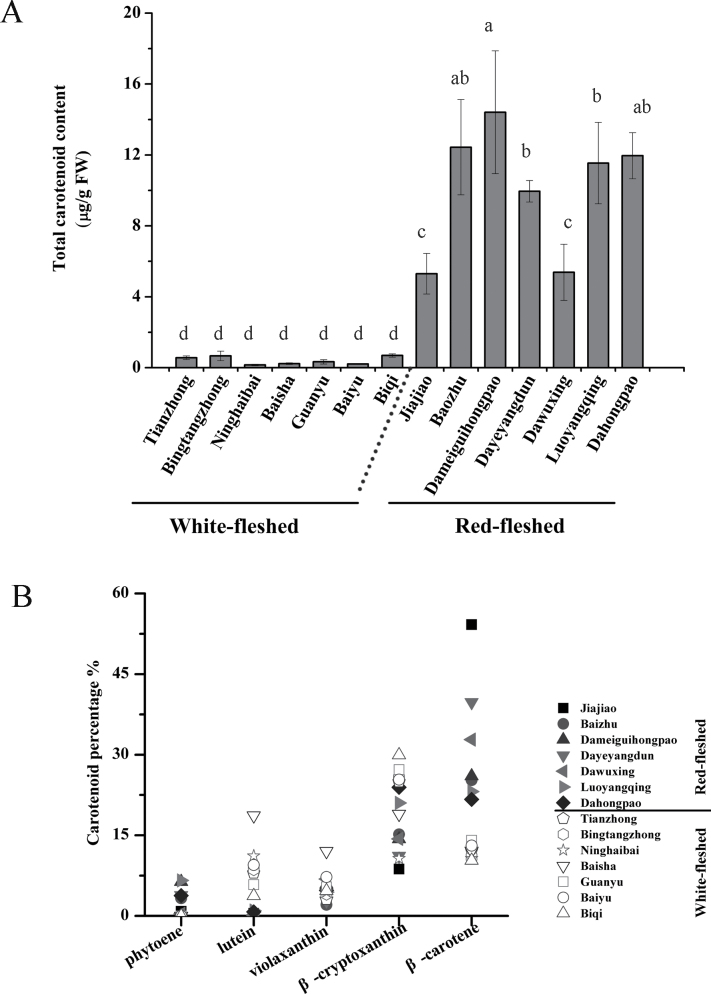
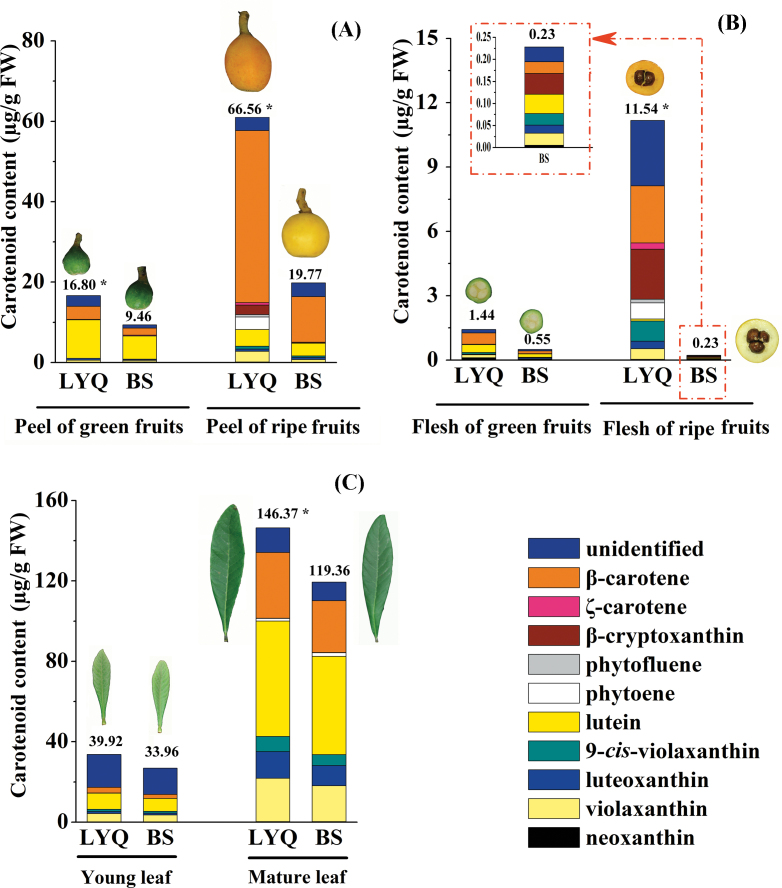
The flesh of red-fleshed cultivars appeared red-orange due to the accumulation of abundant carotenoids [5.30–14.41 μg g–1 fresh weight (FW)]. In contrast, far fewer carotenoids (0.16–0.69 μg g–1 FW) accumulated in the flesh of white-fleshed cultivars (Fig. 1A). Regarding individual carotenoids in flesh, phytoene was present only in red-fleshed cultivars, and the amounts of violaxanthin, β-cryptoxanthin, and β-carotene were much lower in white-fleshed cultivars (Supplementary Table S7 at JXB online). Although no significant difference in the amount of lutein in the flesh was observed between the two groups (Supplementary Table S7 at JXB online), the percentage of lutein in the total carotenoids in the flesh was much higher in white-fleshed cultivars (averaging 9.98%) than in red-fleshed cultivars (averaging 0.33%) (Figs 1B, 2B). In peel, the total amount of carotenoids in green LYQ was ~1.5-fold higher than in BS, but the carotenoid profiles were almost the same, and comprised mainly lutein (60% on average) and β-carotene (19% on average) (Fig. 2A). The peels of a further seven red-fleshed and seven white-fleshed loquat cultivars at ripe stages were also analysed, and contained on average 19.1 μg g–1 FW carotenoids in the white-fleshed cultivars, which was ~25% of the values in the peel of red-fleshed cultivars (Supplementary Fig. S2; Supplementary Table S7 at JXB online).
Fig. 1.
Analysis of the carotenoid content and composition in flesh of different red-fleshed and white-fleshed loquat cultivars. (A) Total carotenoids were extracted from the flesh of red-fleshed [Jiajiao, Baozhu, Dameiguihongpao, Dayeyangdun, Dawuxing, Luoyangqing (LYQ), and Dahongpao] and white-fleshed [Tianzhong, Bingtangzhong, Ninghaibai, Baisha (BS), Guanyu, and Biqi] cultivars and quantified by HPLC. Significance was set at a P-value <0.05. (B) The percentage composition of the main carotenoids (phytoene, lutein, violaxanthin, β-cryptoxanthin, and β-carotene) in the flesh of red- and white-fleshed loquat cultivars; the absolute amount are provided in Supplementary Table S7 at JXB online.
Fig. 2.
Carotenoid analysis in the tissues of LYQ and BS. (A) Carotenoid content and composition were compared in the peel of Luoyangqing (LYQ) and Baisha (BS) at the green and ripe stages. (B) Carotenoid content and composition were compared in the flesh of LYQ and BS at green and ripe stages. (C) Carotenoid content and composition were compared in young and mature leaves of LYQ and BS, respectively. The total carotenoid content is shown at the top of the column, with an asterisk indicating a significant difference, at a P-value <0.05, between the two cultivars. The absolute amount are provided in Supplementary Table S8 at JXB online.
The colour of LYQ and BS leaves appeared similar (Fig. 2). Although the carotenoid colour was masked by chlorophylls, there was no photobleaching of leaves, which might have been expected if the carotenoid content had been low. To determine whether the carotenoid accumulation patterns in BS leaf were affected, the content in young and mature leaves of LYQ and BS was analysed. This comparison showed that young and mature BS leaves contained an ~20% lower amount of carotenoids as compared with LYQ (Fig. 2C; Supplementary Table 8 at JXB online). The leaf carotenoid profiles, however, were very similar between LYQ and BS loquats.
Isolation of four PSY genes from loquat
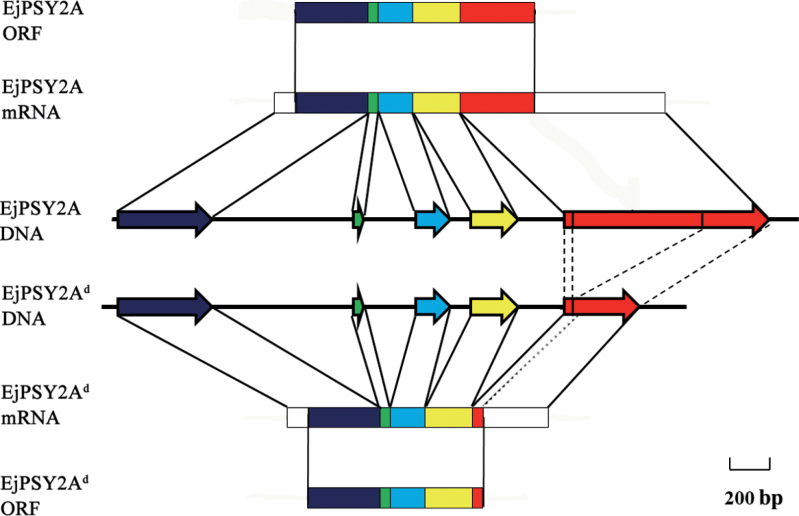
Three unigenes (Unigene13461, Unigene46522, and Unigene26970) were found in the LYQ library and one, Unigene 54535, was identified in the BS library. When the complete sequences of these unigenes were obtained by RACE, Unigene13461 was named EjPSY1; Unigene46522 was named EjPSY2A; Unigene26970, which had high homology with Unigene46522, was named EjPSY2B; and Unigene54535 in BS was 321 bases shorter than the full length of EjPSY2A and was designated EjPSY2A d. Upon sequencing, the cDNA of EjPSY2A d was found to be identical to that of EjPSY2A for the first 861 nucleotides of translated sequence. Thereafter, homology broke down completely for a further 321 nucleotides until the 3′-untranslated region (UTR) sequence was reached. An in-frame stop codon exists within this 321 base sequence, leading to a predicted protein of 33kDa, compared with 45.1kDa for the normal sequence. The first 287 residues of this 290 amino acid truncated sequence were shared with the 397 amino acids encoded by the LYQ EjPSY2A (Supplementary Fig. S3 at JXB online). Further, the genomic DNA of the mutant EjPSY2A d showed that the DNA sequence homology broke down in the fifth exon, resulting in the mutant mRNA of EjPSY2A d (Fig. 3). Degenerate primers (Supplementary Table S3 at JXB online) were designed according to an additional and distinct plant PSY deposited in the Phytozome database (http://www.phytozome.net/), and, following PCR, an additional loquat family member, EjPSY3, was obtained. Sequencing of the EjPSY3 mRNAs found in LYQ and BS varieties showed the occurrence of different sequences that appeared to be due to altered RNA splicing. EjPSY3 in the LYQ variety (named EjPSY3α) lacked 97bp of the fourth exon, whereas the EjPSY3 from the BS variety (named EjPSY3β) lacked 10bp of the third exon and contained the third intron, and both sequences displayed several stop codons (Supplementary Fig. S4). When other loquat varieties were tested, a normal EjPSY3 was also observed, for example in Jiajiao (red-fleshed), Baiyu, and Biqi (white fleshed).
Fig. 3.
The structure of EjPSY2A and EjPSY2A d. The EjPSY2A d sequence is mutated, missing a 694bp nucleotide sequence in the fifth exon of the EjPSY2A gene. The dark blue region is the first exon, the green region is the second exon, the blue region is the third exon, the yellow region is the fourth exon, and the red region is the fifth exon. Thin lines indicate introns. The coloured boxes show the translated sequence and the white boxes the untranslated segments.
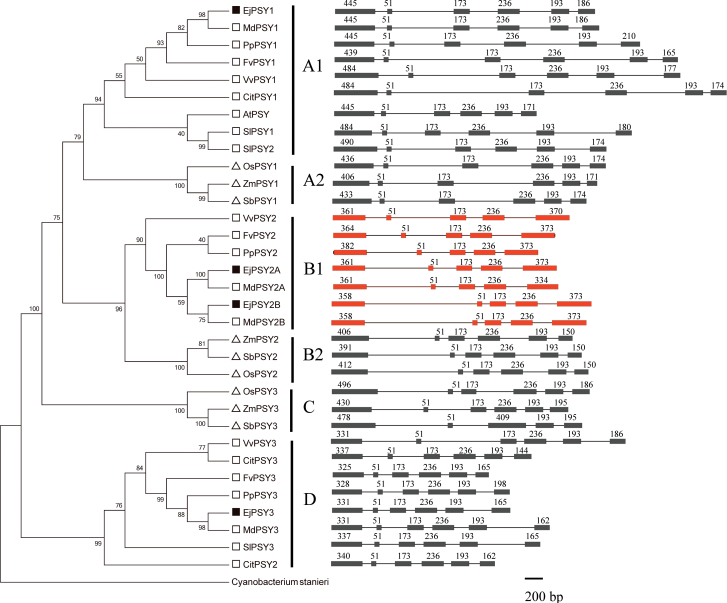
The deduced amino acid sequences for EjPSY1, EjPSY2A, EjPSY2B, and EjPSY3 were determined and aligned (Supplementary Fig. S5 at JXB online). What most distinguished EjPSY1, EjPSY2A, EjPSY2B, and EjPSY3 sequences from each other were the N-terminal regions. To facilitate a more in-depth analysis of the four loquat EjPSY gene family members, the genomic DNA sequences were obtained and the deduced amino acid sequences were used for phylogenetic analysis (Fig. 4). EjPSY1 and EjPSY3 genes possess six exons and five introns, and differ from EjPSY2A and EjPSY2B, which have five exons and four introns. Phylogenetic analysis showed that PSY genes could be divided into four groups, here called A (divided into A1 and A2), B (divided into B1 and B2), C and D. EjPSY1 belongs to group A1, EjPSY2A and EjPSY2B together belong to group B1, and EjPSY3 clustered in another group, D. Notably, the PSY genes in group D are the most closely related to CrtB of Cyanobacterium. For comparison, the three PSY family members in the Poaceae (PSY1-like, PSY2-like, and PSY3-like) were clustered in groups A2, B2, and C, respectively.
Fig. 4.
Phylogenetic tree (left) and the DNA structure (right) of PSYs. Phylogenetic tree: dicot plants are indicated by open squares, monocot plants by triangles, and loquat genes by filled squares. Amino acid sequences were aligned using ClustalW and a Neighbor–Joining tree was constructed with a 1000-bootstap replication support using MEGA5 software. DNA structure: boxes and thin bars indicate exons and introns, respectively; The DNAs having five exons are indicated with red boxes and those with six exons with black boxes.
Reverse transcription–PCR analysis of expression of all EjPSYs transcripts in LYQ and BS tissues
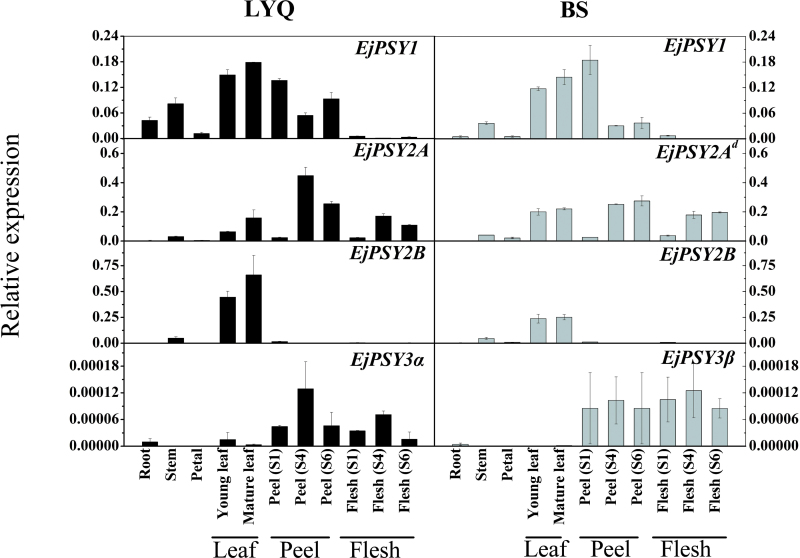
If EjPSY1, EjPSY2A, EjPSY2B, or EjPSY3 mRNA expression correlates with carotenoid accumulation, it would be expected that their transcripts would vary specifically in tissues of peel, flesh, leaf, or root, given that peel and leaf of the BS variety accumulate carotenoids and the flesh does not. To test this possibility, the relative expression of EjPSY1, EjPSY2A (in LYQ), EjPSY2A d (in BS), EjPSY2B, EjPSY3α (in LYQ), and EjPSY3β (in BS) transcript levels were estimated in RNA samples derived from root, stem, petal, young and mature leaf, peel, and flesh at three fruit developmental stages (mature green, breaker, and ripe) using quantitative RT–PCR with gene-specific primers.
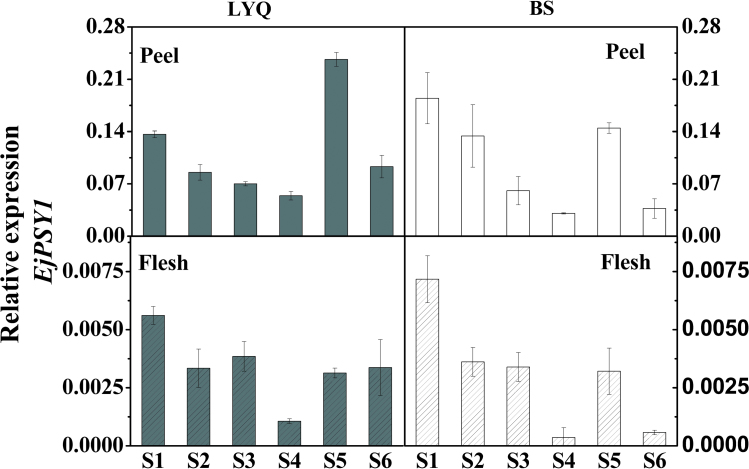
The EjPSY1 transcripts could be detected in almost all the tissues examined and were highest in leaf, stem, and peel, but were present at extremely low levels in petal and mature fruit flesh (Fig. 5). In the flesh tissue, EjPSY1 transcripts were always 15-fold lower than those in the peel during all stages of fruit development in both LYQ and BS (Fig. 6). EjPSY2A (LYQ) transcripts were present at very low levels in the tissues of root, stem, and petal, and in the peel and flesh of fruit at the mature green stage. As expected, however, the EjPSY2A (LYQ) transcript showed a typical increase between the mature green and breaker stage in fruit peel, with a slight reduction between the breaker and ripe stages. The EjPSY2A d transcript levels in BS were not affected by the sequence mutation, and showed a similar expression pattern and level to EjPSY2A in LYQ (Fig. 5).
Fig. 5.
mRNA levels of EjPSY1, EjPSY2A (LYQ), EjPSY2A d (BS), EjPSY2B, EjPSY3α (LYQ), and EjPSY3β (BS) in different tissues. The data show mRNA levels relative to actin mRNA. Note the differences in the scales for different genes. Loquat tissues examined: root, stem, petal, young leaf and mature leaf, peel at the green stage (S1), peel at the breaker stage (S4), peel at the ripe stage (S6), flesh at the green stage (S1), flesh at the breaker stage (S4), and flesh at the ripe stage (S6).
Fig. 6.
The expression patterns of the EjPSY1 gene in peel and flesh of LYQ and BS during fruit ripening.
Accumulation of the EjPSY2B mRNA occurred to the highest level in leaf tissue, and the content was a little higher in LYQ leaves compared with those of BS. The transcripts of EjPSY2B in stem, peel, and flesh at the mature green stage were present at higher concentrations than in the tissues of root, petal, peel, and flesh at breaker and ripe stages, although they were still very low compared with the content in leaf RNA (Fig. 5). This is consistent with the EjPSY2B enzyme playing a pivotal role in providing carotenoids for the assembly of the photosynthetic apparatus in leaves and other green tissues. The EjPSY3α and EjPSY3β transcripts showed similar expression patterns in LYQ and BS and had the lowest expression levels of all the EjPSY family members in loquat, at least 1000-fold less than other members, and were sometimes undetectable by quantitative PCR (Fig. 5). Very few EjPSY3 transcripts were detected by quantitative PCR in the flesh of Jiajiao, Baiyu, or Biqi cultivars, which was similar to the situation for EjPSY3α in LYQ and EjPSY3β in BS cultivars.
Functional analysis of all EjPSYs in loquat
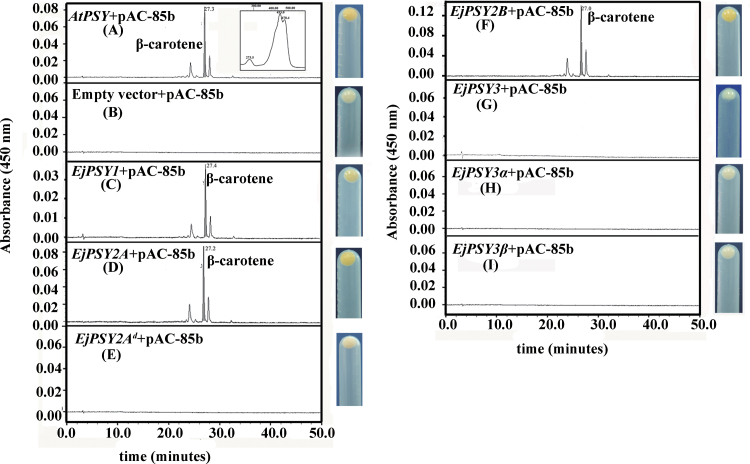
To determine whether the EjPSY members EjPSY1, EjPSY2A, EjPSY2Ad, EjPSY2B, EjPSY3, EjPSY3α, and EjPSY3β are functional in a bacterial carotenoid synthesis system, the ORF of each corresponding gene was subcloned into the E. coli expression vector and co-transformed along with the pAC-85b plasmid, which contains all coding sequences necessary for carotenoid biosynthesis, except PSY. The expected product, β-carotene, confirmed by matching spectra and column chromatography retention times, as well as two other carotenoids, probably β-carotene isomers according to their spectra, were produced in the bacteria transformed with the EjPSY1, EjPSY2A, and EjPSY2B vectors, while no carotenoids were detected with EjPSY3, the mutant EjPSY2Ad, as well as vectors carrying the mis-spliced EjPSY3α and EjPSY3β sequences (Supplementary Fig. S4 at JXB online). This indicates that the EjPSY1, EjPSY2A, and EjPSY2B cDNAs all encoded enzymes that were functional in the bacterial system. In contrast, the mutant EjPSY2A d found in BS was non-functional, presumably due to the loss of the C-terminal region (Figs 3, 7), and the mis-spliced EjPSY3α found in LYQ and EjPSY3β found in BS were also non-functional. Unexpectedly, EjPSY3 was probably also non-functional although it encodes a full-length translatable sequence (Fig. 7).
Fig. 7.
Effectiveness of EjPSY1, EjPSY2A, EjPSY2A d, EjPSY2B, EjPSY3, EjPSY3α, and EjPSY3β from loquat in functional complementaion of carotenoid synthesis in E. coli. Escherichia coli cells were co-transformed with AtPSY+pAC-85b (A); empty vector+pAC-85b (B); EjPSY1+pAC-85b (C); EjPSY2A+pAC-85b (D); EjPSY2A d+pAC-85b (E); EjPSY2B+pAC-85b (F); EjPSY3+pAC-85b (G); EjPSY3α+pAC-85b (H); and EjPSY3β+pAC-85b (I). Chromatograms show HPLC separation of extracted pigments; the inset in (A) shows the spectral fine structure for the pathway end-product, β-carotene. The pellet colours of the cells are shown on the right.
The phenotype of white-fleshed loquat is determined by the recessive gene EjPSY2A d
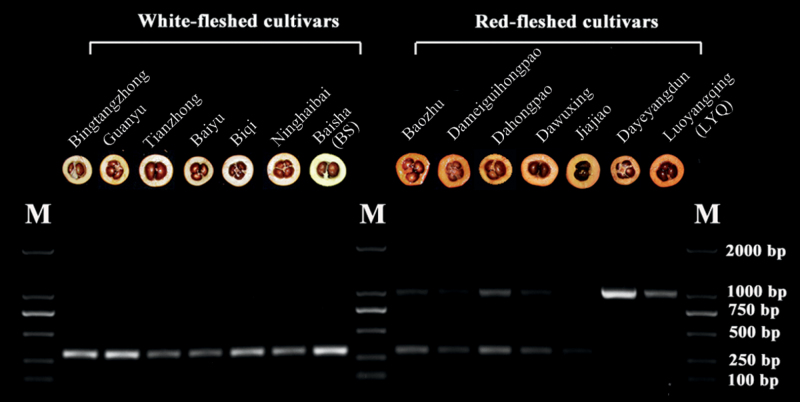
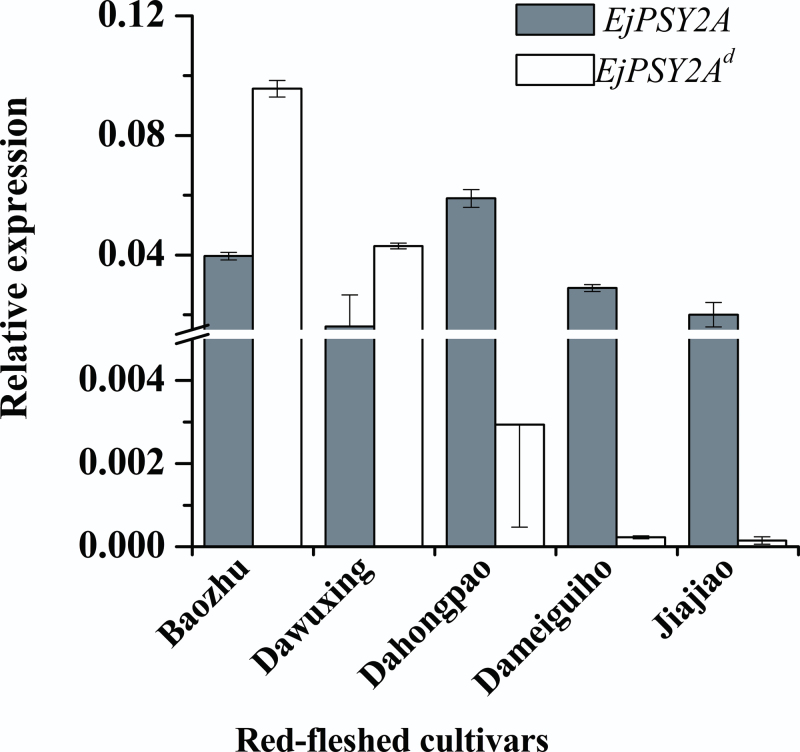
The differences in the expression level of carotenogenic genes between LYQ (red-fleshed) and BS (white-fleshed) loquat seemed insufficient to explain the large differences in carotenoid content between the two cultivars (Fu et al., 2012). Here, the mutant EjPSY2A d cloned from the BS (white-fleshed) variety showed specific expression in mature fruit (Fig. 7), although it was truncated and had no PSY catalytic activity in the bacterial system (Fig. 7), indicating that this mutation is likely to be responsible for the white flesh colour of BS. The flesh of the LYQ (red-fleshed) variety, expressing EjPSY2A, did, on the other hand, accumulate abundant carotenoids. There are several other red- and white-fleshed loquat cultivars cropped in China. To investigate whether the mutant EjPSY2A d occurred in other white-fleshed loquat or also existed in other red-fleshed loquat cultivars, a specific primer pair was designed across the region of the lost sequence in EjPSY2A d, so that only one short band (319bp) could be amplified if the cultivar contained only the mutant sequence EjPSY2A d, whereas only one larger band (1013bp) would be generated if the cultivar had only the normal sequence EjPSY2A. The production of both bands would indicate the presence of both EjPSY2A and EjPSY2A d in the cultivar. The results clearly showed (Fig. 8) that all the white-fleshed loquat cultivars tested possessed only the same mutant EjPSY2A d gene structure, and all the red-fleshed loquat cultivars contained the EjPSY2A sequence encoding the functional enzyme. Interestingly, some red-fleshed loquat cultivars (Baozhu, Dawuxing, Dahongpao, Dameiguihongpao, and Jiajiao) also contained the mutant EjPSY2A d gene (Fig. 8). In addition, the mutant EjPSY2A d mRNA also had high expression levels in the flesh of Baozhu and Dawuxing at the mature stage (Fig. 9), indicating that the white flesh of loquat was controlled by this recessive gene, and that some red-fleshed varieties were heterozygous for this gene.
Fig. 8.
PCR amplification of the EjPSY2A/EjPSY2A d genomic region from red- and white-fleshed loquat varieties. PCR amplification of the EjPSY2A/EjPSY2A d genomic region gave two fragments: a 1013bp fragment (EjPSY2A) present only in red-fleshed varieties and absent in white-fleshed varieties, and a 319bp fragments (EjPSY2A d) present in white-fleshed types and some red-fleshed cultivars, together with the 1013bp fragment.
Fig. 9.
The EjPSY2A and EjPSY2A d mRNA expression levels in flesh of five ripe red-fleshed fruit cultivars. The red-fleshed cultivars Baozhu, Dawuxing, Dahongpao, Dameiguihongpao, and Jiajiao contained the DNA sequence of the normal EjPSY2A gene and the mutant EjPSY2A d.
Discussion
EjPSY2A plays the main role in flesh carotenoid biosynthesis in mature fruit of loquat
Red-fleshed loquat varieties all contain a functional EjPSY2A (Figs 7, 8), which was highly expressed in fruit flesh and was probably responsible for carotenoid accumulation in the flesh (Fig. 5). A mutated non-functional variant of this gene (EjPSY2A d) was detected in all the white-fleshed loquat cultivars examined (Fig. 8). Thus, although EjPSY2A d is expressed in the flesh of white-fleshed cultivars, the non-functional enzyme (Fig. 7) it encodes is unable to participate in carotenoid biosynthesis, showing a convincing correlation with the greatly reduced level of carotenoids accumulated in the flesh of white-fleshed loquat cultivars. This phenomenon is similar to the situation in the yellow-fleshed tomato r mutant, which possessess a non-functional PSY1 gene and does not accumulate carotenoids in the fruit (Fray and Grierson, 1993). Transfer of the EjPSY2A gene to white-fleshed cultivars would be a direct way to validate further whether the failure of carotenoid accumulation in flesh resulted solely from the loss-of-function mutation of EjPSY2A; however, at present, a genetic transformation system is not available for loquat, although such a system has been successfully used for several other Rosaceae plants (Espley et al., 2007; Gambino and Gribaudo, 2012).
It is interesting that some red-fleshed loquat cultivars possess both the functional enzyme EjPSY2A and the non-functional mutant EjPSY2Ad, and high transcript levels of the inactive EjPSY2Ad were present in the flesh of some red-fleshed cultivars. However, this heterozygosity of EjPSY2A and EjPSY2A d in the red-fleshed loquat cultivars did not affect the overall carotenoid accumulation (Fig. 1), which is entirely consistent with the phenotype of the white-fleshed loquat cultivars being controlled by the recessive gene EjPSY2A d. According to the analysis of different cultivars, the first white-fleshed loquat cultivar might have arisen from one of the red-fleshed cultivars by mutation, followed by segregation. Red-fleshed loquat cultivars heterozygous for EjPSY2A and EjPSY2A d might have descended from trees heterozygous for the mutation, or, alternatively, might have arisen by hybridization between red-fleshed and white-fleshed cultivars.
In a previous study, no chromoplast structure was found in the cells of ripe BS fruit flesh (Fu et al., 2012); it would be interesting to investigate in future studies whether the failure of chromoplast development in BS flesh can be due to the non-functional mutant EjPSY2Ad. There is evidence from other studies that plastid morphology can be affected by elevated carotenoid levels in canola endosperm (Shewmaker et al., 1999), and the expression of PSY was tightly related to chromoplast formation (Fraser et al., 2007; Nogueira et al., 2013; Bai et al., 2014). The mutation of critical amino acid residues in maize PSY can result in changes in localization and cause distorted plastid shape and formation of a fibril phenotype (Shumskaya et al., 2012), and cellular structures can be accordingly adapted to facilitate the sequestration of newly formed products (Nogueira et al., 2013).
The expression of EjPSY1 and EjPSY2B is responsible for accumulation of carotenoids in the peel and leaf of white-fleshed loquat
The tissue-specific expression of EjPSY1, EjPSY2A, EjPSY2B, and EjPSY3 revealed unique features of the four gene family members. Transcripts of EjPSY2A were in low abundance in root and petal, detectable in stem, leaf, and green fruits (peel and flesh), and most abundant in breaker fruits. The role and importance of catalytically active EjPSY2A in loquat fruit carotenoid accumulation was established by the lack of carotenoids accumulated in the flesh of white-fleshed loquat expressing the catalytically inactive EjPSY2A d mutant. In contrast to EjPSY2A, EjPSY2B transcripts were only abundant in leaf tissue, and transcripts were present in only very low amounts in other organs and tissues, which supports the proposal that EjPSY2B is responsible for carotenoid production in green tissues, especially in the leaf. This explains why young fruits and leaves of the white-fleshed loquat cultivars with the non-functional EjPSY2A d can still accumulate normal amounts of carotenoids, because EjPSY2B plays the main role in carotenoid accumulation in the young fruit and leaf of both red- and white-fleshed varieties.
According to the expression pattern, EjPSY1 was present in all tissues, with few transcripts in fruit flesh but with a higher expression level in the peel in both LYQ and BS loquat fruits. The observation that EjPSY1 had higher expression in the peel explains very well how the peel of white-fleshed cultivars can accumulate moderate amount of carotenoids during ripening (Fig. 6). Differential carotenoid accumulation patterns have also been discovered in tissues of the Cara Cara citrus fruit, where the flesh accumulates large amounts of lycopene, but the peel tissue does not (Xu et al., 2006), although the underlying mechanism is not yet known.
The function of EjPSY3 remains to be determined
EjPSY3 showed the lowest mRNA accumulation in all tissues examined, and different RNA editing mutations occurred in the LYQ and BS varieties, resulting in the production of non-functional enzymes (Figs 5, 7), although the normal cDNA of EjPSY3 was observed in some other loquat varieties such as Jiajiao, Baiyu, and Biqi. However, the normal sequence of EjPSY3 also lacked catalytic activity in the E. coli system. This might be due to several amino acid differences, compared with other functional EjPSY genes (Supplementary Figs S5, S6 at JXB online), in or near the normally conserved domain 2 (Shumskaya et al., 2012). There have been several reports that a single amino acid change can alter the activity of a PSY enzyme. Welsch et al. (2010) showed that a single nucleotide polymorphism in PSY2, causing a non-conservative amino acid exchange, leads to markedly increased carotenoid formation and accumulation in cassava storage roots. Gady et al. (2012) indicated that the P192L mutation (an amino acid substitution P192L) affects PSY1 activity through misfolding, leading to low phytoene accumulation.
Due to the lack of catalytic activity and much lower expression level of PSY3, it seems not to play a role in carotenoid accumulation in the loquat plant. Different splicing mutations were found in LYQ and BS, which showed there were variations in mRNA processing, although the significance of this is not clear. EjPSY3 might possibly have had a role previously, but lost its catalytic activity during the course of evolution. The amino acid sequence of EjPSY3 was most similar to that of SlPSY3, which has also been reported to be the least expressed PSY gene in tomato. SlPSY3 does not appear to play a major role in fruit lycopene biosynthesis, although when PSY3 was silenced in tomato fruit a small but significant reduction of phytoene, phytofluene, γ-carotene, and δ-carotene was observed (Fantini et al., 2013). However, the catalytic activity of SlPSY3 has not yet been examined. The PSY3 family members from dicot plant shared high homology (Fig. 4) and clustered in group D. A striking difference was found in the highly conserved coding region 2 (Supplementary Fig. S6 at JXB online) of PSY3, at amino acid residue 264, which was glycine (Gly264) in dicot plants, compared with alanine in other plant PSYs, including CrtB. Furthermore, the nearby position, 260, was occupied by tyrosine, asparagine, or histidine in dicot plant PSY3s (Tyr260, Asn260, or His260), in contrast to the alanine present in all other PSYs, also including CrtB. Whether these amino acid differences affect the function of EjPSY3s requires further study, and the properties of PSY3 genes in other dicots (such as tomato or citrus) require further investigation to test whether they are also catalytically inactive, or whether they have retained activity.
PSY expression patterns are independent of gene structure and evolution
Only one phytoene synthase gene family member has been found in Arabidopsis thaliana (Lange and Ghassemian, 2003), and three family members are found in Solanum lycopersicum (Tomato Genome Consortium, 2012). In this study, four family members were found, and the function, evolution, and structure of these genes were analysed. According to the phylogenetic tree, EjPSY3 was clustered with SlPSY3 in group D, which is considered much more ancient than other plant phytoene synthase genes. On the basis of the present evidence, it seems likely that EjPSY1-type genes (six exons) in group A1 possibly evolved from PSY3 genes in group D. EjPSY2A–EjPSY2B-type genes (five exons) in group B1 of some dicot plants (e.g. Eriobotrya japonica, Vitis vinifera, and Prunus persica) probably arose by the fusion of the fifth and sixth exons during evolution.
The PSY evolutionary branches in dicot plants seem quite diverse. For example, one branch has either been lost (A. thaliana, which only has one gene), or has lost catalytic activity (EjPSY3 in group D). Also, where duplication in one of the three branches has taken place (e.g. SlPSY1 and SlPSY2), a new PSY DNA structure (EjPSY2A and EjPSY2B) has developed during the course of evolution.
Although the gene structures and evolutionary relationships (Fig. 4) are different among species, the expression patterns of these genes are conserved. For example, the structures of SlPSY1 and SlPSY2 in tomato (expressed in fruit and leaves, respectively, clustered in group A1, with six exons and five introns) are different from those of EjPSY2A and EjPSY2B in loquat (clustered in group B1, with five exons and four introns), but they play similar roles in carotenoid synthesis in the respective plants (Figs 4, 5; Fraser et al., 1999). Paradoxically, according to the gene structures, although EjPSY1 is closer to SlPSY1 and SlPSY2, clustered in group A1, all with six exons and five introns, the expression patterns are different, with EjPSY1 having few transcripts in flesh. This strongly suggests that different PSY genes have been recruited to perform similar roles during the evolution of different fruits.
GenBank accession numbers
Sequence data from this article have been deposited in the EMBL/GenBank data libraries under accession numbers: EjPSY1 (KF922363), EjPSY2A (KF922364), EjPSY2A d (KF922365), EjPSY2B (KF922366), EjPSY3 (KF922367), EjPSY3α (KF922368), and EjPSY3β (KF922369).
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Unigene sequence of PSY in loquat RNA-Seq libraries.
Figure S2. Carotenoid content and composition in the peel of red- and white-fleshed loquat cultivars.
Figure S3. Comparison of the deduced amino acid sequences of EjPSY2A and EjPSY2Ad.
Figure S4. Analysis of the alternative splicing of EjPSY3α and EjPSY3β.
Figure S5. Alignment of EjPSY amino acid sequences.
Figure S6. Alignment of PSY amino acid sequences.
Table S1. Primers for RACE PCR.
Table S2. Primer sequences for genome walking.
Table S3. Primers for genomic DNA PCR.
Table S4. Primer sequences for PCR amplification of the EjPSY2A/EjPSY2A d genomic fragment.
Table S5. Primers for real-time PCR.
Table S6. Primers for PSY cDNAs subcloned as in-frame translational fusions.
Table S7. Carotenoid content in peel and flesh tissues of white- and red-fleshed loquat cultivars.
Table S8. Carotenoid content in various tissues of LYQ and BS.
Acknowledgments
The pAC-85b and pAtPSY plasmids were kindly provided by Dr Francis Cunningham (University of Maryland). This research was supported by the National Natural Science Foundation in China [30871691, 31030052], the Program of International Science and Technology Cooperation [2011DFB31580], the Special Scientific Research Fund of Agricultural Public Welfare Profession of China [200903044], and the Specialized Research Fund for the Doctoral Program of Higher Education of China [20120101110069].
References
- Abdel-Aal EM, Akhtar H, Zaheer K, Ali R. 2013. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 5, 1169–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C, Rivera SM, Medina V, et al. 2014. An in vitro system for the rapid functional characterization of genes involved in carotenoid biosynthesis and accumulation. The Plant Journal 77, 464–475 [DOI] [PubMed] [Google Scholar]
- Chen Y, Li F, Wurtzel ET. 2010. Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiology 153, 66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett-Dame M, Knutson D. 2011. Vitamin A in reproduction and development. Nutrients 3, 385–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F, Jr, Gantt E. 2007. A portfolio of plasmids for identification and analysis of carotenoid pathway enzymes: Adonis aestivalis as a case study. Photosynthesis Research 92, 245–259 [DOI] [PubMed] [Google Scholar]
- DellaPenna D, Pogson BJ. 2006. Vitamin synthesis in plants: tocopherols and carotenoids. Annual Review of Plant Biology 57, 711–738 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW. 1992. Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology 43, 599–626 [Google Scholar]
- Demmig-Adams B, Adams WW. 2002. Antioxidants in photosynthesis and human nutrition. Science 298, 2149–2153 [DOI] [PubMed] [Google Scholar]
- Egea I, Barsan C, Bian W, Purgatto E, Latché A, Chervin C, Bouzayen M, Pech J-C. 2010. Chromoplast differentiation: current status and perspectives. Plant and Cell Physiology 51, 1601–1611 [DOI] [PubMed] [Google Scholar]
- Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC. 2007. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. The Plant Journal 49, 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi R, Vendramin E, Zanon L, Scalabrin S, Cipriani G, Verde I, Vizzotto G, Morgante M. 2013. Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. The Plant Journal 76, 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini E, Falcone G, Frusciante S, Giliberto L, Giuliano G. 2013. Dissection of tomato lycopene biosynthesis through virus-induced gene silencing. Plant Physiology 163, 986–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser PD, Bramley PM. 2004. The biosynthesis and nutritional uses of carotenoids. Progress in Lipid Research 43, 228–265 [DOI] [PubMed] [Google Scholar]
- Fraser PD, Enfissi EMA, Halket JM, Truesdale MR, Yu D, Gerrish C, Bramley PM. 2007. Manipulation of phytoene levels in tomato fruit: effects on isoprenoids, plastids, and intermediary metabolism. The Plant Cell 19, 3194–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P, Kiano J, Truesdale M, Schuch W, Bramley P. 1999. Phytoene synthase-2 enzyme activity in tomato does not contribute to carotenoid synthesis in ripening fruit. Plant Molecular Biology 40, 687–698 [DOI] [PubMed] [Google Scholar]
- Fray RG, Grierson D. 1993. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Molecular Biology 22, 589–602 [DOI] [PubMed] [Google Scholar]
- Fu X, Kong W, Peng G, Zhou J, Azam M, Xu C, Grierson D, Chen K. 2012. Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya japonica) fruits. Journal of Experimental Botany 63, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gady AF, Vriezen W, Wal MBJ, Huang P, Bovy A, Visser RF, Bachem CB. 2012. Induced point mutations in the phytoene synthase 1 gene cause differences in carotenoid content during tomato fruit ripening. Molecular Breeding 29, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino G, Gribaudo I. 2012. Genetic transformation of fruit trees: current status and remaining challenges. Transgenic Research 21, 1163–1181 [DOI] [PubMed] [Google Scholar]
- Harjes CE, Rocheford TR, Bai L, et al. 2008. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 319, 330–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J. 2001. Carotenoid biosynthesis in flowering plants. Current Opinion in Plant Biology 4, 210–218 [DOI] [PubMed] [Google Scholar]
- Howitt CA, Pogson BJ. 2006. Carotenoid accumulation and function in seeds and non-green tissues. Plant, Cell and Environment 29, 435–445 [DOI] [PubMed] [Google Scholar]
- Huang Y, Yin X, Zhu C, Wang W, Grierson D, Xu C, Chen K. 2013. Standard addition quantitative real-time PCR (SAQPCR): a novel approach for determination of transgene copy number avoiding PCR efficiency estimation. PLoS One 8, e53489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachanovsky DE, Filler S, Isaacson T, Hirschberg J. 2012. Epistasis in tomato color mutations involves regulation of phytoene synthase 1 expression by cis-carotenoids. Proceedings of the National Academy of Sciences, USA 109, 19021–19026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilcrease J, Collins AM, Richins RD, Timlin JA, O’Connell MA. 2013. Multiple microscopic approaches demonstrate linkage between chromoplast architecture and carotenoid composition in diverse Capsicum annuum fruit. The Plant Journal 76, 1074–1083 [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Landrum JT, Bone RA. 2003. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annual Review of Nutrition 23, 171–201 [DOI] [PubMed] [Google Scholar]
- Lange BM, Ghassemian M. 2003. Genome organization in Arabidopsis thaliana: a survey for genes involved in isoprenoid and chlorophyll metabolism. Plant Molecular Biology 51, 925–948 [DOI] [PubMed] [Google Scholar]
- Lu S, Van Eck J, Zhou X, et al. 2006. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of β-carotene accumulation. The Plant Cell 18, 3594–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Zhang L, Matsuta A, Matsutani K, Yamawaki K, Yahata M, Wahyudi A, Motohashi R, Kato M. 2013. Enzymatic formation of β-citraurin from β-cryptoxanthin and zeaxanthin by carotenoid cleavage dioxygenase4 in the flavedo of Citrus fruit. Plant Physiology 163, 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, Jarvik T. 2010. Lateral transfer of genes from fungi underlies carotenoid production in Aphids. Science 328, 624–627 [DOI] [PubMed] [Google Scholar]
- Niyogi KK. 1999. Photoprotection revisited: genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology 50, 333–359 [DOI] [PubMed] [Google Scholar]
- Nogueira M, Mora L, Enfissi EMA, Bramley PM, Fraser PD. 2013. Subchromoplast sequestration of carotenoids affects regulatory mechanisms in tomato lines expressing different carotenoid gene combinations. The Plant Cell 25, 4560–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolillo DJ, Jr, Garvin DF, Parthasarathy MV. 2004. The chromoplasts of Or mutants of cauliflower (Brassica oleracea L. var. botrytis). Protoplasma 224, 245–253 [DOI] [PubMed] [Google Scholar]
- Peng G, Wang C, Song S, Fu X, Azam M, Grierson D, Xu C. 2013. The role of 1-deoxy- d -xylulose-5-phosphate synthase and phytoene synthase gene family in citrus carotenoid accumulation. Plant Physiology and Biochemistry 71, 67–76 [DOI] [PubMed] [Google Scholar]
- Ronen G, Carmel-Goren L, Zamir D, Hirschberg J. 2000. An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proceedings of the National Academy of Sciences, USA 97, 11102–11107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewmaker CK, Sheehy JA, Daley M, Colburn S, Ke DY. 1999. Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. The Plant Journal 20, 401–412 [DOI] [PubMed] [Google Scholar]
- Shumskaya M, Bradbury LMT, Monaco RR, Wurtzel ET. 2012. Plastid localization of the key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. The Plant Cell 24, 3725–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumskaya M, Wurtzel ET. 2013. The carotenoid biosynthetic pathway: thinking in all dimensions. Plant Science 208, 58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium. 2012. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485, 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch R, Arango J, Bär C, Salazar B, Al-Babili S, Beltrán J, Chavarriaga P, Ceballos H, Tohme J, Beyer P. 2010. Provitamin A accumulation in Cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. The Plant Cell 22, 3348–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CJ, Fraser PD, Wang WJ, Bramley PM. 2006. Differences in the carotenoid content of ordinary citrus and lycopene-accumulating mutants. Journal of Agricultural and Food Chemistry 54, 5474–5481 [DOI] [PubMed] [Google Scholar]
- Zhou CH, Xu CJ, Sun CD, Li X, Chen K-S. 2007. Carotenoids in white- and red-fleshed loquat fruits. Journal of Agricultural and Food Chemistry 55, 7822–7830 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.