Abstract
Eating high fat chow increases sensitivity of male rats to some behavioral effects of the direct-acting dopamine receptor agonist quinpirole; it is not known whether sensitivity to quinpirole is similarly enhanced in female rats eating high fat chow. Female Sprague-Dawley rats had free access to standard chow (5.7% fat) or either free or restricted access (i.e., body weight matched to rats eating standard chow) to high fat (34.3% fat) chow. Quinpirole (0.0032–0.32 mg/kg) produced hypothermia and a low frequency of yawning. Eating high fat chow produced insulin resistance without affecting quinpirole-induced yawning or hypothermia. Pretreatment with the dopamine D2 receptor antagonist L-741,626 failed to increase quinpirole-induced yawning, indicating that the low frequency of yawning was not due to enhanced D2 receptor sensitivity. Compared with younger (post natal day [PND] 75), drug-naïve female rats in a previous study, rats in the present study (PND 275) were more sensitive to cocaine-elicited (1–17.8 mg/kg) locomotion and the development of sensitization across 5 weeks; however, eating high fat chow did not further enhance these effects. These results suggest that drug history and age might modulate the effects of diet on sensitivity to drugs acting on dopamine systems.
Keywords: dopamine receptor, direct-acting agonist, indirect-acting agonist, yawning, high-fat chow, cocaine, quinpirole, sensitization, locomotion, rat
Introduction
Feeding condition (i.e. type and quantity of food consumed) can profoundly affect sensitivity to the behavioral effects of drugs acting on dopamine systems (Baladi et al., 2012a; Collins et al., 2008). For example, food restriction or eating high fat chow differentially modify sensitivity to the behavioral effects of drugs acting directly (i.e., dopamine receptor agonists such as quinpirole) or indirectly (i.e., dopamine transporter inhibitors such as cocaine) on dopamine receptors. Direct-acting dopamine receptor agonists (e.g., quinpirole) elicit yawning in male rats (Baladi and France 2010); food restriction attenuates (Collins et al., 2008; Baladi and France 2009) whereas eating high fat chow enhances (Baladi et al., 2011; Baladi and France 2010) agonist-induced yawning. Moreover, male rats with restricted access to high fat chow, such that their body weight is not different from that of rats eating standard chow, also are more sensitive to quinpirole-induced yawning, suggesting that eating fat, rather than gaining weight, is critical for increasing sensitivity to the effects of direct-acting dopamine receptor agonists (Baladi et al., 2011). Dopamine systems are the target of a number of therapeutic drugs (e.g., antipsychotics) and an important site of action of several abused drugs (e.g., cocaine). Thus, diet-induced changes in sensitivity to drugs acting on dopamine systems have implications for both the effectiveness of therapeutic drugs as well as vulnerability to abuse drugs.
Although much research on drugs of abuse research has used male subjects, there are significant differences between male and female subjects in their sensitivity to some drugs of abuse, including cocaine (Craft and Stratmann 1996; Chin et al., 2001; Lynch and Carroll 1999; for a review see Anker and Carroll 2011a). Similarly, most studies investigating the impact of feeding condition on sensitivity to drugs acting on dopamine systems have used male subjects (see Baladi et al., 2012a for a review), although recent evidence suggests that females are more sensitive to the effects of feeding condition on drugs acting on dopamine systems. For example, adult female rats eating high fat chow are more sensitive than adult female rats eating standard chow to the locomotor-stimulating effects of cocaine and they show greater sensitization to cocaine (Baladi et al., 2012b). In contrast, eating high fat chow has little or no effect on the locomotor-stimulating effects of cocaine in adult male rats (Baladi, unpublished observation). These results suggest that females are more sensitive than males to the impact of eating high fat chow on the behavioral effects of indirect-acting dopamine receptor agonists; it is not known whether eating high fat chow also increases sensitivity of female rats to direct-acting dopamine receptor agonists (e.g., quinpirole).
Although it is not known whether feeding conditions differentially affect sensitivity of male and female rats to the effects of direct-acting agonists, there are well characterized sex differences in both spontaneous and drug-induced yawning (Berendsen and Nickolson 1981; Serra et al., 1984; Urba-Holmgren et al., 1990; Diaz-Veliz et al., 1994) with female rats yawning much less than male rats. For example, in a 20-min observation period, direct-acting dopamine receptor agonists can elicit a maximum of 15–20 yawns in male rats (Collins et al., 2007) and only 2–3 yawns in female rats (Berendsen and Nickolson 1981). That eating high fat chow increases sensitivity of male rats to quinpirole-induced yawning, and increases sensitivity of female rats to cocaine-induced locomotion and sensitization suggests that low frequencies of quinpirole-induced yawning might be increased in female rats eating high fat chow. Quinpirole also causes hypothermia (Baladi and France 2009; Baladi et al., 2011), although neither food restriction nor eating high fat chow significantly affect quinpirole-induced hypothermia (Baladi and France 2009; Baladi et al., 2011), supporting the view that diet-induced changes in sensitivity to drug-induced yawning are not due to pharmacokinetic factors.
The mechanism(s) underlying enhanced sensitivity to drugs in rats eating high fat chow is not known, although insulin and insulin-signaling pathways have been suggested to play a role. Eating high fat chow causes insulin resistance in male rats (Baladi et al., 2011; Liu et al., 2013; Serafine and France 2014) that is paralleled by changes in sensitivity to quinpirole-induced yawning (Baladi et al., 2011; Serafine and France 2014), consistent with the hypothesis that insulin signaling pathways might contribute to diet-induced changes in sensitivity to drugs acting on dopamine receptors (Daws et al., 2011).
The present experiment investigated the behavioral (i.e. yawning) and physiological (i.e., hypothermia) effects of cumulative doses of quinpirole in female rats eating standard or high fat chow to determine whether eating high fat chow enhances sensitivity of female rats to the behavioral effects of a direct-acting dopamine receptor agonist (i.e., quinpirole). Sensitivity to insulin (i.e., blood glucose) was also examined to monitor the possible development of insulin resistance. Finally, sensitivity to cocaine-induced locomotion and sensitization was also examined since it is known that feeding condition can impact sensitivity of female rats to cocaine (Baladi et al., 2012b).
Methods
Subjects
Female Sprague–Dawley rats (n = 30; Harlan, Indianapolis, IN, USA), approximately 130 days of age, weighing 250–300 g at the beginning of the experiment, were housed individually in an environmentally controlled room (24 ± 1°C, 50 ± 10% relative humidity) under a 12:12-hr light/dark cycle (light period 0700–1900 hrs). Rats had free access to water in the home cage throughout the experiment. Food was also available in the home cage as described below. The study was conducted in two different cohorts of rats (see Table 1). In one cohort, 4 rats had free access to standard chow, 4 had free access to high fat chow, and an additional 5 had restricted access to high fat chow; in a second cohort 6 had free access to standard chow, 6 had free access to high fat chow, and 5 had restricted access to high fat chow. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, the University of Texas Health Science Center at San Antonio, and with the 2011 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, the National Research Council, and the National Academy of Sciences).
Table 1.
Groups of rats were tested in two separate cohorts, as indicated. After baseline testing, rats had access to standard chow or high fat chow beginning on week 1 and continuing throughout the study.
| Number of Subjects | % macronutrients by weight | % macronutrients by calorie | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Cohort 1 | Cohort 2 | Total | Type of Chow | Fat | Carbohydrates | Protein | Fat | Carbohydrates | Protein |
| Free Feeding Standard | 4 | 6 | 10 | Harlan Teklad 7912 | 5.7 | 44.3 | 19.9 | 17 | 58 | 25 |
| Free Feeding High Fat | 4 | 6 | 10 | Harlan Teklad 06414 | 34.3 | 27.3 | 23.5 | 60 | 21.3 | 18.4 |
| Restricted High Fat | 5 | 5 | 10 | Harlan Teklad 06414 | 34.3 | 27.3 | 23.5 | 60 | 21.3 | 18.4 |
Feeding conditions
Upon arrival, rats were habituated to the laboratory and to the experimental apparatus and procedures. All subjects had free access to standard chow upon arrival, and during the first tests with quinpirole. Rats were ranked based on the total number of yawns observed during 4 initial once per week quinpirole tests and subsequently assigned to groups such that the average number of yawns was not significantly different among the three groups. Thereafter, separate groups of rats had free access to standard laboratory chow (n = 10), free access to high fat chow (n = 10), or restricted access to high fat chow (n = 10; body weights matched to rats eating standard chow) for the duration of the experiment (Table 1). Tests with quinpirole occurred once every week. Since there was no significant difference between cohorts, data for each feeding condition were combined across the two cohorts. All subjects were fed daily at the same time throughout the experiment (11.00 h). The macronutritional content of the standard chow (Harlan Teklad 7912) and the high fat chow (Harlan Teklad 06414) are provided in Table 1.
Yawning
Yawning was defined as an opening and closing of the mouth (~1 s) such that the lower incisors were completely visible (Baladi and France 2009; Sevak et al., 2008). On the day of testing, rats were transferred to test cages (home and test cages were identical with the exception that test cages had no food, water, or bedding) and allowed to habituate for 15 min. Yawning was assessed at the same time each day after injection of vehicle followed by injection of increasing doses of quinpirole (0.0032, 0.01, 0.032, 0.1, 0.32 mg/kg, i.p.) administered every 30 min using a cumulative dosing procedure. Beginning 20 min after each injection, the total number of yawns observed for 10 min was recorded. Yawning dose-response curves were generated once per week for a total of 7 weeks while rats had access to their respective feeding conditions (week 1–7).
The ascending and descending limbs of the quinpirole yawning dose-response curve are mediated by D3 and D2 receptors, respectively (Baladi et al., 2010, 2011). The low frequency of agonist-induced yawning in food-restricted male rats is believed to be due to increased sensitivity at D2 receptors (descending limb of the dose-response curve) because a D2 receptor selective antagonist (L-741,626) restores sensitivity to agonist-induced yawning (Collins et al., 2008). The low frequency of drug-induced yawning observed in female rats, in the current study and in previous studies, might also be due to increased sensitivity at D2 receptors; thus, the D2 receptor selective antagonist L-741,626 was studied to see whether blocking D2 receptors increases quinpirole-induced yawning in female rats. L-741,626 (0.32, 1.0 and 3.2 mg/kg) was administered 30 min before administration of the first cumulative dose of quinpirole (weeks 8–11).
Body temperature
Body temperature was measured in a temperature controlled room (24 ± 1°C and 50 ± 10% relative humidity) by inserting a lubricated thermal probe attached to a thermometer 3 cm into the rectum. During yawning experiments, body temperature was measured upon completion of each 10-min observation period for yawning and prior to the next injection.
Insulin Sensitivity
Insulin sensitivity was measured using blood collected from the tip of the tail following a small incision (using a sterile scalpel blade) expressed on a blood-glucose test strip. Glucose values were measured with a commercially available glucose meter (Accu-Chek Aviva; CVS). Based on a previous study in this laboratory using male rats (Baladi et al., 2011), a dose of 0.75 U/kg insulin was used for the first test. However, that dose failed to produce hypoglycemia in female rats eating standard chow. Therefore, the dose of insulin was increased to 2.0 U/kg for subsequent tests; glucose was measured prior to as well as 15, 30, 45, and 75 min after the administration of insulin. Insulin sensitivity was measured every week for 3 consecutive weeks and on days when no other drug was administered.
Locomotor activity
Following yawning experiments (week 11, on days when rats did not receive quinpirole), for three consecutive days rats were placed in locomotor chambers for a 30-min habituation period, followed by injections of saline administered every 15 minutes for a total of 5 injections (105 minutes). Beginning the following week (week 12), rats received cumulative doses of cocaine (1.0, 3.2, 10, 17.8 mg/kg; i.p.) administered every 15 minutes for a total of 5 injections (105 minutes). These doses include the ascending limb of the cocaine locomotion dose-response curve (Baladi et al., 2012b). Only the ascending limb was examined because it was expected that the dose-response curve would shift leftward after repeated injections of cocaine (i.e., sensitization). Testing with cocaine occurred once per week on the same day and time for a total of 5 weeks (i.e., weeks 12–16).
Data analyses
Results are expressed as the average (± SEM) number of yawns during a 10-min observation period and average body temperature in °C, plotted as a function of dose. Mean (± SEM) locomotor activity counts in the last 5-min of each 15-min observation period are plotted as a function of dose or as mean (± SEM) area under the dose-response curve (AUC) and plotted as a function of week. Data are also expressed as the average (±SEM) change in glucose concentration (milligrams per deciliter) from pre-insulin injection levels as a function of week. Data are also expressed as average (±SEM) body weight and plotted as a function of week of specific feeding conditions. Dose-response curves for quinpirole-induced yawning and cocaine-induced locomotor activity were analyzed using two-way repeated measures ANOVA (feeding condition by week) to examine differences in the AUC (calculated by means of GraphPad Prism [GraphPad Software Inc., San Diego, CA, USA]). Multiple comparisons were made with the Bonferroni test (GraphPad Prism) where appropriate. Body temperature was analyzed using a two-way repeated-measures ANOVA (feeding condition by dose). Tukey post hoc analyses were used to examine significant differences among treatments. For each group of rats, a two-tailed paired t-test with Bonferroni corrections for multiple comparisons was used to examine changes in blood glucose concentration before and after administration of insulin (Durham et al., 2006). For all tests, P<0.05 was considered significant.
Drugs
Quinpirole dihydrochloride (Sigma-Aldrich, St. Louis, MO, USA) and cocaine hydrochloride (NIDA Research Technology Branch, Rockville, MD, USA) were dissolved in sterile 0.9% saline and administered i.p. in a volume of 1 ml/kg body weight. Insulin (protamine zinc recombinant human insulin; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) was dissolved in sterile 0.9% saline and injected i.p. in a volume of 1 mg/kg body weight. L-741,626 (Tocris, Ellisville, MO, USA) was dissolved in 5% ethanol with 1 M HCl and injected s.c., typically in a volume of 1 ml/kg body weight. Due to its limited solubility, L-741,626 was prepared at a concentration of 1.78 mg/mL for the largest dose examined (3.2 mg/kg), resulting in injection volumes larger than 1 ml/kg body weight.
Results
Body Weight
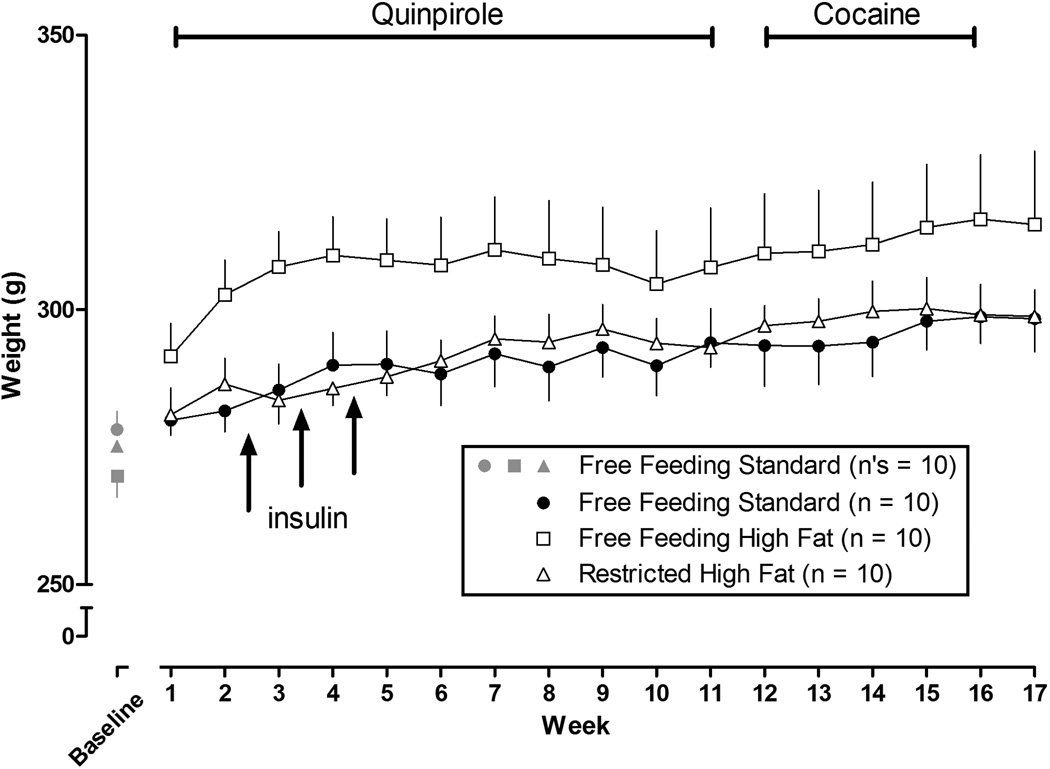
At the beginning of the experiment, when all rats were eating standard chow, the mean (± SEM) body weight for all 30 rats was 266.8 ± 2.6 g and 4 weeks later, after once per week testing with quinpirole, the mean body weight had increased to 275.2 ± 2.6 g (Figure 1, gray symbols above “Baseline” show body weight for each group of 10 rats that subsequently were assigned to different feeding conditions). A one-way ANOVA revealed that, from week 1 through week 17, rats with free access to high fat chow gained significantly more weight than other rats (F (2, 16) = 15.75; P < 0.001). By the end of the experiment (week 17), the average weight of rats with free access to high fat chow was 316.4 ± 5.2 g (Figure 1, open squares; n = 10) while the average weight of those with free access to standard chow was 295.9 ± 1.0 g (Figure 1, closed circles; n = 10). In the third group of rats the amount of high fat chow provided daily was adjusted to match the body weight of rats eating standard chow; in week 17 (Figure 1, open triangles; n = 10) the average weight of rats with restricted access to high fat chow was 296.0 ± 2.0 g.
Figure 1.
Body weight of rats throughout the experiment. Baseline refers to the last of 4 weeks that all subjects (n = 30) had free access to standard chow (gray symbols). Thereafter rats had free access to standard chow (n = 10), free access to high fat chow (n = 10), or restricted access to high fat chow (n = 10; body weight was matched to rats eating standard chow). Quinpirole was tested alone once per week during weeks 1–7; quinpirole was tested in combination with different doses of L-741,626 during weeks 8–11; and cocaine was tested alone once per week during weeks 12–16. Insulin testing is indicated by arrows and occurred on days 10, 17 and 24. Vertical axis: body weight in g recorded at the end of each week. Horizontal axis: week in study.
Yawning
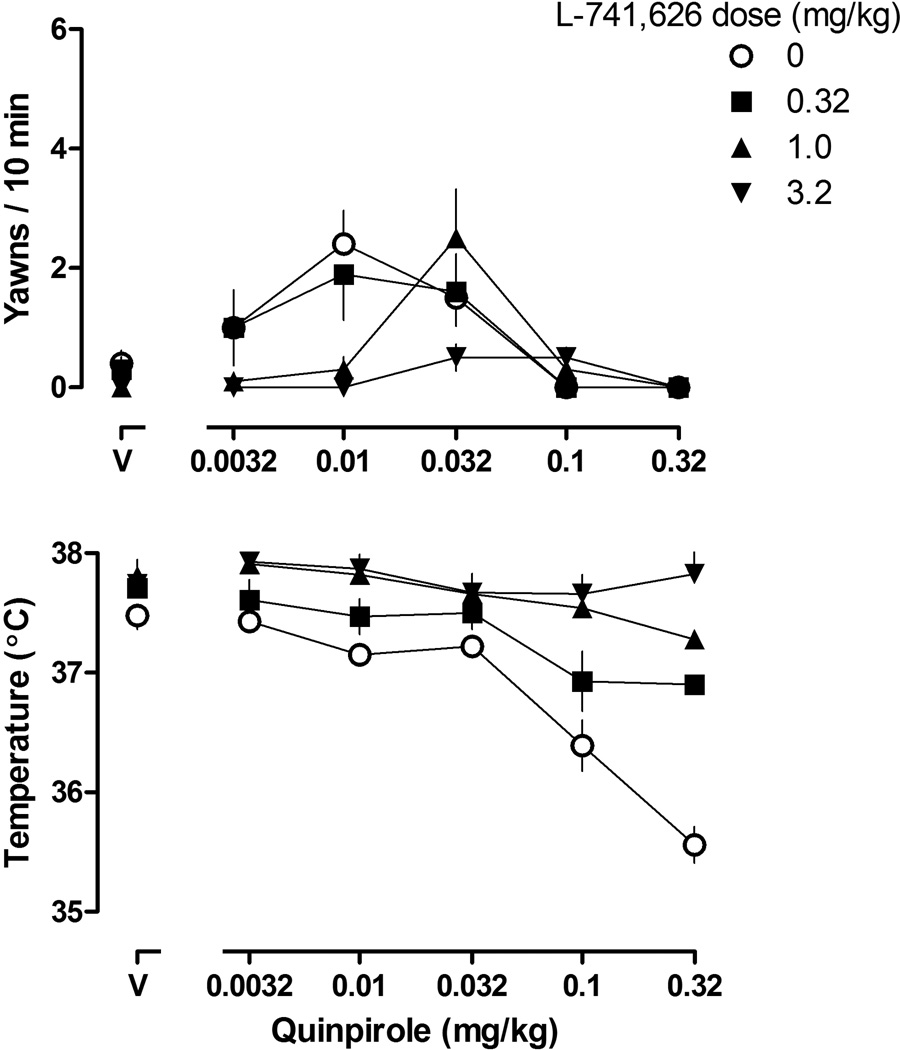
With increasing doses of quinpirole, yawning increased then decreased in all rats resulting in inverted U-shaped dose-response curves (Figure 2, upper panels). Quinpirole generated a low frequency of yawning in female rats, with a maximum of 2–4 yawns occurring per 10-min observation period. A two-way ANOVA (week and feeding condition) for AUC failed to reveal a significant difference in yawning across weeks between rats eating standard chow and those eating high fat chow (both groups). Thus, free or restricted access to high fat chow for up to 7 weeks did not significantly affect quinpirole-induced yawning in female rats. A one-way repeated measures ANOVA examining AUC over time within groups also failed to reveal any difference among quinpirole dose-response curves from week 1 through week 7. Administration of L-741,626 (0.32 – 3.2 mg/kg; s.c.) did not increase quinpirole-induced yawning in any group; thus, only data from rats eating standard chow are presented in the upper panel of Figure 3. The largest dose of L-741,626 (3.2 mg/kg) significantly decreased quinpirole-induced yawning in all groups (Figure 3, inverted triangles, rats eating standard chow; data not shown for rats eating high fat chow).
Figure 2.
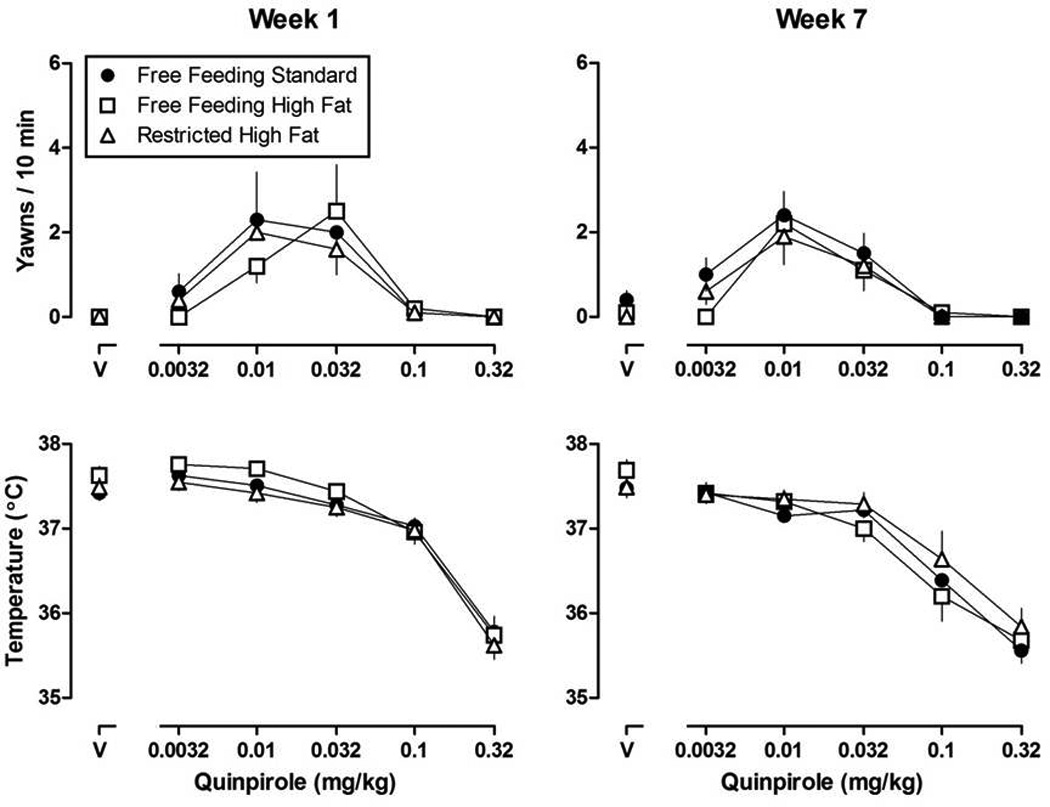
Dose-response curves for quinpirole-induced yawning (upper panels) and quinpirole-induced changes in body temperature (lower panels) during week 1 (left panels) and week 7 (right panel) in rats with free access to standard chow, free access to high fat chow, or restricted access to high fat chow. Vertical axes: upper panels, mean (±SEM) yawns per 10 min; lower panels, body temperature in °C. Horizontal axes: dose of quinpirole in mg/kg body weight (V = vehicle).
Figure 3.
Dose-response curves for quinpirole-induced yawning (upper panels) and quinpirole-induced changes in body temperature (lower panels) during weeks 8–11 in rats eating standard chow; quinpirole was studied alone and in combination with three different doses of L-741,626. Vertical axes: upper panel, mean (±SEM) yawns per 10 min; lower panel, body temperature in °C. Horizontal axes: dose of quinpirole in mg/kg body weight (V = vehicle).
Body Temperature
Quinpirole decreased body temperature in a dose-dependent manner in all rats (maximal average decrease of 2.2 °C), although there was no significant difference in hypothermic effect among groups and no difference within groups from week 1 to week 7 (Figure 2, lower panels). All three doses of L-741,626 attenuated quinpirole-induced hypothermia and that attenuation was not different among the three groups (Figure 3, lower panel, rats eating standard chow; data not shown for rats eating high fat chow). The hypothermic effects of quinpirole were completely attenuated by a dose of 3.2 mg/kg of L-741, 626.
Insulin Sensitivity
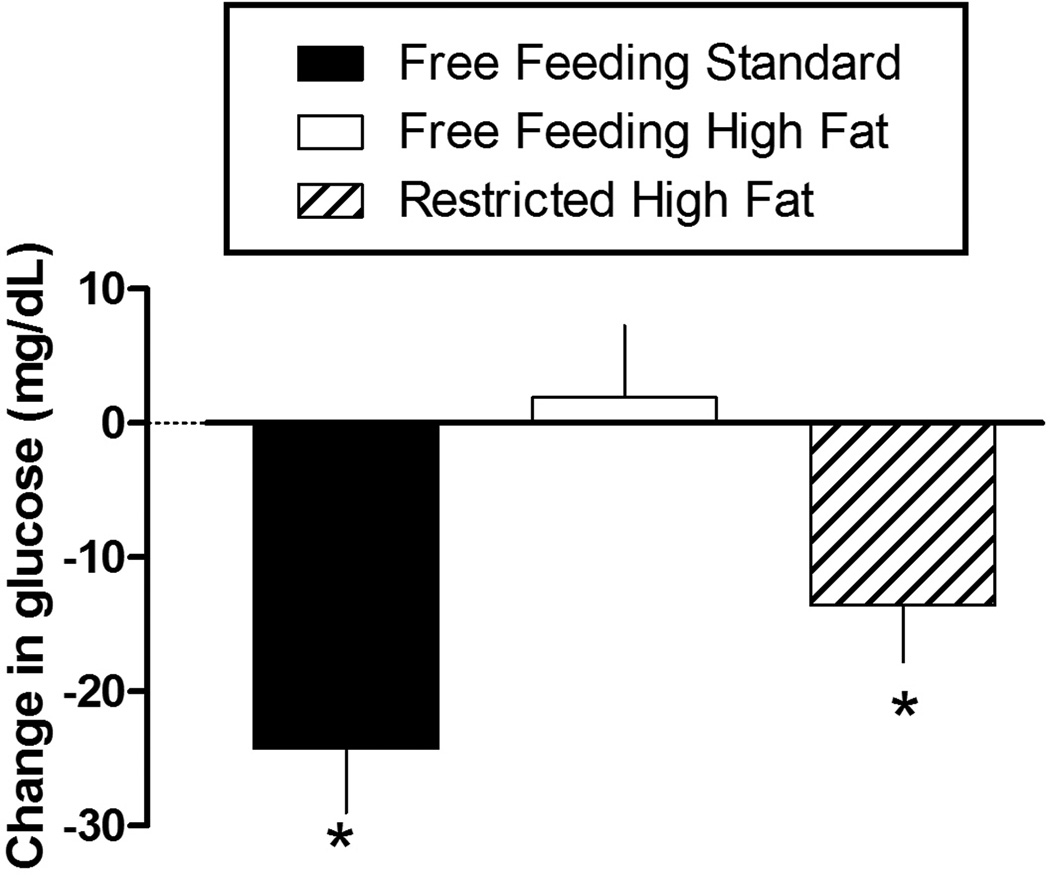
Prior to the administration of insulin, blood glucose concentration was not different among the three groups of rats (data not shown). Maximal decreases in blood glucose were measured 30 min after i.p. administration of 2.0 U/kg insulin. Only the rats with free access to high fat chow for 10 days were insulin resistant (Figure 4). Paired t-tests revealed significant decreases between blood glucose concentrations measured before and 30 min after the administration of insulin for rats with free access to standard chow (t (9) = 4.83; P < 0.001) and rats with restricted access to high fat chow (t (9) = 3.15; P < 0.02) but not for rats with free access to high fat chow (Figure 4). A one-way ANOVA revealed that maximum decreases in blood glucose were significantly different between rats with free access to standard chow and rats with free access to high fat chow (F(2, 27) = 7.42; P < 0.005). There was no difference between rats with restricted access to high fat chow and any other group. Insulin-induced changes in blood glucose concentration measured after 17 and 24 days of eating standard or high fat chow were not different from values shown in Figure 4 (10 days).
Figure 4.
Change in blood glucose concentration (mean ± SEM, milligrams per deciliter; vertical axis) determined after 10 days of free access to standard chow (n = 10), free access to high-fat chow (n = 10), or restricted access to high fat chow (n = 10). * = Significantly different from baseline (prior to insulin) blood glucose concentration.
Locomotor Activity
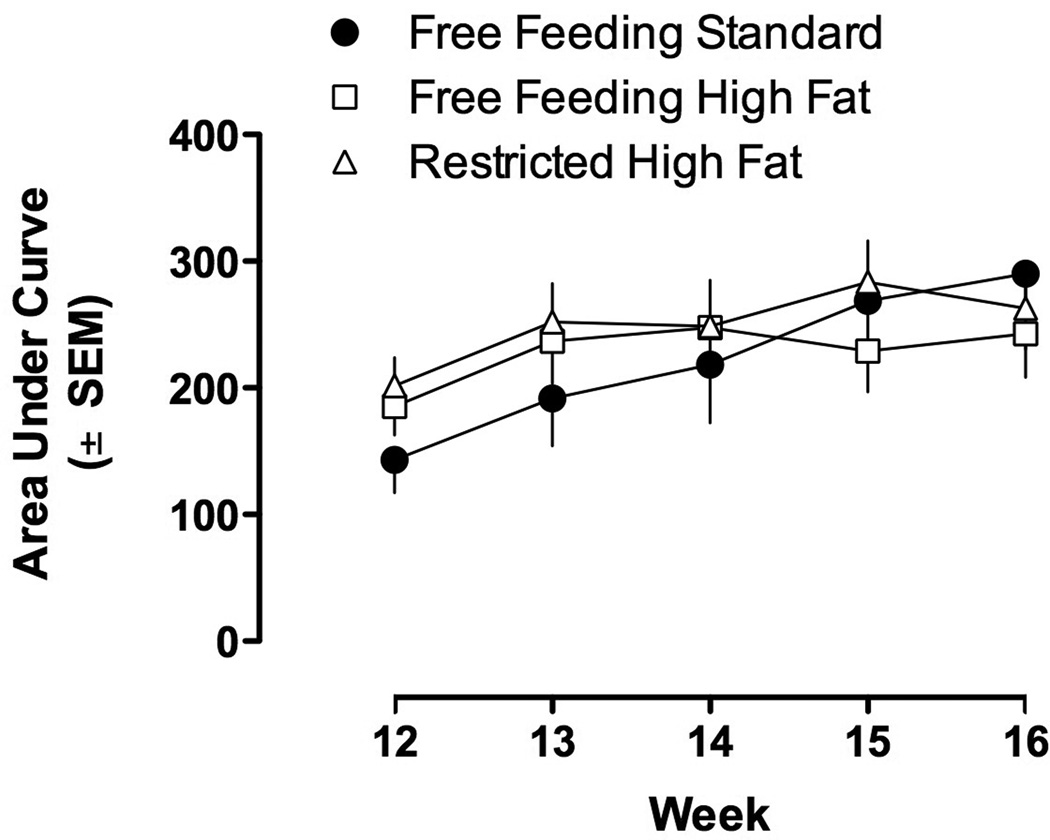
Cocaine dose-dependently increased locomotor activity with maximal increases occurring at 17.8 mg/kg; there was no significant difference in the locomotor-stimulating effects of cocaine among the three groups of rats (Figure 5, upper panel). Once per week testing with cocaine increased sensitivity to cocaine-induced locomotion (i.e. Figure 5, upper and lower panels; Figure 6); however, there was no significant difference in the development of sensitization among groups. Two-way repeated measures ANOVA revealed a significant main effect of week (F(4,27)=13.47, P<0.001). That is, the AUC for cocaine dose-response curves in week 16 were significantly increased as compared with the AUC for cocaine dose-response curves in week 12 (Figure 6); however, there was no difference among groups of rats eating standard chow or high fat chow (free or restricted access).
Figure 5.
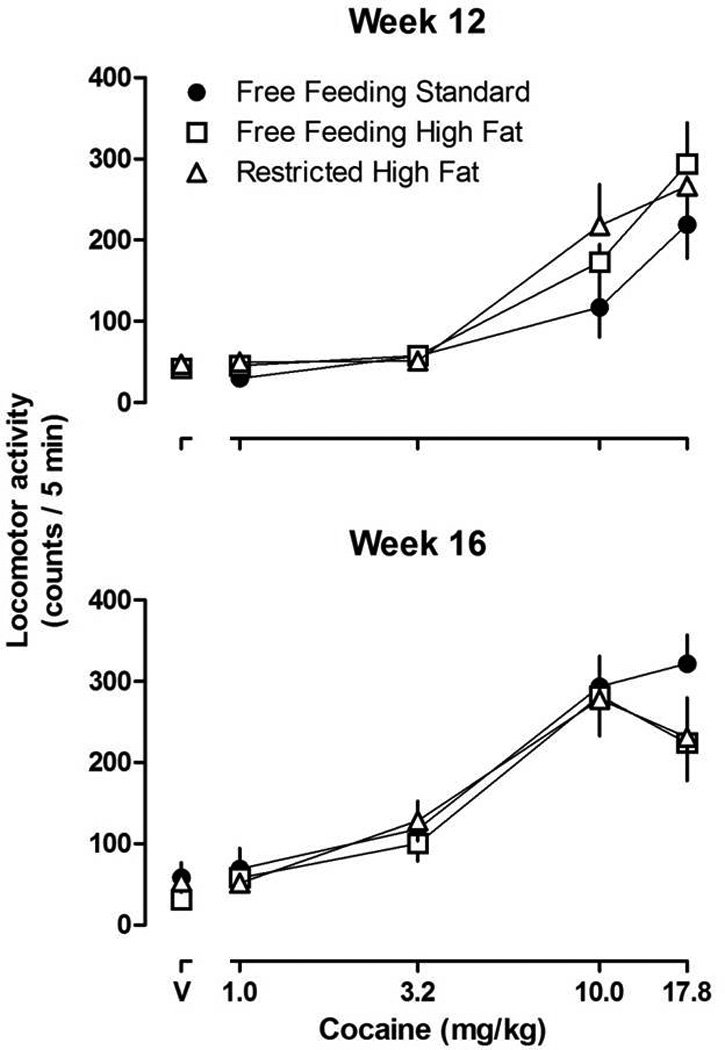
Locomotor activity counts/5 min period after the administration of cocaine in female rats that had eaten standard or high fat chow for 12 or 16 weeks (upper and lower panels, respectively). Vertical axes: mean ± SEM locomotor activity counts / 5 min period. Horizontal axes: dose of cocaine in mg/kg body weight (V = vehicle).
Figure 6.
AUC for cocaine dose-response curves determined once per week during weeks 12–16 in rats with free access to standard chow, free access to high fat chow, or restricted access to high fat chow. Vertical axis: mean (± SEM) AUC. Horizontal axis: week in study.
Discussion
Feeding condition can profoundly impact the sensitivity of male rats to the behavioral effects of drugs acting directly on dopamine receptors (Baladi et al., 2012a); however, the extent to which feeding condition impacts sensitivity of female rats to drugs acting directly on dopamine receptors is not known. This study examined the effects of eating high fat chow on sensitivity of female rats to both direct- (quinpirole) and indirect- (cocaine) acting dopamine receptor agonists. Quinpirole elicited relatively little yawning in female rats, as compared with male rats, with a maximum of 2–4 yawns observed in 10 min. These results are consistent with previous reports using female rats and a different dopamine receptor agonist, apomorphine (Berendsen and Nickolson, 1981). Despite increased weight gain and the development of insulin resistance, quinpirole-induced yawning and hypothermia were not different in female rats eating high fat chow as compared with female rats eating standard chow. After 2 weeks of eating high fat chow, sensitivity to quinpirole is enhanced in insulin-resistant male rats, and after 4 weeks of eating high fat chow, both the ascending and the descending limbs of the quinpirole-induced yawning dose-response curve are shifted upward and to the left (Baladi et al., 2011). After eating high fat chow for nearly twice as long (i.e. 7 consecutive weeks), yawning was not significantly changed in female rats in the current study.
These contrasting results between male (Baladi and France 2009; Baladi et al., 2011) and female rats suggest that females are less sensitive than males to the influence of eating high fat chow on sensitivity to the effects of direct-acting dopamine receptor agonists. However, the lower frequency of drug-elicited yawning in female rats might limit the value of this procedure for examining feeding conditions and sensitivity to drugs. A low frequency of spontaneous and drug-induced yawning in female rats has been reported previously (Berendsen & Nickolson, 1981; Serra et al., 1984; Urba-Holmgren et al., 1990; Diaz-Veliz et al., 1994). Even in rats that were bred selectively for a higher frequency of spontaneous yawning, female rats yawn less than male sub-line counterparts (although yawning was greater in selectively bred female rats as compared to control female rats; Urba-Holmgren et al., 1990).
This difference in frequency of yawning between male and female rats appears to be influenced by sex hormones (Urba-Holmgren et al., 1990; Berendsen and Nickolson 1981; Serra et al., 1984; Diaz-Veliz et al., 1994). For example, apomorphine-induced yawning is similar between intact and ovariectomized female rats and enhanced only when ovariectomy is accompanied by daily injections of dihydrotestosterone (Berendsen and Nickolson 1981). Thus, androgens appear to be necessary for dopamine receptor agonist-induced yawning. When estrous cycle is monitored, more apomorphine-induced yawning is observed during estrous and diestrus; however, the slightly elevated frequency of yawning during these phases never reaches the higher frequency of yawning observed in male rats (Diaz-Veliz et al., 1994). While it is possible that estrous cycle influenced the results in the present report (phase was not monitored), the low frequency of yawning induced by quinpirole was very stable across repeated testing and across individual rats in two separate cohorts, likely spanning multiple phases of the estrous cycle for each individual. In contrast to the striking sex difference in agonist-induced yawning, quinpirole-induced hypothermia was similar in female rats as compared with previous results obtained with male rats (e.g., Baladi et al., 2011). That comparable hypothermic effects are observed with quinpirole in male and female rats suggests that the sex difference in drug-induced yawning is not due to pharmacokinetic factors.
The low frequency of yawning observed in female rats does not appear to be due to an increased sensitivity at dopamine D2 receptors (i.e. the receptors that mediate the descending limb of the quinpirole yawning dose-response curve) since pretreatment with the selective dopamine D2 receptor antagonist L-741,626 did not increase quinpirole-induced yawning. Food restriction significantly decreases agonist-induced yawning in male rats (Baladi et al., 2011; Collins et al., 2008) and that suppression of yawning is reversed by 1.0 mg/kg of L-741,626 (Baladi et al., 2011; Collins et al., 2008), indicating that decreased agonist-induced yawning in food-restricted rats is related to enhanced sensitivity at dopamine D2 receptors. In female rats there was no enhancement of quinpirole-induced yawning by doses of L-741,626 that significantly and completely (3.2 mg/kg) attenuated the hypothermic effects of quinpirole. The present results might suggest that female rats are more sensitive than male rats to L-741,626. A dose of L-741,626 that selectively impacts the D2-mediated descending limb of the quinpirole yawning dose-response curve in male rats (1.0 mg/kg), shifted the ascending limb of the quinpirole dose-response curve to the right in female rats in the present study (Figure 3). Selectivity for D2 over D3 receptors decreases with larger doses of L-741,626, resulting in a similar rightward shift in the ascending limb of the quinpirole dose-response curve in male rats (3.2 mg/kg L-741,626; see Baladi et al., 2010 for an example). Complete attenuation of quinpirole-induced hypothermia in the present study suggests that female rats are more sensitive than male rats to L-741,626 since the same doses of L-741,626 only modestly attenuated quinpirole-induced hypothermia in male rats (Baladi et al., 2010).
In contrast to the striking effect feeding conditions can have on drug effects in male rats, in the present study eating high fat chow did not impact the sensitivity of female rats to the locomotor stimulating effects of cocaine. Age can also influence sensitivity to some behavioral effects of cocaine (Anker and Carroll 2011b). In the current study, rats were 275 days of age on the day locomotor testing began with cocaine. Female rats eating standard chow in the current study were more sensitive to cocaine (Figure 5; upper panel) than younger female adult rats that ate standard chow in a previous study (Baladi et al., 2012b), suggesting that older female rats might be more sensitive to the behavioral effects of cocaine. The impact of eating high fat chow on sensitivity to the behavioral effects of cocaine might also be age-dependent (Baladi et al., 2012b). Taken together with previous studies, the present results suggest that, while adolescent female rats are more sensitive than adult female rats to the effects of eating high fat chow on sensitivity to cocaine-induced locomotion and sensitization (Baladi et al., 2012b), with increasing age (i.e., PND 275 versus 75) sensitivity of female rats to cocaine might become less affected by feeding conditions.
Another potentially important factor for the current study is the duration that rats ate high fat chow (Baladi et al., 2011, 2012a). Sensitivity to cocaine-induced locomotion and sensitization has been shown to occur after 4 weeks of eating high fat chow (Baladi et al., 2012b). In the present experiment, testing with cocaine was preceded by 11 consecutive weeks of eating high fat chow. It is possible that enhancement of the effects of cocaine in rats eating high fat chow (Baladi et al., 2012b) occurs during the early but not the later stages of development and, therefore, that there is a critical window of vulnerability when feeding condition can most dramatically impact the effects of cocaine.
That eating high fat chow did not impact sensitivity to the behavioral effects of cocaine in the present study might also be a function of the specific drug history of the animals prior to cocaine testing. Specifically, repeated testing with quinpirole might increase sensitivity of rats to cocaine-induced locomotion, regardless of feeding condition, thereby limiting further increases in sensitivity that might occur as a consequence of eating high fat chow. There are reports that repeated administration of direct-acting dopamine receptor agonists does not enhance sensitivity to the behavioral effects of indirect-acting dopamine receptor agonists (Mattingly et al., 2001; Cope et al., 2010); however, repeated administration of the indirect-acting agonist cocaine can increase sensitivity to the behavioral effects of the direct-acting agonist quinpirole (Collins and Woods 2008; Blaylock et al., 2011). The conditions under which sensitization develops to the effects of drugs acting on dopamine systems are highly dependent upon the specific parameters used (Emmett-Oglesby 1995). Although a drug-naïve control group was not included in the present experiment, cocaine-induced locomotion in female rats with a history of once weekly quinpirole testing appears to be greater than the effects reported for drug-naïve female rats in a previous study (Baladi et al., 2012b).
Previous studies showed that eating high fat chow enhances sensitivity of male rats to quinpirole-induced yawning; the results of the current study suggest that this effect does not occur in female rats. Previous studies also showed that eating high fat chow enhances sensitivity of female rats to cocaine-induced locomotion and sensitization; the failure of the current study to replicate those findings suggests that other factors (e.g., age and/or drug history) influence the extent to which feeding conditions alter sensitivity to cocaine. The impact of diet, age, sex, and drug history on sensitivity to drugs acting on dopamine systems could have important implications for both the therapeutic effectiveness of drugs and for vulnerability to abuse drugs. Given that female rats are more sensitive than male rats to cocaine (Craft and Stratmann 1996; Chin et al., 2001; Lynch and Carroll 1999), future investigations should consider drug history and feeding condition as well as other factors (e.g., age) that contribute to differences in drug sensitivity among females.
Acknowledgements
CPF is supported, in part, by United States Public Health Service Grant K05DA17918 from the National Institute on Drug Abuse, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Disclosure/conflict of interest: The authors have no conflict of interest.
References
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011a;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology. 2011b;215:785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur J Pharmacol. 2009;610:55–60. doi: 10.1016/j.ejphar.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. Eating high-fat chow increases the sensitivity of rats to quinpirole-induced discriminative stimulus effects and yawning. Behav Pharmacol. 2010;21:615–620. doi: 10.1097/FBP.0b013e32833e7e5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Dopamine D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats. J Pharmacol Exp Ther. 2010;322:308–315. doi: 10.1124/jpet.109.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Influence of body weight and type of chow on the sensitivity of rats to the behavioral effects of the direct-acting dopamine-receptor agonist quinpirole. Psychopharmacology. 2011;217:573–585. doi: 10.1007/s00213-011-2320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Daws LC, France CP. You are what you eat: influence of type and amount of food consumed on central dopamine systems and the behavioral effects of direct- and indirect-acting dopamine receptor agonists. Neuropharmacology. 2012a;63:76–86. doi: 10.1016/j.neuropharm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Koek W, Aumann M, Velasco F, France CP. Eating high fat chow enhances the locomotor-stimulating effects of cocaine in adolescent and adult female rats. Psychopharmacology. 2012b;222:447–457. doi: 10.1007/s00213-012-2663-7. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Nickolson VJ. Androgenic influences on apomorphine-induced yawning in rats. Behav Neural Biol. 1981;33:123–128. doi: 10.1016/s0163-1047(81)92306-2. [DOI] [PubMed] [Google Scholar]
- Blaylock BL, Gould RW, Banala A, Grundt P, Luedtke RR, Newman AH, Nadar MA. Influence of cocaine history on the behavioral effects of dopamine D(3) receptor-selective compounds in monkeys. Neuropsychopharmacology. 2011;36:1104–1113. doi: 10.1038/npp.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Fletcher H, Perrotti Ll, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral sensitization. Cell Mol Biol (Noisy-le-grand) 2001;47:1089–1095. [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N'-propyl–4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Truong YN, Levant B, Chen J, Wang S, Woods JH. Behavioral sensitization to cocaine in rats: evidence for temporal differences in dopamine D3 and D2 receptor sensitivity. Psychopharmacology. 2011;215:609–620. doi: 10.1007/s00213-010-2154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope Z, Huggins KN, Sheppard AB, Noel DM, Roane DS, Brown RW. Neonatal quinpirole treatment enhances locomotor activation and dopamine release in the nucleus accumbens core in response to amphetamine treatment in adulthood. Synapse. 2010;64:289–300. doi: 10.1002/syn.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA. Discriminative stimulus effects of cocaine in female versus male rats. Drug Alcohol Depend. 1996;42:27–37. doi: 10.1016/0376-8716(96)01259-8. [DOI] [PubMed] [Google Scholar]
- Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011;61:1123–1129. doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Veliz G, Baeza R, Benavente F, Dussaubat N, Mora S. Influence of the estrous cycle and estradiol on the behavioral effects of amphetamine and apomorphine in rats. Pharmacol Biochem Behav. 1994;49:819–825. doi: 10.1016/0091-3057(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Durham HA, Truett GE. Development of insulin resistance and hyperphagia in Zucker fatty rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R652–R658. doi: 10.1152/ajpregu.00428.2004. [DOI] [PubMed] [Google Scholar]
- Emmett-Oglesby MW. Sensitization and tolerance to the motivational and subjective effects of psychostimulants. In: Hammer RP, editor. The Neurobiology of Cocaine: Cellular and Molecular Mechanisms. Boca Raton: CRC Press; 1995. pp. 31–47. [Google Scholar]
- Liu S, Labouebe G, Karunakaran S, Clee SM, Borgland SL. Effect of insulin on excitatory synaptic transmission onto dopamine neurons of the ventral tegmental area in a mouse model of hyperinsulinemia. Nutr Diabetes. 2013;3:e97. doi: 10.1038/nutd.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 2013;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Caudill A, Abel M. Differential effects of 7-OH-DPAT on the development of behavioral sensitization to apomorphine and cocaine. Pharmacol Biochem Behav. 2001;68:417–426. doi: 10.1016/s0091-3057(00)00471-8. [DOI] [PubMed] [Google Scholar]
- Serafine KM, France CP. Restricted access to standard or high fat chow alters sensitivity of rats to the 5-HT2A/2C receptor agonist 1-(2,5-dimethoxy–4-methylphenyl)-2-aminopropane. Behav Pharmacol. 2014;25:44–52. doi: 10.1097/FBP.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra G, Collu M, Serra A, Gessa GL. Estrogens antagonize apomorphine-induced yawning in rats. Eur J Pharmacol. 1984;104:383–386. doi: 10.1016/0014-2999(84)90418-7. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs in male rats. Eur J Pharmacol. 2008;592:109–115. doi: 10.1016/j.ejphar.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urba-Holmgren R, Trucios N, Holmgren B, Eguibar JR, Gavito A, Cruz G, Santos A. Genotypic dependency of spontaneous yawning frequency in the rat. Behav Brain Res. 1990;40:29–35. doi: 10.1016/0166-4328(90)90039-h. [DOI] [PubMed] [Google Scholar]