Abstract
Importance
Retrospective studies have demonstrated disparate outcomes following acute optic neuritis in individuals of African descent compared with Caucasians. However, published analyses of the prospectively collected Optic Neuritis Treatment Trial (ONTT) data did not identify an association between worse visual outcomes and African race.
Objective
To investigate the association among race, gender, and age with visual outcomes following acute optic neuritis through application of longitudinal data analysis techniques to the ONTT data set.
Design
Secondary analysis of the ONTT (a prospective, randomized, controlled trial) data set. Our models included effects of treatment (placebo, oral prednisone, IV methylprednisolone), time and treatment-time interaction as well as demographic covariates of age, gender and race.
Setting
ONTT data were collected at multiple centers in the United States.
Participants
Black(n=58) and White(n=388) subjects presenting with acute optic neuritis who enrolled in the ONTT within 8 days of symptom onset were included in analyses.
Main outcome measures
logMAR visual acuity (VA) and contrast sensitivity (CS) in the affected eye were modeled using two-stage mixed effect regression techniques. All available follow-up data from baseline to 15–18 years were included.
Results
The data did not identify a relationship of age, gender, or treatment groups with VA or CS outcomes. Race was significantly related to CS (p<0.001) and VA (p<0.001) over a 15 year period following acute optic neuritis with black race being associated with worse scores for both.
Conclusions and Relevance
Race appears to be associated with contrast sensitivity and visual acuity outcomes in affected eyes following acute optic neuritis. To our knowledge, this is the largest cohort of black subjects with optic neuritis that has been analyzed, and the first evidence from a prospectively collected data set that supports a hypothesis of race-dependent visual outcomes in optic neuritis.
Keywords: optic neuritis, outcomes, vision, race, longitudinal analysis
Introduction
Though most individuals recover excellent vision after optic neuritis, some affected individuals have poor visual recovery that limits independence and reduces quality of life. Current management strategies, based on population studies, do not account for this outcome heterogeneity. Improved understanding of prognostic factors is necessary to tailor treatment of this potentially blinding condition. In the landmark optic neuritis treatment trial (ONTT), 7% of subjects had vision worse than 20/50 and 3% had vision worse than 20/200 after 1 year1. Age and baseline visual acuity were significantly associated with visual outcome at 6 months2. In this prospective randomized treatment trial, race and ethnicity were not associated with visual outcome using cross-sectional analysis techniques at 6 months, 5 years, 10 years and 15 years after acute optic neuritis3–5. However, in a retrospective case series from two hospitals in Atlanta, black patients (n=33) had worse visual outcomes than white patients (n=63) with 36% of black patients seeing less than 20/50 and 18% less than 20/200 compared with 8% and 5% of white patients respectively at 1 year6. In a small retrospective study of black subjects in South Africa, 9 of 10 patients had vision below 20/200 at last follow up, suggesting that poor visual outcomes in black individuals may be a global phenomenon7.
One possible explanation for the lack of an association between race/ethnicity with visual outcomes in the ONTT is the sensitivity of the analysis techniques employed. Cross sectional visual outcomes were compared at each time point in the study;3–5 however, these do not capture change over time in individuals. Longitudinal data analysis techniques are more efficient than cross sectional analyses because they model individual changes as well as differences between groups and are less susceptible to missing data8. We sought to further investigate demographic associations with visual outcomes following optic neuritis through application of longitudinal data analysis techniques to the ONTT data set to test the hypothesis that visual outcomes following optic neuritis are associated with race.
Methods
We performed a longitudinal analysis on the prospectively collected data set from the North American Optic Neuritis Treatment Trial (ONTT), which was obtained from http://lons.jaeb.org/. Our study of these publicly available, existing data that are recorded such that subjects cannot be identified is exempt from institutional review board review under U.S. Department of Health and Human Services regulations. Full study details are provided elsewhere9. Briefly, 457 subjects were enrolled who presented with a first episode of optic neuritis in the affected eye. Major trial inclusion criteria were age 18–45 years and a clinical exam consistent with acute unilateral optic neuritis with symptoms starting within 8 days prior to enrollment. Major exclusion criteria were a prior episode of optic neuritis in the affected eye and evidence of systemic disease other than multiple sclerosis that can be associated with optic neuritis. The Investigational Review Board at each of 15 clinical centers approved the study and participants signed an informed consent form prior to enrollment. Participants were randomized to one of three treatment arms (intravenous steroids, oral steroids and placebo) and followed for outcomes of vision and development of multiple sclerosis. The primary vision outcome was contrast sensitivity (CS), measured using Pelli-Robson chart steps (Range 0–16 triplets of letters correctly identified, where 16 is best). Secondary visual outcomes included visual acuity (VA), measured with Snellen retro-illuminated EDTRS charts and converted to logMAR (0 equivalent to 20/20, < 0 is better, > 0 is worse). Demographic variables of age, gender and race/ethnicity (white, black, Asian or Hispanic) were recorded based on self-report. Follow up visits occurred 8 times in the first year, then annually through 10 years with a final follow up at 15–18 years. 438 subjects (96%) completed the 6 month examination, which was the primary endpoint2. Subsequent published analyses were based on 5–8 year follow up (397 subjects, 87% retention)3, 10–14 year follow up (319 subjects, 70% retention)4, and 15–18 year follow up (294 subjects, 65% retention)5.
Data on black and white ONTT subjects were included in our analysis. First, demographic and clinical factors were compared between black and white groups using two sample t-test/Mann-Whitney and Chi-square test. Contrast sensitivity and visual acuity (logMAR) were modeled as continuous outcomes using mixed effects linear models. All available follow up data were included. Time was represented by visit number in order to discriminate early change in visual outcomes typical of optic neuritis. Multiple models were run, with different sets of potential confounders, to explore effects of treatment, time, age, gender and race on visual outcomes following optic neuritis. Pairs of significant variables were multiplied at each time point to generate interaction variables. These were included as terms in the models to explore combined effects of potential confounders. Models were calculated for follow up of 0 to 8 years, 0 to 10–14 years and 0 to 15–18 years to determine effect of declining sample size on model precision. Models were analyzed using SAS version 9.2 (SAS Institute Inc.). P values less than 0.05 were considered significant.
Results
58 black and 388 white subjects from the ONTT database met criteria for analysis (Table 1). Subjects of other races (Asian n=7, Hispanic n=2) and subjects found to have compressive optic neuropathies (n=2) were excluded. Neither black nor white subjects with 5–8 year follow up differed from those without 5–8 year follow up with regards to gender, age or baseline visual function (Table e-1 (on-line only)). White subjects with 10–14 year and 15–18 year follow up were older than those without follow up (p=0.018, 0.046, t-test for independent samples). Black subjects with 10–14 year follow up did not differ from those without follow up. Black subjects with 15–18 year follow up were younger than those without follow up (p=0.041, t-test for independent samples). Subjects lost to follow up at 10–14 years had worse baseline visual acuity than those with follow up (p=0.027 t-test for independent samples). This difference was not significant within race groups and was not observed at 15–18 year follow up.
Table 1.
Demographic and Clinical factors of Study Subjects by Race
| White (n=388) |
Black (n=58) |
p | |
|---|---|---|---|
| Female gender (number (%)) | 297 (76.5%) | 47 (81.0%) | 0.45* |
| Age (mean +/−SD years) | 32.2 +/− 6.7 | 30.5 +/− 6.2 | 0.082** |
| Affected eye at enrollment | |||
| Contrast sensitivity (mean +/− SD PR steps) | 8.2 +/− 4.7 | 5.3 +/− 4.9 | <0.0001** |
| Visual acuity (mean +/− SD logMAR) | 0.69 +/− 0.65 | 1.09 +/− 0.63 | <0.0001** |
| Follow up time (median years (median visit)) | 16 (17) | 11.1 (16) | <0.0001*** |
| Follow up visual outcome data complete | |||
| 6 mo completed (visit 7) (number (%)) | 376 (96.9%) | 53 (91.4%) | 0.04* |
| 5–8 year CS completed (visit 12–15) (number (%)) | 335 (86.3%) | 38 (65.5%) | <0.0001* |
| Female gender | 261 (77.9%) | 33 (86.8%) | 0.20* |
| Age | 32.4 +/− 6.8 | 30.3 +/− 6.6 | 0.07** |
| CS at enrollment | 8.0 +/− 4.7 | 5.26 +/− 4.8 | <0.0001** |
| VA at enrollment | 0.7 +/− 0.66 | 1.08 +/− 0.64 | <0.0001** |
| 10–14 year CS completed (visit 16)(number (%)) | 245 (63.1%) | 29 (50%) | 0.06* |
| 15–18 year CS completed (visit 17)(number (%)) | 196 (50.5%) | 22 (37.9%) | 0.07* |
Chi square test
t-test for independent samples
Mann-Whitney (Wilcoxon rank sum test)
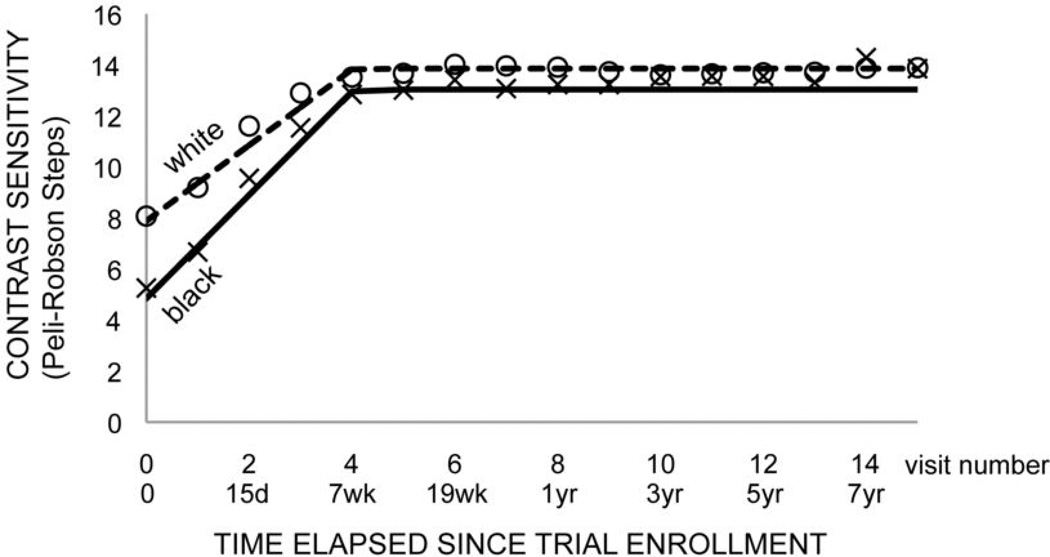
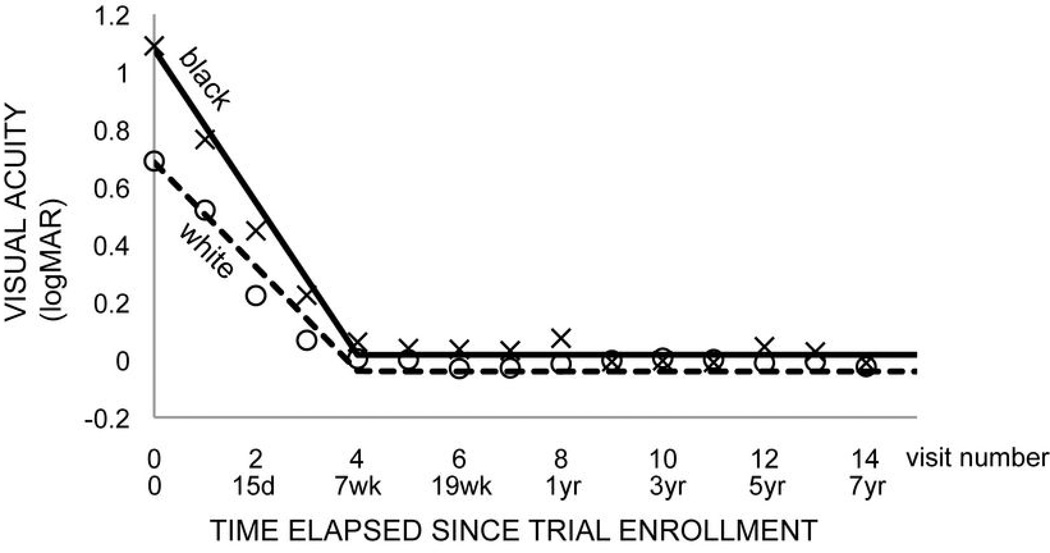
A two-stage model (with different time coefficients for visits 1–4 (stage 0) and 4–17 (stage 1)) provided the best fit for both contrast sensitivity and visual acuity outcomes with transition from time-dependent (i.e. improving) to time-independent (i.e. stable) outcome at visit 4 (7 weeks) (Fig. 1,2). Model results for maximum 15–18 year and 8 year follow up were statistically identical, though the 8 year follow up models had greater precision in coefficient estimates due to larger sample sizes at the later time points. We chose to present the models with up to 8 years (15 visits) of follow up for this reason.
Figure 1. Final model of contrast sensitivity versus time.
Two stage mixed model for black (solid) and white (dashed) populations (model 5, Table 2). Markers indicate mean contrast sensitivity at each visit for black (x) and white (circle) ONTT subjects.
Figure 2. Final model of visual acuity versus time.
Two stage mixed model for black (solid) and white (dashed) populations (model 5, Table 3). Markers indicate mean contrast sensitivity at each visit for black (x) and white (circle) ONTT subjects.
The simplest model of contrast sensitivity (CS) did not identify a relationship between treatment, interaction effects of treatment and time or stage and CS (Table 2, model 1). The data did not identify a relationship between age (p=0.27) or gender (p=0.19) and CS. Race was significantly related to CS (p=0.009) with black race being associated with worse (lower) scores (Table 2, model 2). The race effect persisted in a model that incorporated age, gender and race (p=0.005) (Table 2, model 3). There were no significant interaction effects for race and treatment, which means that the association between race and CS was not different for different treatments (Table 2, model 4). There were significant interactions between race and time (p<0.0001), and race and stage (p<0.0001), which means that the association between race and CS were different according to time and stage (Table 2, model 5).
Table 2.
Mixed effects two-stage linear models of contrast sensitivity from 0 to 8 years following optic neuritis
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 (final) | |
|---|---|---|---|---|---|
| Time (visit 1,2,3,4) | 1.56 (1.47–1.65)∝ | 1.56 (1.47–1.65) ∝ | 1.56 (1.47–1.65) ∝ | 1.56 (1.47–1.65) ∝ | 1.49 (1.39–1.58) ∝ |
| Stage (0 = visits 1–4, 1 = visit 5 onward) | −1.52 (−1.62–−1.43)∝ | −1.52 (−1.62–−1.43) ∝ | −1.52 (−1.62–−1.43) ∝ | −1.52 (−1.62–−1.43) ∝ | −1.46 (−1.55–−1.36) ∝ |
| Treatment (IV vs. placebo) | 0.58 (−0.27–1.43) | 0.61 (−0.23–1.45) | 0.62 (−0.21–1.46) | 0.63 (−0.24–1.5) | 0.66 (−0.17–1.49) |
| Treatment (PO vs. placebo) | 0.32 (−0.52–1.17) | 0.27 (−0.57–1.10) | 0.27 (−0.56–1.10) | 0.25 (−0.60–1.11) | 0.13 (−0.70–0.95) |
| Treatment * time (IV vs. placebo) | −0.05 (−0.18–0.07) | −0.05 (−0.18–0.07) | −0.05 (−0.18–0.07) | −0.05 (−0.18–0.07) | −0.06 (−0.19–0.06) |
| Treatment * time (PO vs. placebo) | −0.08 (−0.21–0.04) | −0.08 (−0.21–0.04) | −0.08 (−0.21–0.04) | −0.08 (−0.21–0.04) | −0.05 (−0.17–0.07) |
| Treatment * stage (IV vs. placebo) | −0.05 (−0.18–0.09) | −0.05 (−0.18–0.09) | −0.05 (−0.18–0.09) | −0.05 (−0.18–0.09) | −0.04 (−0.17–0.10) |
| Treatment * stage (PO vs. placebo) | −0.02 (−0.16–0.11) | −0.02 (−0.16–0.11) | −0.02 (−0.15–0.11) | −0.02 (−0.15–0.11) | −0.05 (−0.18–0.08) |
| Race (B=1, W=0) | −0.87 (−1.54–−0.20) ∂ | −0.93 (−1.60–−0.26) ∂ | −0.87 (−1.99–0.26) | −3.04 (−4.05–−2.03) ∝ | |
| Gender (F=1, M=0) | 0.36 (−0.17–0.89) | ||||
| Age (years) | −0.02 (−0.05–0.01) | ||||
| Race * treatment (IV vs. placebo) | −0.13 (−1.66–1.40) | ||||
| Race * treatment (PO vs. placebo) | −0.20 (−1.59–1.99) | ||||
| Race * time | 0.54 (0.39–0.70) ∝ | ||||
| Race * stage | −0.50 (−0.67–−0.33) ∝ | ||||
Each cell contains the coefficient estimate indicating the amount contrast sensitivity improves (by triplet steps) for each increment in variable (95% CI).
indicates interaction term between two variables.
= p<0.0001,
= p<0.01, B=black, W=white, F=female, M=male
The simplest model of visual acuity (VA) did not identify a relationship between treatment or interaction effects of treatment and time and VA (Table 3, model 1). The data did not identify a relationship between age (p=0.77) or gender (p=0.87) and VA. Race was significantly related to VA (p=0.03) with black race being associated with worse (higher) VA (Table 3, model 2). The race effect persisted in a model that incorporated age, gender and race (Table 3, model 3). There were no significant interaction effects for race and treatment (Table 3, model 4). There were significant interactions between IV treatment and time (p=0.04), race and time (p<0.0001) and race and stage (p<0.0001) (Table 3, model 5).
Table 3.
Mixed effect two-stage linear models of visual acuity from 0 to 8 years following optic neuritis
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 (final) | |
|---|---|---|---|---|---|
| Time (visit 1,2,3,4) | −0.19 (−0.21–−0.18)∝ | −0.19 (−0.21–−0.18)∝ | −0.19 (−0.21–−0.18)∝ | −0.19 (−0.21–−0.18)∝ | −0.18 (−0.19–−0.17)∝ |
| Stage (0 = visits 1–4,,1= visit 5 onward) | 0.19 (0.18–0.20)* | 0.19 (0.18–0.20)* | 0.19 (0.18–0.20)* | 0.19 (0.18–0.20)∝ | 0.18 (0.17–0.19)∝ |
| Treatment (IV vs. placebo) | −0.09 (−0.20–0.03) | −0.09 (−0.21–0.02) | −0.09 (−0.20–0.02) | −0.10 (−0.22–0.02) | −0.10 (−0.21–0.02) |
| Treatment (PO vs. placebo) | −0.01 (−0.12–0.10) | −0.003 (−0.12–0.11) | −0.004 (−0.12–0.11) | −0.01 (−0.13–0.10) | 0.02 (−0.10–0.13) |
| Treatment * time (IV vs. placebo) | 0.02 (−0.00–0.03) | 0.02 (−0.00–0.03) | 0.02 (−0.00–0.03) | 0.02 (−0.00–0.03) | 0.02 (0.00–0.04)◦ |
| Treatment * time (PO vs. placebo) | 0.01 (−0.01–0.03) | 0.01 (−0.01–0.03) | 0.01 (−0.01–0.03) | 0.01 (−0.01–0.03) | 0.00 (−0.01–0.02) |
| Treatment * stage (IV vs. placebo) | −0.01 (−0.02–0.01) | −0.01 (−0.02–0.01) | −0.01 (−0.03–0.01) | −0.01 (−0.02–0.01) | −0.01 (−0.03–0.01) |
| Treatment *stage (PO vs. placebo) | 0.003 (−0.02–0.02) | 0.003 (−0.02–0.02) | 0.01 (−0.01–0.03) | 0.01 (−0.02–0.02) | 0.01 (−0.01–0.03) |
| Race (B=1, W=0) | 0.09 (0.01–0.17)◦ | 0.09 (0.01–0.18)◦ | 0.04 (−0.10–0.18) | 0.39 (0.26–0.53)∝ | |
| Gender (F=1, M=0) | −0.01 (−0.07–0.06) | ||||
| Age (years) | 0.001 (−0.00–0.01) | ||||
| Race * treatment (IV vs. placebo) | 0.07 (−0.12–0.27) | ||||
| Race * treatment (PO vs. placebo) | 0.08 (−0.15–0.30) | ||||
| Race * time | −0.08 (−0.11–−0.06)∝ | ||||
| Race * stage | 0.09 (0.06–0.11)∝ | ||||
Each cell contains the coefficient estimate indicating the amount VA worsens for each increment in that variable (95% CI).
indicates interaction term between two variables.
p<0.0001,
= p<0.05, B=black, W=white, F=female, M=male
Comment
The Optic Neuritis Treatment Trial is a landmark study in neuro-ophthalmology, hailed for its prospective nature, high enrollment and excellent follow up. From it we have learned much about visual outcomes following optic neuritis and the association with multiple sclerosis. A conflict exists between published analyses of the ONTT and retrospective studies regarding demographic associations with optic neuritis visual outcomes, particularly with regards to race and ethnicity. We applied longitudinal data analysis techniques to the ONTT data set in order to further define demographic associations, and identified a statistically significant association between black race and visual outcomes. Strengths of our analysis include 1) the use of a prospectively collected data set, which is less prone to selection bias than retrospective data; 2) a data set containing the largest cohort of black subjects that has been reported for optic neuritis; and. 3) the use of efficient, longitudinal analysis statistical techniques.
Black race appears to be associated with worse contrast sensitivity (CS) and visual acuity (VA) outcomes in the affected eye from 0 to 15 years following optic neuritis. Black subjects had worse vision at baseline as demonstrated by a significant race term in the models. The trajectory of vision is associated with race during the early recovery period such that black subjects recovered vision faster. This is demonstrated by the significance of the race*time variable in the models. Our models of the ONTT data set demonstrate that though black subjects had worse vision at onset and, and recovered at a faster rate than white patients, their final vision remained worse than that of white subjects. The magnitude of the effect is within the range of significant changes based on the variability of the Pelli-Robson test10 and is clinically significant due to correlations between CS and vision associated quality of life. There was not a differential treatment response associated with race for the acute treatments studied (i.e. the race*treatment variable was not significant in the models). The reasons for the race associations with visual function both at the time of optic neuritis diagnosis and during long term follow up are not known. Possibilities include biological differences in disease, as well as sociocultural differences such as health care seeking behavior11,12 or differences in healthcare access and barriers. Further study is necessary to determine the relative contributions of each and appropriate interventions to improve outcomes in all racial groups.
We attribute our statistically significant differences in visual outcomes according to race where the initial ONTT analysis did not find differences to the use of more efficient data analysis techniques. In addition to the new finding regarding race association with visual outcome, our longitudinal analysis captures previous conclusions from the primary ONTT analysis. We found expected associations with time and interaction between treatment and time. Our models showing linear improvement in vision as a function of time until visit 4 (7 weeks) with plateau following this as well as differences in the vision time relationship depending on treatment. We did not find an association between age and visual outcome, which was reported in cross sectional analysis of the 6 month ONTT outcomes, but not at 5, 10 or 15 year follow ups3–5. This illustrates the value of longitudinal analysis to capture overall trends. We also did not find an association with gender, which is in line with prior ONTT analyses.
Though our analysis supports the hypothesis of a race association with optic neuritis vision outcomes proposed by retrospective studies, this does not account for the difference in the proportion of subjects of black race with poor vision at one year in the ONTT (3% less than 20/200 at one year) versus the Atlanta case series (18% less than 20/200 at one year)6. This may reflect differences in patient selection between the two studies, emphasizing the complementary roles for randomized trials and observational cohorts in studying the epidemiology of visual outcomes. Both the case series and prospective randomized study are likely to have selection bias due to differences in eye care seeking behavior based on insurance, socioeconomic status, race and severity of symptoms, and this is also likely to affect follow up11,12. There is also likely a degree of selection bias in the ONTT caused by the tendency for minorities to be under represented in clinical trials13. The ONTT prospective data set does not capture potential covariates such as socioeconomic status and insurance status. The Atlanta case series captured this in a preliminary manner by comparing African American patients from an inner city hospital with those from a tertiary referral practice and showed no difference in vision at presentation or after one year of follow up between sites6. However, insurance and socioeconomic status on an individual patient level was not analyzed. Further study is necessary to determine if these variables play a confounding role in the association between race and optic neuritis visual outcomes.
Demyelinating disease is another important outcome following optic neuritis. Retrospective studies have demonstrated associations between race and development of neuromyelitis optica (NMO), but not multiple sclerosis (MS). This distinction is important given evidence that NMO associated optic neuritis is associated with worse visual outcomes14, worse optic nerve injury15, and may respond differentially to treatment16. The retrospective case series from Atlanta that demonstrated worse visual outcomes after optic neuritis in African American patients also suggested racial disparity in development of NMO following optic neuritis6. 21% and 15% of black patients developed NMO and MS respectively following optic neuritis, compared with 5% and 15% of white patients. However, this analysis was performed prior to updated NMO diagnostic criteria17 and did not report anti-aquaporin 4 testing. A British study of 64 patients with optic neuritis found that 33% of African heritage subjects versus 10% of white subjects had optic neuritis associated with a steroid requiring condition, such as NMO, the diagnosis of which was based on positive serum testing for aquaporin 4 antibodies18. NMO has emerged as a distinct demyelinating disease since the inception of the ONTT and therefore was not captured as distinct from MS in this trial. Thus we were unable to extend our analysis to this important question.
Limitations of our study include definition of race by self report. While self report of race has demonstrated an association with predominant genetic ancestry in multiple studies, it is generally not associated with genetic admixture, a potentially important variable19,20. In addition, we did not analyze other, potentially important demographic variables such as socioeconomic status and insurance status, as these were not included in the ONTT data set. Another limitation is the use of an older data set, although we feel the ONTT is still relevant since it remains the landmark and most recent study regarding treatment of optic neuritis, and treatment patterns have not changed in the interim.
Through application of longitudinal data analysis techniques to the ONTT, we find race to be associated with contrast sensitivity and visual acuity outcomes in the affected eye at all time points up to 15 years following optic neuritis. To our knowledge, this is largest cohort of black subjects with optic neuritis to be studied and the first evidence from a prospectively collected data set to support a hypothesis of race dependent outcomes of optic neuritis. These results have implications for understanding prognostic determinants of optic neuritis outcomes.
Supplementary Material
Acknowledgements
Financial support: NIH K12 EY02147 (design & conduct of the study; collection, analysis, and interpretation of the data; preparation, review or approval of the manuscript), NIH UL1TR000050 (collection, analysis, and interpretation of the data; preparation, review or approval of the manuscript) NIH P60MD003424 and NIH P60 MD003424-02S1 (design & conduct of the study; collection, analysis, and interpretation of the data; preparation, review or approval of the manuscript).
Footnotes
Author contributions: Study design (HM, LB, CJ), Analysis (HM, WG, CJ), interpretation of the data (HM, WG, LB, CJ), preparation of the manuscript (HM), revision of the manuscript (HM, WG, LB, CJ). Dr. Moss had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest disclosures: Dr. Moss, Ms. Gao and Dr. Joslin report no conflicts of interest. Dr. Balcer has received consulting fees and speaking honoraria for work to develop visual function and OCT outcomes for MS trials from Biogen-Idec, Questcor and Accorda
A preliminary version of this analysis was presented at the North American Neuro-ophthalmology Society 2013 Annual Meeting (Snowbird, UT; February, 2013)
Contributor Information
Heather E. Moss, Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago School of Medicine (M/C 648), 1855 W. Taylor St, 3.158, Chicago, IL 60612, 312-996-9120, F 312-413-7895, hemoss@uic.edu.
Weihua Gao, Center for Clinical and Translational Research, University of Illinois at Chicago.
Laura J. Balcer, Department of Neurology, New York University School of Medicine.
Charlotte E Joslin, Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago School of Medicine; Division of Epidemiology and Biostatistics, University of Illinois at Chicago School of Public Health.
References
- 1.Beck RW, Cleary PA, Anderson MM, Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326(9):581–588. doi: 10.1056/NEJM199202273260901. [DOI] [PubMed] [Google Scholar]
- 2.Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis. Experience of the Optic Neuritis Treatment Trial. Ophthalmology. 1994;101(11):1771–1778. doi: 10.1016/s0161-6420(94)31103-1. [DOI] [PubMed] [Google Scholar]
- 3.Optic Neuritis Study Group. Visual function 5 years after optic neuritis: experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1997;115(12):1545–1552. [PubMed] [Google Scholar]
- 4.Beck R, Gal R, Bhatti M, et al. Visual function more than 10 years after optic neuritis: experience of the optic neuritis treatment trial. Am J Ophthalmol. 2004;137(1):77–83. doi: 10.1016/s0002-9394(03)00862-6. [DOI] [PubMed] [Google Scholar]
- 5.Optic Neuritis Study Group. Visual function 15 years after optic neuritis: a final follow-up report from the Optic Neuritis Treatment Trial. Ophthalmology. 2008;115(6):1079–1082. doi: 10.1016/j.ophtha.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Phillips PH, Newman NJ, Lynn MJ. Optic neuritis in African Americans. Arch Neurol. 1998;55(2):186–192. doi: 10.1001/archneur.55.2.186. [DOI] [PubMed] [Google Scholar]
- 7.Pokroy R, Modi G, Saffer D. Optic neuritis in an urban black African community. Eye. 2001;15(4):469–473. doi: 10.1038/eye.2001.157. [DOI] [PubMed] [Google Scholar]
- 8.Hedeker D, Gibbons RD. Longitudinal Data Alanysis. 1 ed. Wiley-Interscience; 2006. [Google Scholar]
- 9.Optic Neuritis Study Group. The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1991;109(12):1673–1678. doi: 10.1001/archopht.1991.01080120057025. [DOI] [PubMed] [Google Scholar]
- 10.Elliott DB, Sanderson K, Conkey A. The reliability of the Pelli�Robson contrast sensitivity chart. Ophthalmic Physiol Opt. 1990;10(1):21–24. [PubMed] [Google Scholar]
- 11.Lee DJ, Lam BL, Arora S, et al. Reported eye care utilization and health insurance status among US adults. Arch Ophthalmol. 2009;127(3):303–310. doi: 10.1001/archophthalmol.2008.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Lee PP, Thompson TJ, et al. Health insurance coverage and use of eye care services. Arch Ophthalmol. 2008;126(8):1121–1126. doi: 10.1001/archopht.126.8.1121. [DOI] [PubMed] [Google Scholar]
- 13.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes DB, Renata de Iracema PR, Falcochio C, et al. Comparison of visual acuity and automated perimetry findings in patients with neuromyelitis optica or multiple sclerosis after single or multiple attacks of optic neuritis. J Neuroophthalmol. 2012;32(2):102–106. doi: 10.1097/WNO.0b013e31823a9ebc. [DOI] [PubMed] [Google Scholar]
- 15.Naismith R, Tutlam N, Xu J, et al. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology. 2009;72(12):1077–1082. doi: 10.1212/01.wnl.0000345042.53843.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merle H, Olindo S, Jeannin S, et al. Treatment of Optic Neuritis by Plasma Exchange (Add-On) in Neuromyelitis OpticaOptic Neuritis Treatment by PE (Add-On) in NMO. Arch Ophthalmol. 2012;130(7):858–862. doi: 10.1001/archophthalmol.2012.1126. [DOI] [PubMed] [Google Scholar]
- 17.Wingerchuk D, Lennon V, Pittock S, Lucchinetti C, Weinshenker B. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 18.Storoni M, Pittock SJ, Weinshenker BG, Plant GT. Optic neuritis in an ethnically diverse population: Higher risk of atypical cases in patients of African or African-Caribbean heritage. J Neurol Sci. 2012;312(1):21–25. doi: 10.1016/j.jns.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang H, Quertermous T, Rodriguez B, et al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet. 2005;76(2):268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinha M, Larkin EK, Elston RC, Redline S. Self-reported race and genetic admixture. N Engl J Med. 2006;354(4):421–422. doi: 10.1056/NEJMc052515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.