Abstract
We analyzed plasma 8OHdG concentrations in 20 individuals enrolled in the Pre-2CARE study before and after treatment with CoQ. Treatment resulted in a mean reduction in 8OHdG of 2.9 ± 2.9 pg/ml for the cohort (p = 0.0003) and 3.0 ± 2.6 pg/ml, for the HD group (p = 0.002). Baseline 8OHdG levels were not different between individuals with HD and controls (19.3 ± 3.2 pg/ml vs. 19.5 ± 4.7 pg/ml, p = 0.87) though baseline CoQ levels were elevated in HD compared with controls (p < 0.001). CoQ treatment reduces plasma 8OHdG and this reduction may serve as a marker of pharmacologic activity of CoQ in HD.
Keywords: Huntington disease, coenzyme Q10, 8OHdG, oxidative injury
INTRODUCTION
Huntington Disease (HD) is an autosomal dominant neurodegenerative disease caused by the cytosine-adenine-guanine (CAG) trinucleotide repeat expansion on chromosome 4 [1] and is characterized clinically by movement disorder, behavioral disturbances and dementia. Growing evidence implicates mitochondrial dysfunction and oxidative injury in the pathophysiology of HD [2].
Serum 8-hydroxy-2′-deoxyguanosine (8OHdG) is a marker of oxidative damage to DNA. It has been shown to be elevated in individuals with HD, ALS and Friedreich’s Ataxia [3–5]. Hersch et al. have shown that this 8OHdG elevation in HD can be reduced by treatment with creatine [3]. CoQ exerts antioxidant properties in the mitochondrial electron transport chain and decreases 8OHdG levels in a dose-dependent fashion in the R6/2 transgenic mouse model of HD [6]. However, the relationship between CoQ dosing and 8OHdG levels in humans is not known.
We completed a secondary analysis of 8OHdG levels and response to CoQ treatment in 20 participants from the Pre-2CARE study.
MATERIALS AND METHODS
Overview of Pre-2CARE
The design and methods for Pre-2Care have been previously described [7]. Twenty individuals with HD and 8 healthy controls consented to a 20-week open-label, dose-escalation, safety and tolerability trial of CoQ. Participants started on 1200 mg/day of CoQ, titrated to 3600 mg/day at week 8 and followed for an additional 12 weeks. Venous sampling for CoQ levels at baseline and weeks 4, 8, 12 and 20.
Participants
Twenty out of the 28 individuals enrolled in Pre-2CARE (14 HD/6 Healthy Controls) had a sufficient amount of stored plasma samples available from baseline and 20 weeks and were thus included in this analysis.
CoQ analysis
Trough-level assays for CoQ plasma levels were performed for the primary Pre-2CARE analysis using techniques previously described [8].
8OHdG analysis
Pre2-Care plasma samples were stored in a minus 80 degree freezer at the University of Rochester. Samples were shipped to the Matson laboratory and processed using a standard solid-phase extraction (SPE) protocol. A carbon column switching system, blindly assayed with a duplicate, was then used for measurements of 8OHdG [9].
Statistical analyses
Baseline and change in plasma 8OHdG and CoQ levels were compared between HD and healthy controls and between individuals with HD taking CoQ and those not taking CoQ at baseline using Wilcoxon two-sample exact tests. Wilcoxon one-sample tests evaluated the within-subject change from baseline to 20 weeks in 8OHDG levels and CoQ levels. The relationship between baseline and 20 week changes in plasma 8OHDG and CoQ levels were evaluated using Pearson’s correlations. All tests were 2-sided at the 5% significance level.
RESULTS
Baseline characteristics
Baseline characteristics of individuals with HD and healthy controls are detailed in Table 1. HD participants were generally early disease with 5 in Stage I, 8 in Stage II and one in Stage III. Four individuals in the HD group reported taking CoQ at baseline.
Table 1.
Baseline characteristics
| Characteristics | Manifest HD (n = 14)
|
Healthy Controls (n = 6)
|
P-value | ||
|---|---|---|---|---|---|
| Gender (female/male) | (8/6) | (4/2) | 1.00 | ||
| Mean (SD) | Median (range) | Mean (SD) | Median (range) | ||
| Age | 52.65 (8.61) | 50.35 (26.64) | 51.57 (8.64) | 47.52 (22.90) | 0.72 |
| UHDRS motor (mean ± sd) | 43.71 (12.63) | 43.50 (41.00) | N/A | N/A | N/A |
| TFC (mean ± sd) | 9.64 (1.86) | 9.50 (6.00) | N/A | N/A | N/A |
| Baseline plasma CoQ (mcg/ml) | 1.65 (1.13) | 1.21 (3.73) | 0.74 (0.26) | 0.68 (0.66) | 0.02 |
| Baseline plasma 8OHdG (pg/ml) | 18.98 (3.26) | 18.70 (12.00) | 19.57 (5.47) | 17.70 (14.70) | 0.97 |
HD = Huntington Disease; UHDRS = Unified Huntington Disease Rating Scale; TFC = Total Functional Capacity; CoQ = Coenzyme Q10; 8OHdG = 8-hydroxy-2′-deoxyguanosine; SD = standard deviations; N/A = not applicable.
CoQ levels
Baseline mean (SD) CoQ levels (Table 1) were elevated in individuals with HD as compared with healthy controls, 1.65 (1.13) mcg/ml vs. 0.74 (0.26) mcg/ml, p = 0.02. CoQ levels were higher in individuals with HD who reported taking CoQ at baseline compared with those who did not, 2.8 (1.2) vs. 1.2 (0.77) mcg/ml, p = 0.02. However, baseline CoQ levels remained higher in individuals with HD compared with healthy controls even when those individuals taking CoQ at baseline were excluded, 1.6 (1.5) mcg/ml vs. 0.74 (0.26) mcg/ml, p < 0.001. CoQ levels increased at the final visit by a mean (SD) of 4.47 (3.50) mcg/ml in the HD group compared with 3.52 (2.06) mcg/ml in healthy controls (p = 0.66).
8OHdG levels
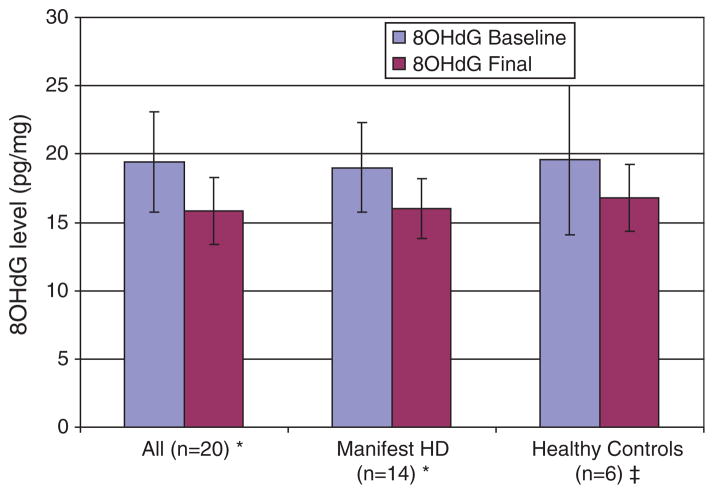
Baseline mean (SD) 8OHdG levels were not significantly different between HD and healthy controls, 18.98 (3.28) pg/ml vs. 19.57 (5.47) pg/ml, p = 0.97. There was no difference in baseline 8OHdG levels between the HD participants who reported taking CoQ at baseline and those who did not, 19.6 (3.2) pg/ml vs. 18.8 (3.4) pg/ml, p = 0.45. Administration of CoQ led to a mean (SD) reduction in 8OHdG of 2.9 (2.9) pg/ml for the entire cohort, p = 0.0003, and 3.0 (2.6) pg/ml, for the HD group, p = 0.002. Healthy controls had a reduction in 8OHdG of 2.8 (4.0) pg/ml, p = 0.15. Figure 1 shows the baseline and final 8OHdG levels by group.
Fig. 1.
8OHdG levels at baseline and after 20 weeks of treatment with CoQ in manifest HD and healthy controls. For the change in 8OHdG from baseline: *p < 0.001, ‡p = 0.79. Error bars represent the SD of the mean 8OHdG level.
DISCUSSION
In this study, CoQ administration reduced plasma 8OHdG levels in individuals with HD. These findings are consistent with data showing a reduction in 8OHdG in the R6/2 mouse model treated with CoQ [6] and in HD individuals treated with creatine, a cytoplasmic antioxidant [3]. While not significant, we found a similar reduction in 8OHdG in healthy controls treated with CoQ, suggesting the effect of CoQ on 8OHdG may be non-specific. Together these results suggest that 8OHdG may serve as a biomarker of the pharmacological activity of CoQ in HD.
A leading hypothesis of HD pathophysiology is that mitochondrial dysfunction leads to increased oxidative stress [2]. 8OHdG is a marker of oxidative injury to DNA. Increases in 8OHdG have been found in postmortem HD brain tissue, [10] in the R6/2 mouse model [6], and in other neurodegenerative diseases where oxidative injury is important [4].
CoQ has demonstrated neuroprotective properties in experimental models of HD [11] and may slow progression in patients with HD [12] and Parkinson disease, [13] through stabilization of the mitochondrial membrane, improving ATP production, and as an antioxidant [14]. Our study supports the hypothesis that CoQ may reduce oxidative injury in HD as measured by 8OHdG.
These findings have potential implications for disease modifying therapies in HD. While the current data are insufficient to address the validity of 8OHdG as a surrogate marker of clinical effectiveness, identifying 8OHdG as a marker of the pharmacological activity of an intervention is a critical step in validating 8OHdG as a potential surrogate marker [15]. In addition, ongoing Huntington Study Group trials of CoQ and Creatine in manifest HD (2CARE, NCT00608881; CREST-E, NCT00712426) and pre-manifest HD (PREQUEL, NCT00920699), as well as the Prospective Hunting-ton At-Risk Observational Study (PHAROS) [16] will provide additional information on the effectiveness of CoQ and the utility of 8OHdG as a pharmacological activity and natural history biomarker in HD.
In contrast to previous studies [3] we found no difference in plasma 8OHdG levels comparing individuals with HD and healthy controls. Plasma 8OHdG levels in participants with HD were in the range previously reported for healthy controls and were less than half the levels reported for HD [3]. A plausible explanation is the elevated baseline CoQ levels in our HD group. While this is partially accounted for by the fact that four participants reported taking CoQ at baseline, CoQ levels remained higher in the HD group even when those participants were excluded. Perhaps individuals with HD have naturally high levels of CoQ; however, in a study of CoQ and remacemide in HD (CARE-HD) mean (SD) baseline CoQ levels were 0.81 (0.28) mcg/ml, much lower than the HD participants and similar to the healthy controls in this study (K. Kieburtz, personal communication, [12]). Alternatively, some HD subjects may have been taking CoQ and either did not report CoQ use or had recently discontinued it, as CoQ levels can remain elevated for weeks following discontinuation of CoQ [17]. The duration of this effect on 8OHdG is not known. Other potential explanations include laboratory error, differences in sampling and storage, or that our findings simply reflect the normal distribution of 8OHdG in this population. Future and ongoing research exploring 8OHdG in HD may clarify this issue.
This study has several limitations. The small sample size may account for our findings of no difference in 8OHdG between HD and healthy controls. However, despite the sample size, we found reduction in 8OHdG with CoQ and differences in baseline CoQ between groups. Also, 8OHdG levels in our study are comparable to levels reported in healthy controls and are lower than reported in HD [3]. Prior CoQ use was not excluded or systematically evaluated, which may have influenced the results. Finally, the relationship of 8OHdG to disease stage and progression in HD remains unclear and requires further study.
The current study demonstrates that plasma 8OHdG is reduced with CoQ treatment and therefore may serve as a useful biomarker in HD to screen compounds with putative antioxidant mechanisms. We did not confirm previous reports of elevated 8OHdG in manifest HD though this may be secondary to concurrent CoQ use in our HD participants. Future studies need to ensure that participants do not have significant prior antioxidant exposure at baseline. Ongoing observational studies and clinical trials of CoQ and creatine in HD will refine our understanding of 8OHdG as a biomarker in HD.
Acknowledgments
The authors would like to thank Matt Grana and Michael Bull for assistance in the preparation of this manuscript. The original Pre-2Care study was performed with funding from the CHDI/HiQ foundation.
This study was partially supported by NIH grants NS16375 (C.A. Ross) and NS058793 (S. Hersch).
Huntington study group Pre-2Care investigators
Steering Committee
University of Western Ontario: H. Christopher Hyson, MD (Medical Monitor). University of Rochester, Rochester, NY: Karl Kieburtz, MD, MPH (Principal Investigator); Ira Shoulson, MD; Michael McDermott, PhD (Chief Biostatistician); Bernard Ravina, MD; Elisabeth A. de Blieck, MPA CRCC (Project Coordinator). Massachusetts General Hospital, Charlestown, MA: Merit E. Cudkowicz, MD, MSc (Co-Principal Investigator). Boston University School of Medicine, Boston, MA and the Edith Nourse Rogers Veterans Administration Hospital, Bedford, MA: Robert J. Ferrante, MSc, PhD.
Site Investigators and Coordinators
University of Rochester Medical Center, Rochester, NY: Peter Como, PhD; Sam Frank, MD; Carol Zimmerman, RN. Massachusetts General Hospital, Charlestown, MA: Merit E. Cudkowicz, MD, MSc; Kimberly Ferrante; Kristyn Newhall, BS. Institute for Neurodegenerative Disorders, New Haven, CT: Danna Jennings, MD; Tammie Kelsey, LPN. Wake Forest University School of Medicine, Winston-Salem, NC: Francis Walker, MD; Vicki Hunt, RN.
Biostatistics and Clinical Trial Coordination Center
University of Rochester, Rochester, NY: Susan Daigneault (Data Control Clerk); Michele Goldstein, BS (Information Analyst); Joseph Weber, BS (Database Manager); Arthur Watts, BS (Biostatistics Programmer).
Laboratory Support
Weill Medical College of Cornell University, New York, NY: M. Flint Beal, MD; Susan E. Browne, PhD; Linda J. Metakis.
References
- 1.Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF. Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Ann Neurol. 1997;41:160–5. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- 3.Hersch SM, Gevorkian S, Marder K, et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2′dG. Neurology. 2006;66:250–2. doi: 10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- 4.Bogdanov M, Brown RH, Matson W, et al. Increased oxidative damage to DNA in ALS patients. Free Radic Biol Med. 2000;29:652–8. doi: 10.1016/s0891-5849(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 5.Schulz JB, Dehmer T, Schols L, et al. Oxidative stress in patients with Friedreich ataxia. Neurology. 2000;55:1719–21. doi: 10.1212/wnl.55.11.1719. [DOI] [PubMed] [Google Scholar]
- 6.Smith KM, Matson S, Matson WR, et al. Dose ranging and efficacy study of high-dose coenzyme Q10 formulations in Huntington’s disease mice. Biochim Biophys Acta. 2006;1762:616–26. doi: 10.1016/j.bbadis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 7.The Huntington Study Group Pre-2CARE Investigators. Safety and tolerability of high dosage coenzyme Q10 in Huntington’s disease and healthy subjects. Movement Disorders. 2010;25:1924–8. doi: 10.1002/mds.22408. [DOI] [PubMed] [Google Scholar]
- 8.Shults CW, Haas RH, Passov D, Beal MF. Coenzyme Q10 levels correlate with the activities of complexes I and II/III in mitochondria from parkinsonian and nonparkinsonian subjects. Ann Neurol. 1997;42:261–4. doi: 10.1002/ana.410420221. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanov MB, Acworth IN, editors. The Use of HPLC/EC for Measurements of Oxidative DNA Damage. World Scientific; 2003. [Google Scholar]
- 10.Polidori MC, Mecocci P, Browne SE, Senin U, Beal MF. Oxidative damage to mitochondrial DNA in Huntington’s disease parietal cortex. Neurosci Lett. 1999;272:53–6. doi: 10.1016/s0304-3940(99)00578-9. [DOI] [PubMed] [Google Scholar]
- 11.Ferrante RJ, Andreassen OA, Dedeoglu A, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J Neurosci. 2002;22:1592–9. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57:397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- 13.Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–50. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 14.Pepping J. Coenzyme Q10. Am J Health Syst Pharm. 1999;56:519–21. doi: 10.1093/ajhp/56.6.519. [DOI] [PubMed] [Google Scholar]
- 15.Mildvan D, Landay A, De Gruttola V, Machado SG, Kagan J. An approach to the validation of markers for use in AIDS clinical trials. Clin Infect Dis. 1997;24:764–74. doi: 10.1093/clinids/24.5.764. [DOI] [PubMed] [Google Scholar]
- 16.At risk for Huntington disease: The PHAROS (Prospective Huntington At Risk Observational Study) cohort enrolled. Arch Neurol. 2006;63:991–6. doi: 10.1001/archneur.63.7.991. [DOI] [PubMed] [Google Scholar]
- 17.Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol. 2007;47:19–28. doi: 10.1016/j.yrtph.2006.07.001. [DOI] [PubMed] [Google Scholar]