Abstract
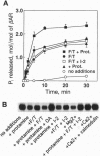
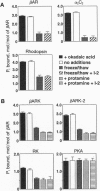
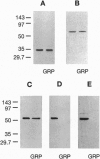
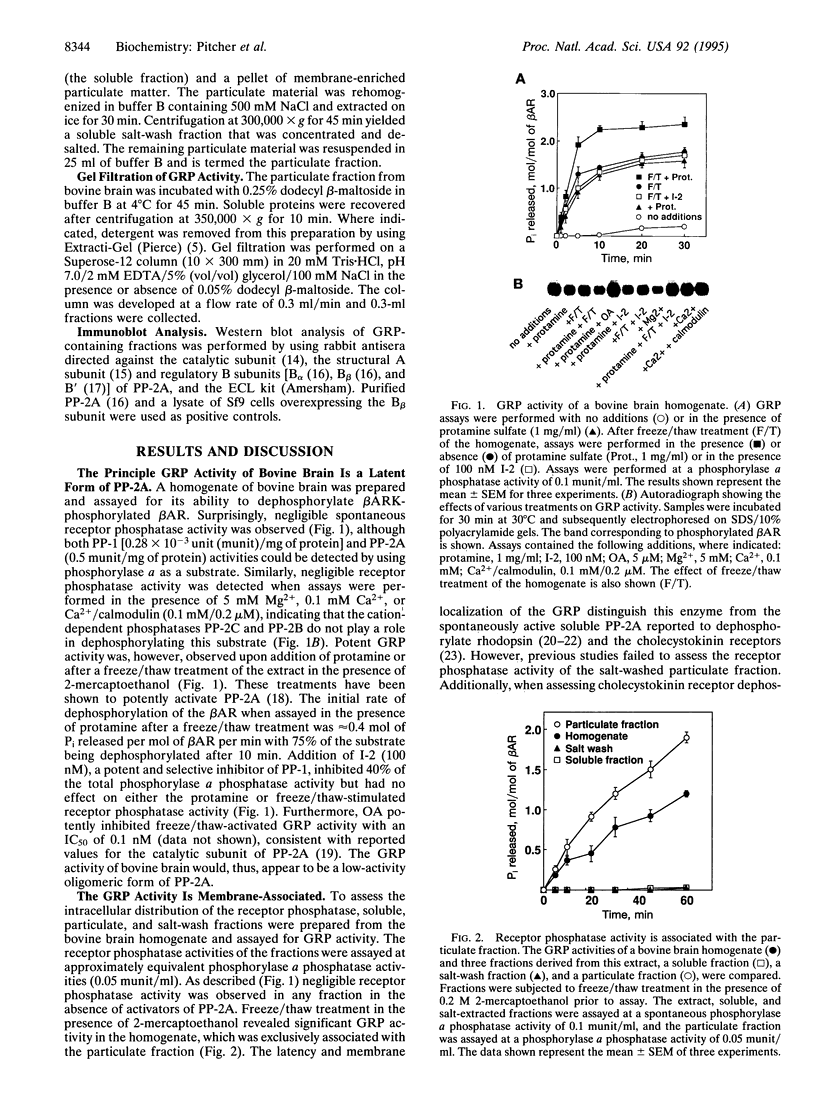
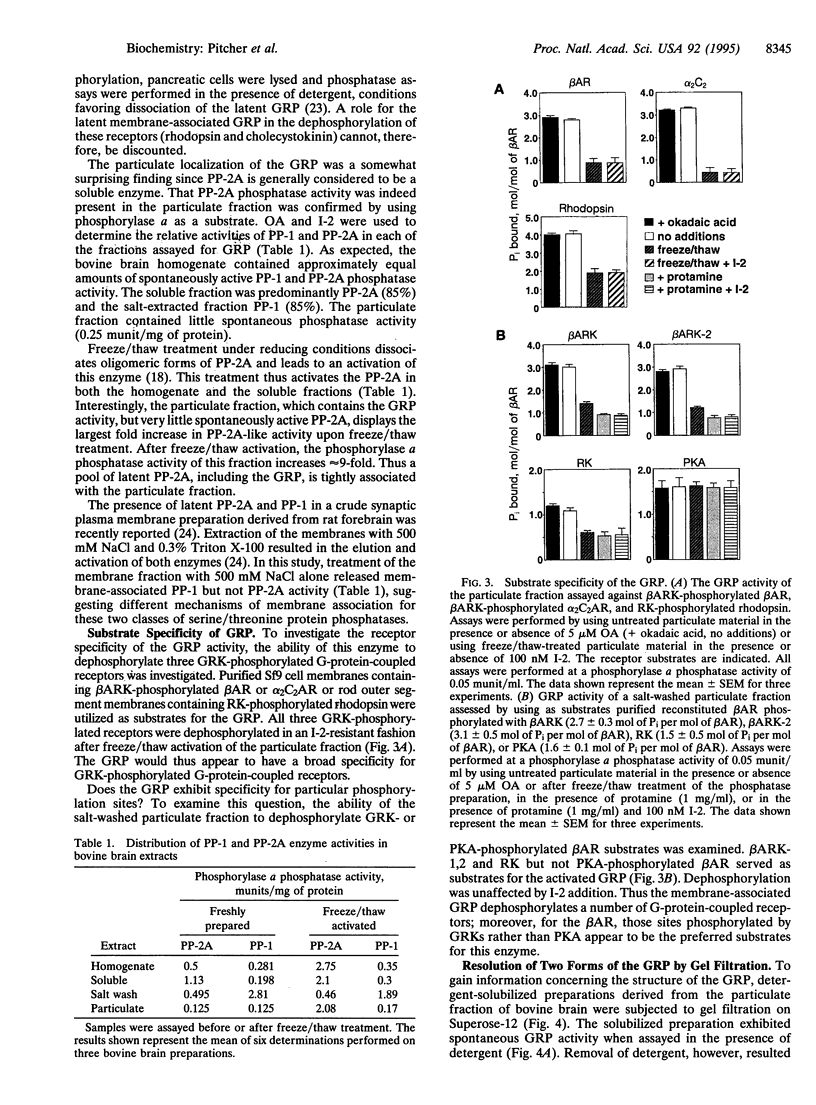
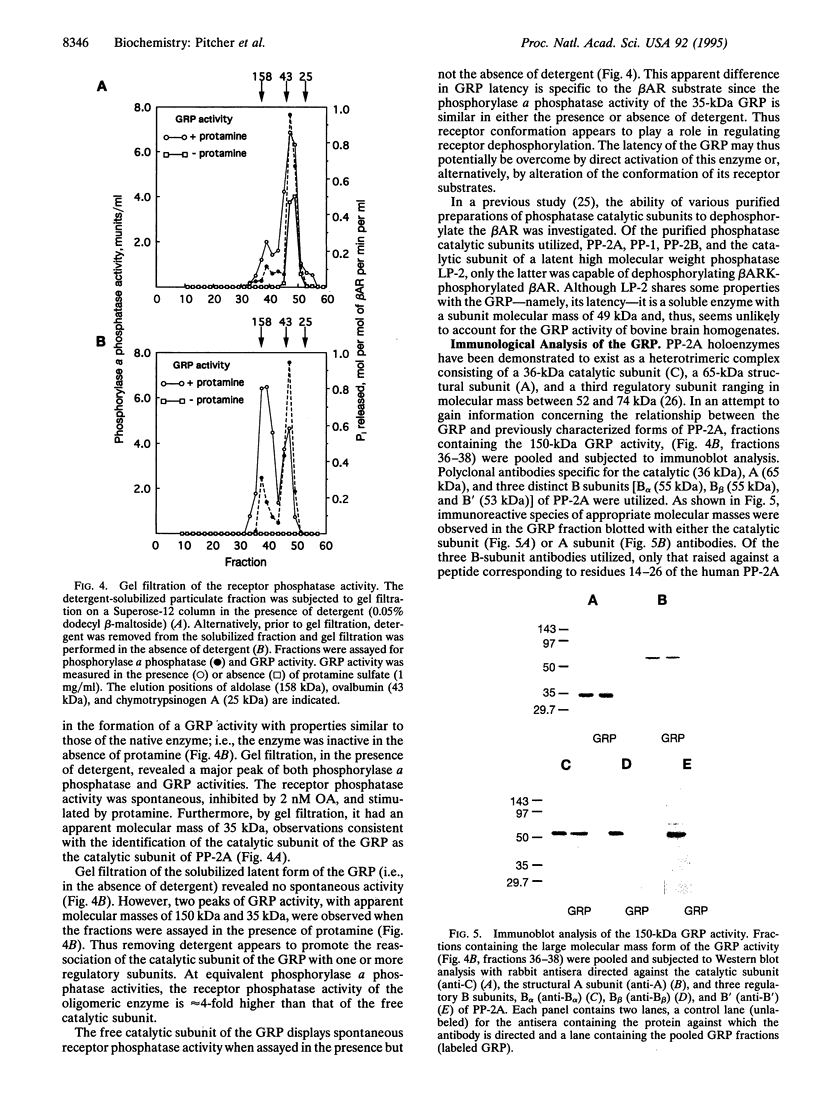
Phosphorylation of G-protein-coupled receptors plays an important role in regulating their function. In this study the G-protein-coupled receptor phosphatase (GRP) capable of dephosphorylating G-protein-coupled receptor kinase-phosphorylated receptors is described. The GRP activity of bovine brain is a latent oligomeric form of protein phosphatase type 2A (PP-2A) exclusively associated with the particulate fraction. GRP activity is observed only when assayed in the presence of protamine or when phosphatase-containing fractions are subjected to freeze/thaw treatment under reducing conditions. Consistent with its identification as a member of the PP-2A family, the GRP is potently inhibited by okadaic acid but not by I-2, the specific inhibitor of protein phosphatase type 1. Solubilization of the membrane-associated GRP followed by gel filtration in the absence of detergent yields a 150-kDa peak of latent receptor phosphatase activity. Western blot analysis of this phosphatase reveals a likely subunit composition of AB alpha C. PP-2A of this subunit composition has previously been characterized as a soluble enzyme, yet negligible soluble GRP activity was observed. The subcellular distribution and substrate specificity of the GRP suggests significant differences between it and previously characterized forms of PP-2A.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arino J., Woon C. W., Brautigan D. L., Miller T. B., Jr, Johnson G. L. Human liver phosphatase 2A: cDNA and amino acid sequence of two catalytic subunit isotypes. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4252–4256. doi: 10.1073/pnas.85.12.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Martin B. L., Brautigan D. L. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992 Aug 28;257(5074):1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- Cohen P., Alemany S., Hemmings B. A., Resink T. J., Strålfors P., Tung H. Y. Protein phosphatase-1 and protein phosphatase-2A from rabbit skeletal muscle. Methods Enzymol. 1988;159:390–408. doi: 10.1016/0076-6879(88)59039-0. [DOI] [PubMed] [Google Scholar]
- Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Fowles C., Akhtar M., Cohen P. Interplay of phosphorylation and dephosphorylation in vision: protein phosphatases of bovine rod outer segments. Biochemistry. 1989 Nov 28;28(24):9385–9391. doi: 10.1021/bi00450a020. [DOI] [PubMed] [Google Scholar]
- Gierschik P., Simons C., Woodard C., Somers R., Spiegel A. Antibodies against a retinal guanine nucleotide-binding protein cross-react with a single plasma membrane protein in non-retinal tissues. FEBS Lett. 1984 Jul 9;172(2):321–325. doi: 10.1016/0014-5793(84)81149-7. [DOI] [PubMed] [Google Scholar]
- Guo H., Damuni Z. Autophosphorylation-activated protein kinase phosphorylates and inactivates protein phosphatase 2A. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2500–2504. doi: 10.1073/pnas.90.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Hemmings B. A., Adams-Pearson C., Maurer F., Müller P., Goris J., Merlevede W., Hofsteenge J., Stone S. R. alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry. 1990 Apr 3;29(13):3166–3173. doi: 10.1021/bi00465a002. [DOI] [PubMed] [Google Scholar]
- Kamibayashi C., Estes R., Lickteig R. L., Yang S. I., Craft C., Mumby M. C. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J Biol Chem. 1994 Aug 5;269(31):20139–20148. [PubMed] [Google Scholar]
- Kamibayashi C., Lickteig R. L., Estes R., Walter G., Mumby M. C. Expression of the A subunit of protein phosphatase 2A and characterization of its interactions with the catalytic and regulatory subunits. J Biol Chem. 1992 Oct 25;267(30):21864–21872. [PubMed] [Google Scholar]
- Kim C. M., Dion S. B., Onorato J. J., Benovic J. L. Expression and characterization of two beta-adrenergic receptor kinase isoforms using the baculovirus expression system. Receptor. 1993 Spring;3(1):39–55. [PubMed] [Google Scholar]
- King A. J., Andjelkovic N., Hemmings B. A., Akhtar M. The phospho-opsin phosphatase from bovine rod outer segments. An insight into the mechanism of stimulation of type-2A protein phosphatase activity by protamine. Eur J Biochem. 1994 Oct 1;225(1):383–394. doi: 10.1111/j.1432-1033.1994.00383.x. [DOI] [PubMed] [Google Scholar]
- Lutz M. P., Pinon D. I., Gates L. K., Shenolikar S., Miller L. J. Control of cholecystokinin receptor dephosphorylation in pancreatic acinar cells. J Biol Chem. 1993 Jun 5;268(16):12136–12142. [PubMed] [Google Scholar]
- Mayer-Jaekel R. E., Hemmings B. A. Protein phosphatase 2A--a 'ménage à trois'. Trends Cell Biol. 1994 Aug;4(8):287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Mumby M. C., Russell K. L., Garrard L. J., Green D. D. Cardiac contractile protein phosphatases. Purification of two enzyme forms and their characterization with subunit-specific antibodies. J Biol Chem. 1987 May 5;262(13):6257–6265. [PubMed] [Google Scholar]
- Palczewski K., Benovic J. L. G-protein-coupled receptor kinases. Trends Biochem Sci. 1991 Oct;16(10):387–391. doi: 10.1016/0968-0004(91)90157-q. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Hargrave P. A., McDowell J. H., Ingebritsen T. S. The catalytic subunit of phosphatase 2A dephosphorylates phosphoopsin. Biochemistry. 1989 Jan 24;28(2):415–419. doi: 10.1021/bi00428a001. [DOI] [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Jakes S., Ingebritsen T. S., Hargrave P. A. Regulation of rhodopsin dephosphorylation by arrestin. J Biol Chem. 1989 Sep 25;264(27):15770–15773. [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Park I. K., Roach P., Bondor J., Fox S. P., DePaoli-Roach A. A. Molecular mechanism of the synergistic phosphorylation of phosphatase inhibitor-2. Cloning, expression, and site-directed mutagenesis of inhibitor-2. J Biol Chem. 1994 Jan 14;269(2):944–954. [PubMed] [Google Scholar]
- Pei G., Tiberi M., Caron M. G., Lefkowitz R. J. An approach to the study of G-protein-coupled receptor kinases: an in vitro-purified membrane assay reveals differential receptor specificity and regulation by G beta gamma subunits. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3633–3636. doi: 10.1073/pnas.91.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. J., Trukawinski S., Cerione R. A. An antibody-induced enhancement of the transducin-stimulated cyclic GMP phosphodiesterase activity. J Biol Chem. 1989 Oct 5;264(28):16679–16688. [PubMed] [Google Scholar]
- Pitcher J. A., Inglese J., Higgins J. B., Arriza J. L., Casey P. J., Kim C., Benovic J. L., Kwatra M. M., Caron M. G., Lefkowitz R. J. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992 Aug 28;257(5074):1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Pitcher J. A., Touhara K., Payne E. S., Lefkowitz R. J. Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J Biol Chem. 1995 May 19;270(20):11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Ingebritsen T. S. Protein (serine and threonine) phosphate phosphatases. Methods Enzymol. 1984;107:102–129. doi: 10.1016/0076-6879(84)07007-5. [DOI] [PubMed] [Google Scholar]
- Sim A. T., Ratcliffe E., Mumby M. C., Villa-Moruzzi E., Rostas J. A. Differential activities of protein phosphatase types 1 and 2A in cytosolic and particulate fractions from rat forebrain. J Neurochem. 1994 Apr;62(4):1552–1559. doi: 10.1046/j.1471-4159.1994.62041552.x. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J., Hemmings B. A. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry. 1987 Nov 17;26(23):7215–7220. doi: 10.1021/bi00397a003. [DOI] [PubMed] [Google Scholar]
- Walter G., Ruediger R., Slaughter C., Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Clarke S. Protein phosphatase 2A is reversibly modified by methyl esterification at its C-terminal leucine residue in bovine brain. J Biol Chem. 1994 Jan 21;269(3):1981–1984. [PubMed] [Google Scholar]
- Yang S. D., Fong Y. L., Benovic J. L., Sibley D. R., Caron M. G., Lefkowitz R. J. Dephosphorylation of the beta 2-adrenergic receptor and rhodopsin by latent phosphatase 2. J Biol Chem. 1988 Jun 25;263(18):8856–8858. [PubMed] [Google Scholar]
- Yu S. S., Lefkowitz R. J., Hausdorff W. P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem. 1993 Jan 5;268(1):337–341. [PubMed] [Google Scholar]
- Zolnierowicz S., Csortos C., Bondor J., Verin A., Mumby M. C., DePaoli-Roach A. A. Diversity in the regulatory B-subunits of protein phosphatase 2A: identification of a novel isoform highly expressed in brain. Biochemistry. 1994 Oct 4;33(39):11858–11867. doi: 10.1021/bi00205a023. [DOI] [PubMed] [Google Scholar]