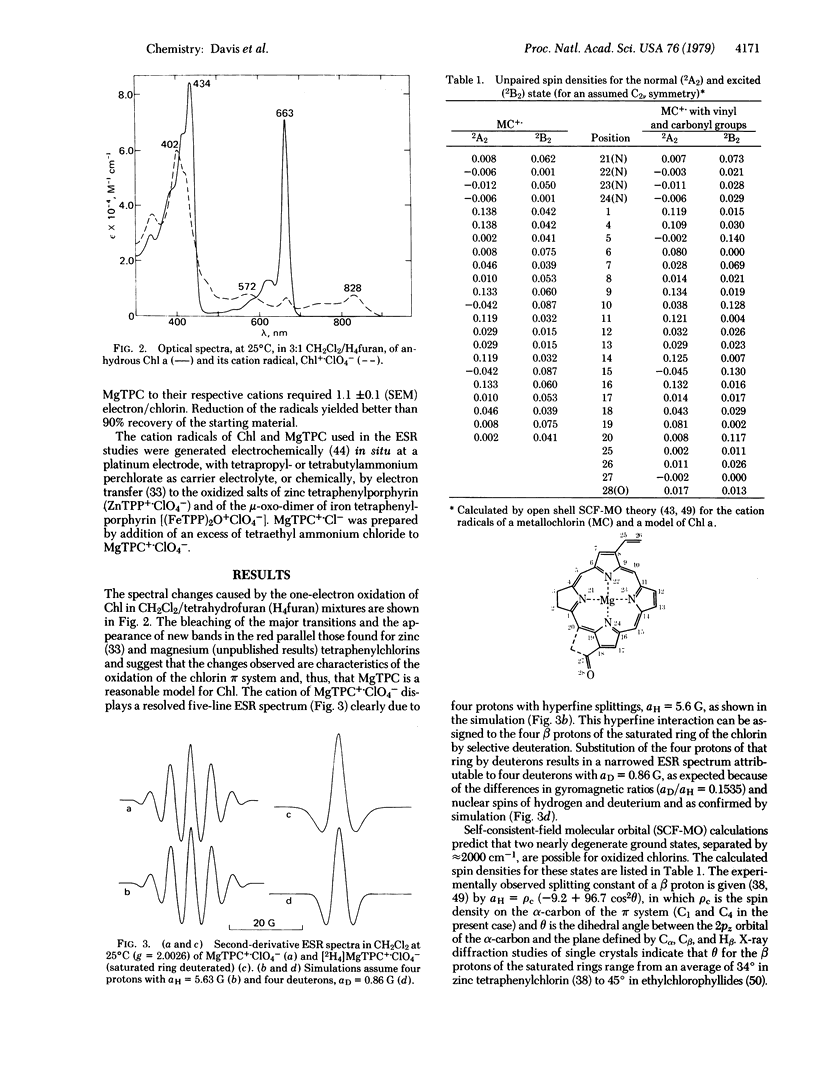
Abstract
Magnesium tetraphenylchlorin, a synthetic model for chlorophyll, exhibits significant variations in the unpaired spin densities of its cation radicals with concomitant changes in oxidation potentials as a function of solvent and axial ligand. Similar effects are observed for chlorophyll (Chl) a and its cation radicals. Oxidation potentials for Chl → Chl+. as high as +0.9 V (against a normal hydrogen electrode) are observed in nonaqueous solvents, with linewidths of the electron spin resonance signals of monomeric Chl+. ranging between 9.2 and 7.8 G in solution. These changes in electronic configuration and ease of oxidation are attributed to mixing of two nearly degenerate ground states of the radicals theoretically predicted by molecular orbital calculations. Comparison of the properties of chlorophyll in vitro with the optical, redox, and magnetic characteristics attributed to P-680, the primary donor of photosystem II which mediates oxygen evolution in plant photosynthesis, leads us to suggest that P-680 may be a ligated chlorophyll monomer whose function as a phototrap is determined by interactions with its (protein?) environment.
Keywords: electron spin resonance, monomeric chlorophyll, P-680, primary donor, photosynthetic oxygen evolution
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bearden A. J., Malkin R. Primary photochemical reactions in chloroplast photosynthesis. Q Rev Biophys. 1974 May;7(2):131–177. doi: 10.1017/s0033583500001396. [DOI] [PubMed] [Google Scholar]
- Borg D. C., Fajer J., Felton R. H., Dolphin D. The pi-Cation Radical of Chlorophyll a. Proc Natl Acad Sci U S A. 1970 Oct;67(2):813–820. doi: 10.1073/pnas.67.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg D. C., Forman A., Fajer J. ESR and ENDOR studies of the pi cation radical of bacteriochlorophyll. J Am Chem Soc. 1976 Oct 27;98(22):6889–6893. doi: 10.1021/ja00438a021. [DOI] [PubMed] [Google Scholar]
- Diehn B., Seely G. R. The oxidation of chlorophyll a in alcohols. Biochim Biophys Acta. 1968 May 28;153(4):862–867. doi: 10.1016/0005-2728(68)90013-3. [DOI] [PubMed] [Google Scholar]
- Döring G., Renger G., Vater J., Witt H. T. Properties of the photoactive chlorophyll-aII in photosynthesis. Z Naturforsch B. 1969 Sep;24(9):1139–1143. doi: 10.1515/znb-1969-0911. [DOI] [PubMed] [Google Scholar]
- Döring G., Stiehl H. H., Witt H. T. A second chlorophyll reaction in the electron chain of photosynthesis--registration by the repetitive excitation technique. Z Naturforsch B. 1967 Jun;22(6):639–644. doi: 10.1515/znb-1967-0614. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Sihra C. K., Slabas A. R. The oxidation-reduction potential of the reaction-centre chlorophyll (P700) in Photosystem I. Evidence for multiple components in electron-paramagnetic-resonance signal 1 at low temperature. Biochem J. 1977 Jan 15;162(1):75–85. doi: 10.1042/bj1620075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Felton R. H., Dolphin D., Vegh L. The cation radicals of free base and zinc bacteriochlorin, bacteriochlorophyll, and bacteriopheophytin. Proc Natl Acad Sci U S A. 1974 Mar;71(3):994–998. doi: 10.1073/pnas.71.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer J., Davis M. S., Brune D. C., Spaulding L. D., Borg D. C., Forman A. Chlorophyll radicals and primary events. Brookhaven Symp Biol. 1976 Jun 7;(28):74–104. [PubMed] [Google Scholar]
- Fajer J., Forman A., Davis M. S., Spaulding L. D., Brune D. C., Felton R. H. Anion radicals of bacteriochlorophyll a and bacteriopheophytin a. Electron spin resonance and electron nuclear double resonance studies. J Am Chem Soc. 1977 Jun 8;99(12):4134–4140. doi: 10.1021/ja00454a037. [DOI] [PubMed] [Google Scholar]
- Feher G., Hoff A. J., Isaacson R. A., Ackerson L. C. ENDOR experiments on chlorophyll and bacteriochlorophyll in vitro and in the photosynthetic unit. Ann N Y Acad Sci. 1975 Apr 15;244:239–259. doi: 10.1111/j.1749-6632.1975.tb41534.x. [DOI] [PubMed] [Google Scholar]
- Fenna R. E., Matthews B. W. Structure of a bacteriochlorophyll a-protein from Prosthecochloris aestuarii. Brookhaven Symp Biol. 1976 Jun 7;(28):170–182. [PubMed] [Google Scholar]
- Goldfield M. G., Halilov R. I., Hangulov S. V. Correlation of the light-induced change of absorbance with ESR signal of photosystem II in presence of silicomolybdate. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1199–1203. doi: 10.1016/0006-291x(78)90669-1. [DOI] [PubMed] [Google Scholar]
- Haveman J., Mathis P. Flash-induced absorption changes of the primary donor of photosystem II at 820 nm in chloroplasts inhibited by low pH or tris-treatment. Biochim Biophys Acta. 1976 Aug 13;440(2):346–355. doi: 10.1016/0005-2728(76)90069-4. [DOI] [PubMed] [Google Scholar]
- KOK B. On the reversible absorption change at 705 mu in photosynthetic organisms. Biochim Biophys Acta. 1956 Nov;22(2):399–401. doi: 10.1016/0006-3002(56)90172-x. [DOI] [PubMed] [Google Scholar]
- KOK B. Partial purification and determination of oxidation reduction potential of the photosynthetic chlorophyll complex absorbing at 700 millimicrons. Biochim Biophys Acta. 1961 Apr 15;48:527–533. doi: 10.1016/0006-3002(61)90050-6. [DOI] [PubMed] [Google Scholar]
- Katz J. J., Norris J. R., Shipman L. L., Thurnauer M. C., Wasielewski M. R. Chlorophyll function in the photosynthetic reaction center. Annu Rev Biophys Bioeng. 1978;7:393–434. doi: 10.1146/annurev.bb.07.060178.002141. [DOI] [PubMed] [Google Scholar]
- Ke B. The primary electron acceptor of photosystem. I. Biochim Biophys Acta. 1973 Feb 12;301(1):1–33. doi: 10.1016/0304-4173(73)90010-4. [DOI] [PubMed] [Google Scholar]
- Klimov V. V., Klevanik A. V., Shuvalov V. A., Kransnovsky A. A. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977 Oct 15;82(2):183–186. doi: 10.1016/0014-5793(77)80580-2. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Malkin R. The oxidation-reduction potentials of electron carriers in chloroplast photosystem I fragments. Arch Biochem Biophys. 1973 Nov;159(1):555–562. doi: 10.1016/0003-9861(73)90488-8. [DOI] [PubMed] [Google Scholar]
- Lutz M. Antenna chlorophyll in photosynthetic membranes. A study by resonance Raman spectroscopy. Biochim Biophys Acta. 1977 Jun 9;460(3):408–430. doi: 10.1016/0005-2728(77)90081-0. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Laser-flash-activated electron paramagnetic resonance studies of primary photochemical reactions in chloroplasts. Biochim Biophys Acta. 1975 Aug 11;396(2):250–259. doi: 10.1016/0005-2728(75)90039-0. [DOI] [PubMed] [Google Scholar]
- Mathis P., Vermeglio A. Chlorophyll radical cation in photosystem II of chloroplasts. Millisecond decay at low temperature. Biochim Biophys Acta. 1975 Sep 8;396(3):371–381. doi: 10.1016/0005-2728(75)90143-7. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Scheer H., Druyan M. E., Katz J. J. An electron-nuclear double resonance (ENDOR) study of the special pair model for photo-reactive chlorophyll in photosynthesis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4897–4900. doi: 10.1073/pnas.71.12.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. R., Uphaus R. A., Crespi H. L., Katz J. J. Electron spin resonance of chlorophyll and the origin of signal I in photosynthesis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):625–628. doi: 10.1073/pnas.68.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson K. D., Sato V. L., Sauer K. Exciton interaction in the photosystem I reaction center from spinach chloroplasts. Absorption and circular dichroism difference spectra. Biochemistry. 1972 Nov 21;11(24):4591–4595. doi: 10.1021/bi00774a027. [DOI] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Verschoor G. A. Primary reactions of photosystem II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):98–106. doi: 10.1016/0005-2728(76)90116-x. [DOI] [PubMed] [Google Scholar]
- Radmer R., Kok B. Energy capture in photosynthesis: photosystem II. Annu Rev Biochem. 1975;44:409–433. doi: 10.1146/annurev.bi.44.070175.002205. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Dolan E., Ke B. Spectral and kinetic evidence for two early electron acceptors in photosystem I. Proc Natl Acad Sci U S A. 1979 Feb;76(2):770–773. doi: 10.1073/pnas.76.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorkom H. J., Pulles M. P., Wessels J. S. Light-induced changes of absorbance and electron spin resonance in small photosystem II particles. Biochim Biophys Acta. 1975 Dec 11;408(3):331–339. doi: 10.1016/0005-2728(75)90134-6. [DOI] [PubMed] [Google Scholar]
- Visokinskas A. A. Iavlenie kristallizatsii biopolimerov v zlokachestvennoi opukholi. Biofizika. 1976 May-Jun;21(3):445–452. [PubMed] [Google Scholar]
- Visser J. W., Rijgersberg C. P., Gast P. Photooxidation of chlorophyll in spinach chloroplasts between 10 and 180 K. Biochim Biophys Acta. 1977 Apr 11;460(1):36–46. doi: 10.1016/0005-2728(77)90149-9. [DOI] [PubMed] [Google Scholar]
- Witt H. T. Coupling of quanta, electrons, fields, ions and phosphrylation in the functional membrane of photosynthesis. Results by pulse spectroscopic methods. Q Rev Biophys. 1971 Nov;4(4):365–477. doi: 10.1017/s0033583500000834. [DOI] [PubMed] [Google Scholar]
- Witt H. T. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979 Mar 14;505(3-4):355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]
- van Gorkom H. J., Tamminga J. J., Haveman J. Primary reactions, plastoquinone and fluorescence yield in subchloroplast fragments prepared with deoxycholate. Biochim Biophys Acta. 1974 Jun 28;347(3):417–438. doi: 10.1016/0005-2728(74)90080-2. [DOI] [PubMed] [Google Scholar]



