Abstract
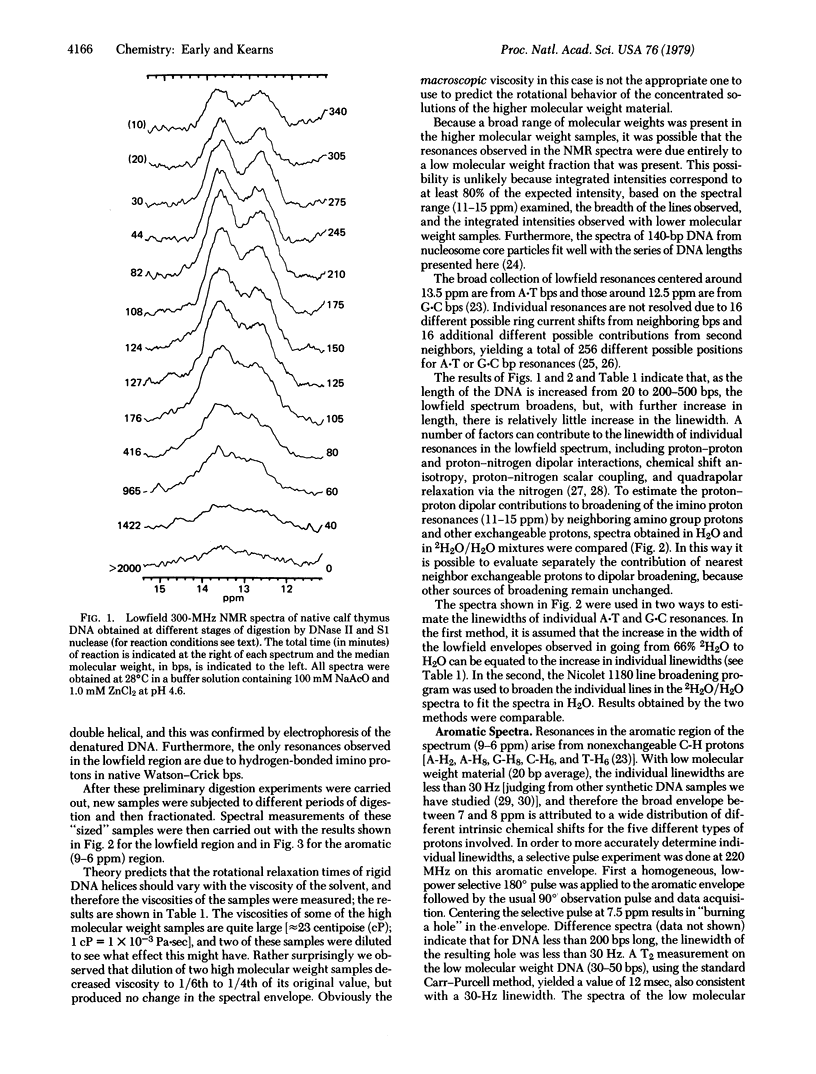
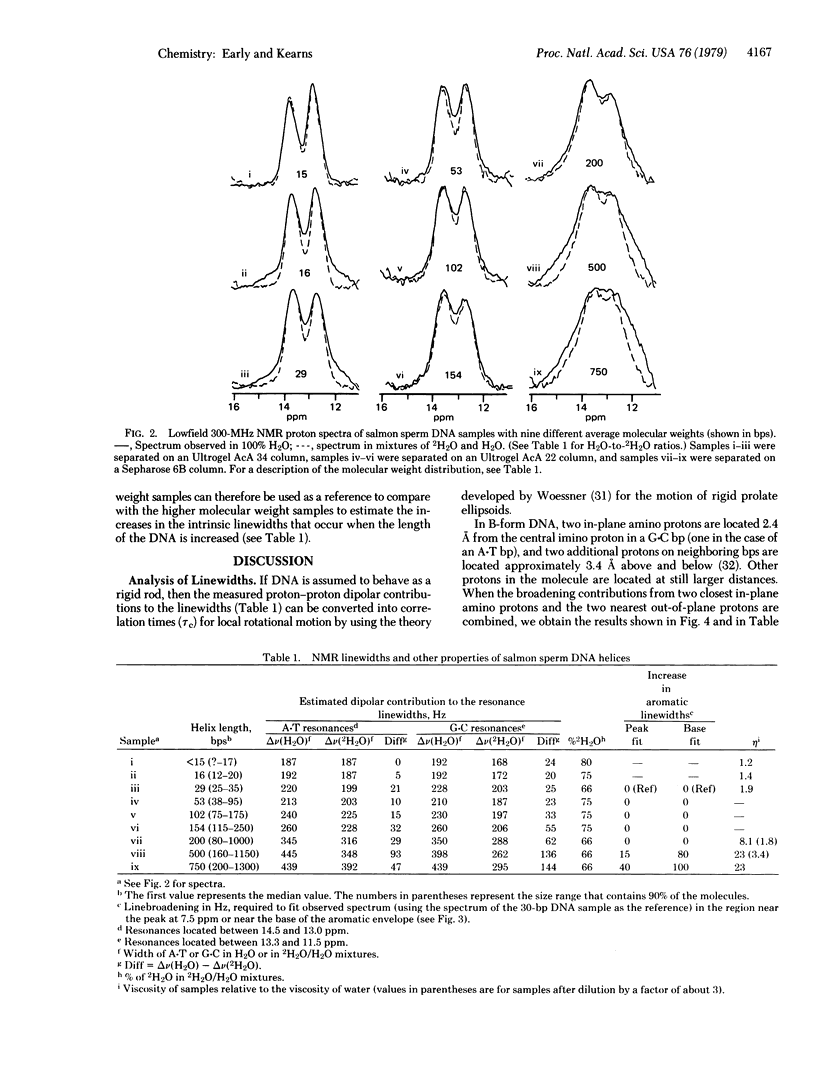
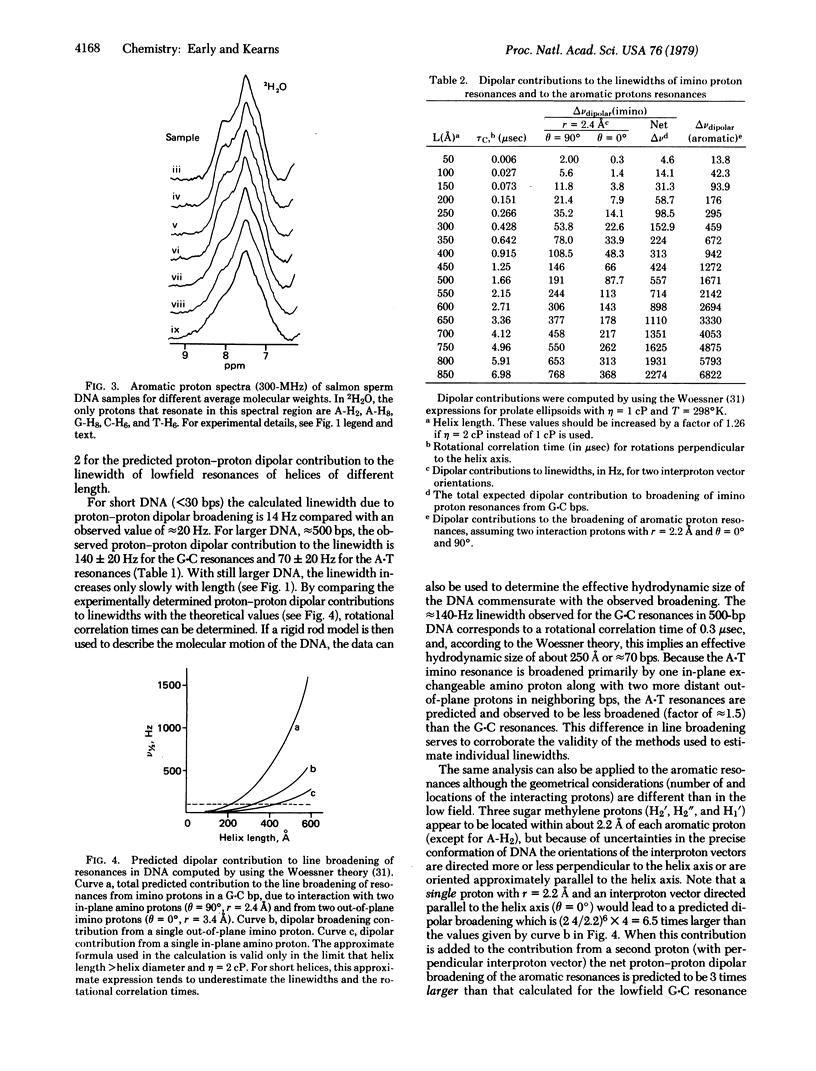
In this paper we report successful observations of proton NMR spectra of native double helical salmon sperm and calf thymus DNA of various lengths. Measurements of the linewidths arising from proton--proton dipolar interactions are used to obtain information about the dynamic behavior of DNA helices in solution. Depending upon which protons are used to monitor the local internal motions of the DNA, different results are obtained. The lowfield resonances from hydrogen-bonded imino protons in the base pairs indicate that the correlation time for reorientation of base pairs is less than 3 x 10(-7) sec, whereas correlation times for motion of neighboring sugar protons relative to the aromatic protons are less than 5 x 10(-8) sec for DNA that is over 200 base pairs long. These observations indicate that there is considerably more internal flexibility in the DNA molecues, especially in the backbone, than is indicated by classic hydrodynamic studies of DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Arter D. B., Schmidt P. G. Ring current shielding effects in nucleic acid double helices. Nucleic Acids Res. 1976 Jun;3(6):1437–1447. doi: 10.1093/nar/3.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. J. Elastic model of supercoiling. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2397–2401. doi: 10.1073/pnas.74.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner W. C., Maestre M. F. Circular dichroism of films of polynucleotides. Biopolymers. 1974;13(2):345–357. doi: 10.1002/bip.1974.360130210. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Felsenfeld G. A simple model of DNA superhelices in solution. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1708–1712. doi: 10.1073/pnas.75.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter R. I., Lilley D. M. The conformation of DNA and protein within chromatin subunits. FEBS Lett. 1977 Oct 1;82(1):63–68. doi: 10.1016/0014-5793(77)80886-7. [DOI] [PubMed] [Google Scholar]
- Crick F. H., Klug A. Kinky helix. Nature. 1975 Jun 12;255(5509):530–533. doi: 10.1038/255530a0. [DOI] [PubMed] [Google Scholar]
- Early T. A., Kearns D. R., Burd J. F., Larson J. E., Wells R. D. High resolution proton nuclear magnetic resonance investigation of the structural and dynamic properties of d(C15A15)-d(T15G15). Biochemistry. 1977 Feb 8;16(3):541–551. doi: 10.1021/bi00622a031. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Bond P. J., Peticolas W. L. Characterization of the A in equilibrium B transition of DNA in fibers and gels by laser Raman spectroscopy. Biopolymers. 1975 Jun;14(6):1245–1257. doi: 10.1002/bip.1975.360140613. [DOI] [PubMed] [Google Scholar]
- Feigon J., Kearns D. R. 1H NMR investigation of the conformational states of DNA in nucleosome core particles. Nucleic Acids Res. 1979;6(6):2327–2337. doi: 10.1093/nar/6.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. Intermolecular nuclear shielding values for protons of purines and flavins. J Theor Biol. 1970 Apr;27(1):87–95. doi: 10.1016/0022-5193(70)90130-x. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Eisenberg H. The flexibility of low molecular weight double-stranded DNA as a function of length. I. Light scattering measurements and the estimation of persistence lengths from light scattering, sedimentation and viscosity. Biophys Chem. 1976 Sep;5(3):301–318. doi: 10.1016/0301-4622(76)80042-7. [DOI] [PubMed] [Google Scholar]
- Hanlon S., Glonek T., Chan A. Comparison of the phosphorus magnetic resonance and circular dichroism properties of calf thymus DNA and chromatin. Biochemistry. 1976 Aug 24;15(17):3869–3875. doi: 10.1021/bi00662a034. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Zimm B. H. Flexibility and stiffness in nicked DNA. J Mol Biol. 1970 Mar 14;48(2):297–317. doi: 10.1016/0022-2836(70)90162-2. [DOI] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transient electric dichroism of rod-like DNA molecules. Proc Natl Acad Sci U S A. 1978 Jan;75(1):195–199. doi: 10.1073/pnas.75.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach N. R., Appleby D. W., Bradley C. H. 31P magnetic resonance of DNA in nucleosome core particles of chromatin. Nature. 1978 Mar 9;272(5649):134–138. doi: 10.1038/272134a0. [DOI] [PubMed] [Google Scholar]
- Kearns D. R. High-resolution nuclear magnetic resonance studies of double helical polynucleotides. Annu Rev Biophys Bioeng. 1977;6:477–523. doi: 10.1146/annurev.bb.06.060177.002401. [DOI] [PubMed] [Google Scholar]
- Levitt M. How many base-pairs per turn does DNA have in solution and in chromatin? Some theoretical calculations. Proc Natl Acad Sci U S A. 1978 Feb;75(2):640–644. doi: 10.1073/pnas.75.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D., Lazar J. Nuclear magnetic resonance determination of thymine nearest neighbor base frequency ratios in deoxyribonucleic acid. J Am Chem Soc. 1967 Aug 2;89(16):4166–4170. doi: 10.1021/ja00992a035. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. Nuclear magnetic resonance studies of the helix-coil transition of poly (dA-dT) in aqueous solution. Proc Natl Acad Sci U S A. 1976 Mar;73(3):674–678. doi: 10.1073/pnas.73.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J. Mutagen-nucleic acid complexes at the polynucleotide duplex level in solution: intercalation of proflavine into poly(dA-dT) and the melting transition of the complex. Biopolymers. 1977 Dec;16(12):2739–2754. doi: 10.1002/bip.1977.360161212. [DOI] [PubMed] [Google Scholar]
- Pilet J., Blicharski J., Brahms J. Conformations and structural transitions in polydeoxynucleotides. Biochemistry. 1975 May 6;14(9):1869–1876. doi: 10.1021/bi00680a011. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Woodbury C. P., Inman R. B. Characterization of rodlike RNA fragments. Biopolymers. 1975 Feb;14(2):393–408. doi: 10.1002/bip.1975.360140212. [DOI] [PubMed] [Google Scholar]
- Schellman J. A. Flexibility of DNA. Biopolymers. 1974 Jan;13(1):217–226. doi: 10.1002/bip.1974.360130115. [DOI] [PubMed] [Google Scholar]
- Selsing E., Wells R. D., Early T. A., Kearns D. R. Two contiguous conformations in a nucleic acid duplex. Nature. 1978 Sep 21;275(5677):249–250. doi: 10.1038/275249a0. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Gilbert S. G., Jain S. C., Sakore T. D. Organization of DNA in chromatin. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3068–3072. doi: 10.1073/pnas.73.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. L., Trifonov E. N. Possibility of nonkinked packing of DNA in chromatin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):103–107. doi: 10.1073/pnas.75.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider H. J., Koller T., Parello J., Sogo J. M. Superstructure of linear duplex DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4125–4129. doi: 10.1073/pnas.73.11.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]



