Summary
This prospective, field-based study examined the association between actigraphically-measured total sleep time and incident illness including cold, flu, gastroenteritis, and other common infectious diseases (e.g., strep throat) in adolescents over the course of a school semester. Participants were 56 adolescents ages 14–19 years (mean = 16.6 (standard deviation = 1.2), 39% male) from 5 high schools in Rhode Island. Beginning in late January, adolescents wore actigraphs (mean 91 (19) days, range 16 – 112 days) and were assigned post-hoc to Longer or Shorter sleep groups based on median splits. Adolescents were interviewed weekly across as many as 16 weeks (modal number of interviews = 13) using a structured protocol that included 14 health event questions. Illness events and illness-related school absences were coded for 710 completed interviews, with 681 illness events and 90 school absences reported. Outcomes (illness bouts, illness duration, and absences) were compared among sex, sleep, and academic year groups using non-parametric regression. In a subset of 18 subjects, mean actigraphically estimated total sleep time 6 nights before matched illness/wellness events was compared using MANOVA. Longer sleepers and males reported fewer illness bouts; total sleep time effects were more apparent in males than females. A trend was found for shorter total sleep time before ill events. The present findings in this small naturalistic sample indicate that acute illnesses were more frequent in otherwise healthy adolescents with shorter sleep, and illness events were associated with less sleep during the prior week than comparable matched periods without illness.
Keywords: adolescent, total sleep time, common illness, actigraphy, sleep duration
INTRODUCTION
A common belief is that shorter sleep and infection susceptibility are linked, particularly for viral infection. Indeed this “old wives tale” has been substantiated by research showing interactions between sleep and both the innate and adaptive immune systems (Majde and Krueger, 2005). This putative link between sleep and infection susceptibility is particularly critical for adolescents, as they frequently obtain too little sleep, getting by with 7 – 7.5 hours on weeknights (Hansen et al., 2005; Wolfson and Carskadon, 1998) vs. the 9+ hours researchers have shown to be adequate for individuals with still-developing bodies and brains (Carskadon, 1999; Dahl and Lewin, 2002).
Using short-duration laboratory studies, cross-sectional epidemiological studies, and longitudinal studies of chronic illness, researchers have shown an association between shorter sleep and illness. Researchers have demonstrated interactions between sleep and both the innate and adaptive immune systems, and the interaction of sleep with both of these systems may indicate a critical function of sleep (Majde and Krueger, 2005). Total and partial sleep deprivation have been implicated in the dysregulation of immune (Faraut et al., 2012; Opp et al., 2007) and endocrine factors (Okun, 2011). Such outcomes as decreased T-cell function and suppressed immune response to vaccination are consistent across total and partial sleep deprivation, while others, such as natural killer cell activity, reverse under conditions of total vs. partial deprivation (see Lange et al., 2010 for a review). These findings suggest that shorter or absent sleep may increase susceptibility to diseases, especially those mediated by inflammation including cardiovascular disease, diabetes, metabolic disease and depression (Okun, 2011). Most sleep and immune response studies, however, have been performed in the laboratory under conditions of total sleep deprivation and may not mirror immune response to an illness challenge under natural conditions. Partial sleep deprivation studies also have been set in artificial conditions for a limited time frame (1–5 days) (cf., Bryant et al., 2004).
In addition to laboratory studies of sleep and immunity, findings from studies of vaccinations given under varying circumstances support an association of shortersleep and reduced immune response. Investigators have shown that limiting sleep to 4 hours for 4 nights before influenza vaccination is related to a significant negative effect on mean antibody titers in men ten days after immunization (Spiegel et al., 2002). Prather and colleagues showed in a recent study that shorter sleep duration, measured by actigraphy, was associated with a lower secondary antibody response to Hepatitis B immunization, and that shorter sleep, whether measured by actigraphy or self-report sleep diary, predicted a decreased likelihood of being clinically protected from Hepatitis B six months after vaccination (Prather et al., 2012). This work parallels earlier work on sleep and Hepatitis A immunity (Lange et al., 2003, 2011)
While researchers have explored associations between sleep and dysregulated immune factors in humans, few have addressed effects of shorter sleep on common illnesses. In an experimental study, Cohen et al. (2009) found that participants with less than 7 hours of sleep were 2.94 times more likely to develop a cold when infected with a cold virus than those with 8 hours or more of sleep. Patel and colleagues also examined incident pneumonia in the Nurse’s Health study among women (ages 37 to 57) who were free of cancer, cardiovascular disease, diabetes and asthma, with no prior history of pneumonia (n= 56,953); compared to 8-hour sleepers, women sleeping <= 5 hours/night or >= 9 hours/night had significantly higher relative risk of contracting pneumonia (Patel et al., 2012).
Our project complements existing studies by examining prospective, naturalistic data collected in 1997–2000 on total sleep time and illness in 56 adolescents. Links between sleep and illness behaviors in this sample were addressed from as many as 112 nights of actigraphically-measured sleep and 16 weeks of weekly in-person interviews, allowing examination of the association between shorter total sleep time and susceptibility to common illnesses over a school term. From detailed interview notes we also prepared qualitative case reports capturing factors contributing to short sleep and illness in adolescents.
The mixed methods analyses presented in this paper extend sleep literature by (1) drawing on quantitative and qualitative data collected in a naturalistic setting and (2) rigorously measuring total sleep time and incident illness among healthy adolescents across a school semester to draw conclusions about associations between total sleep time and common illness. Our specific hypotheses for this study were that (1) shorter sleepers would experience more frequent illness across the study period and (2) adolescents would experience shorter sleep duration prior to illness than prior to a matched period of wellness.
METHODS
Participants
Participants were 56 adolescents ages 14.5 to 19.3 years (M = 16.6, SD = 1.2) who were high school students in Rhode Island between 1997 and 2000. Males made up 39% of the sample. Adolescents participated in the current study for one school semester from late January to mid-May. The sample included students from independent and public (state-supported and administered) schools in Rhode Island: 11 from a coeducational parochial high school (1997), 9 from an independent girls’ school (1998), 11 from an independent coeducational school (1998), 15 from a suburban public high school (1999), and 10 from an urban public high school (2000). All were non-boarding day students. See Table 1 for participant demographics. The Lifespan Institutional Review Board (IRB) approved the study, and informed consent was obtained from parents (for participants under age 18) or students aged 18 or older (n=6). Students and their parents were compensated with gift certificates to local stores. Adolescents were recruited from a larger pool of students (N=309) who participated in a prior study (described in Wolfson et al., 2003). Students were not invited to participate in the current study if they had been diagnosed with sleep apnea, narcolepsy, restless legs, major depressive disorder, bipolar disorder, schizophrenia or a major chronic medical condition such as cancer. Students with a current eating disorder diagnosis, head trauma with a loss of consciousness, repeated involvement with the juvenile court system, or current use of psychoactive substances known to affect the sleep/wake cycle or daytime alertness were excluded. Adolescents were assigned to Longer (n= 27, 11 male) or Shorter (n= 29, 11 male) sleep groups based on their weeknight actigraphically-measured sleep from January to May (see Measures section for details of actigraphy). Longer sleepers slept an average of 7 hrs 28 minutes [SD = 30 minutes] on school nights across the semester, while Shorter sleepers slept 6 hrs 24 minutes [SD = 40 minutes] on average.
Table 1.
Participant Demographics (n = 56)
| Characteristic | Number | Percentage |
|---|---|---|
| Age | ||
| 14 | 10 | 17.9 |
| 15 | 12 | 21.4 |
| 16 | 19 | 33.9 |
| 17 | 9 | 16.1 |
| 18 | 5 | 8.9 |
| 19 | 1 | 1.8 |
| Mean Age (SD) | 16.6 years (1.2) | |
| Sex | ||
| Male | 22 | 39.3 |
| Female | 34 | 60.7 |
| Ethnicity | ||
| White | 47 | 83.9 |
| Black | 3 | 5.4 |
| Hispanic | 3 | 5.4 |
| Multi-Ethnic | 2 | 3.6 |
| Other | 1 | 1.8 |
| School Type | ||
| Independent | 31 | 55.4 |
| Public | 25 | 44.6 |
Note. SD = standard deviation.
To assess whether sleep differed before episodes of illness vs. wellness, a subsample of eighteen adolescents (ages 14–18 years, 7 male) was selected based on the presence within the same participant of useable ill and well events preceded by six well days (see below). Of these eighteen adolescents, 14 had one matched event and 4 had two. Adolescents in the subsample reported 215 illness events in 247 interviews, and 100% completed 12 or more interviews (mean 13.7 interviews). Useable ill events were defined as a report of illness preceded by six well days that did not occur within one week after the spring daylight saving time (DST) shift. Well events for the same participants were also sought within the same month and were preceded by six well days. Well events also did not occur within one week after the DST shift. Of 52 participants who reported illness, only 18 met these strict criteria for illness and wellness timing. A particular challenge was clusters of illness where participants were not well for six consecutive days, but the six-day window was maintained to limit potential sleep duration bias.
Measures
Actigraphy
Each participant wore an actigraph (Mini-motionlogger; Ambulatory Monitoring Inc, Ardsley, NY) on the nondominant wrist throughout the day and night for the duration of the study (mean = 91 days, SD = 19 days, range 16 – 112 days), except when the actigraph might get wet or while engaging in a contact sport. The actigraph was set for 1-minute recording bins, zero crossing mode, and a sensitivity of .05 g in a frequency range of 2 to 3 Hz. Participants completed a daily sleep diary indicating total sleep times (“slept this much last night” answered as hours and minutes), bedtimes (“attempted to fall asleep at” answered as specific time), and wake times (“finally woke at”), as well as times when the actigraph was off (i.e., shower or sports practice). Participants called our time-stamped telephone answering machine before bedtime and after risetime. Actigraph exchanges took place weekly during an in-person interview (described below), and daily sleep diaries were also collected from participants at that time, allowing the research assistant interviewers to check the diary for completeness and evaluate the actigraph data in the context of the diary data.
Actigraph data were analyzed according to the procedures of Acebo and colleagues (Acebo et al., 1999) to estimate sleep using Action-W2 software (AMI) and the validated Actigraphic Scoring Analysis (ASA) algorithm, also called the “Sadeh” algorithm (Sadeh et al., 1994). This procedure relies heavily upon the concurrent behavioral self-report obtained by the sleep diaries. For actigraphy and diary data, nights were defined as school nights if the participant went to school the following day and weekend nights were Friday and Saturday nights only. We excluded individual nights of actigraphy data if the actigraph was off or not working for all or part of the documented nocturnal sleep episode or if the actigraph record included unusual external motion. Our senior research team (CA, RS, MAC) examined such questionable records and made consensus judgments regarding acceptability of the data. For this report we examined total sleep time (minutes of scored sleep during sleep period between reported bedtime and waketime) from nightly actigraphy scoring.
In-Person Interviews
Adolescents were interviewed weekly from January to May. Interviews were performed face to face by trained research assistants using a structured interview protocol that covered 10 life domains1 and included 14 questions about health events asked for each day of the week since the participant’s previous interview. Absences from school and the reason for those absences were also queried. 87% of participants completed 12 or more weekly interviews. Events were coded and entered into SPSS for 710 completed interviews across the 56 participants. Participants reported 681 illness events and 90 illness-related school absences. Outcome variables derived from interview data included number of illness bouts (calculated as reported illness separated by at least one calendar day), illness duration (calculated as the sum of days ill within a single illness bout), and absences from school, (included if participants attributed absence to illness). For analysis, mean illness duration was calculated as total days ill/number of illness bouts.
Identification of illnesses was determined from analysis of weekly interviews. In the health section of the interview, students were asked “were you sick this week?” and interviewers wrote down what type of illness they reported (often symptoms like sore throat, menstrual cramps, or back pain) and the days and times this illness had affected them since their last interview. If a student missed an interview because of illness, the interview was rescheduled and the interviewer collected daily illness data back to the date of the previous interview. Illness did not include accidental injuries, such as cuts, sprains, falls, etc. that might have been caused by lack of sleep, nor did it include status changes in chronic illnesses such as asthma. Type of illness was classified into the following categories by a research assistant, based on the participant’s report of their illness or the symptoms noted on the interview sheet: cold, flu-like, gastroenteritis, menstrual complication, pain, and other. KMO and a second research assistant subsequently checked the classifications for accuracy. In instances where the illness type was not clear, a consensus meeting among several of the authors (KMO, RS, MAC) determined the final coded illness.
Data Analysis
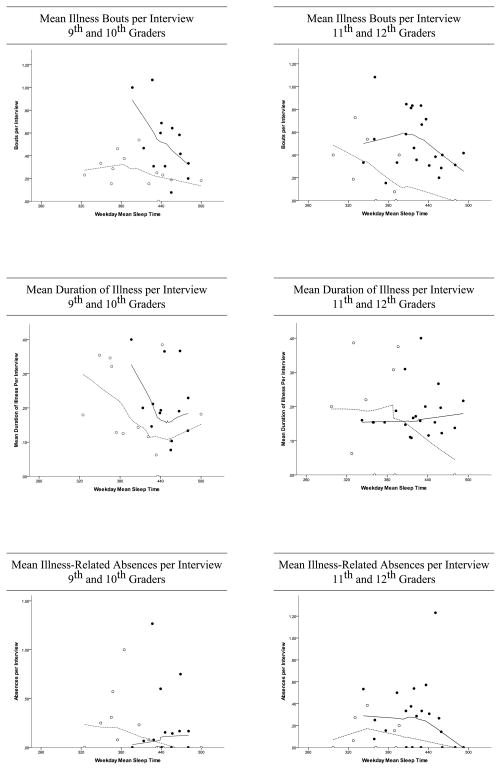
Due to variation in the number of completed interviews, variables were computed as a function of the number of interviews the participant completed: mean illness bouts per interview, mean duration of illness per interview, and mean days absent per interview. Non-parametric regression (i.e., localized regression (Cleveland, 1979)) was used to graphically examine the relationships between total sleep time and the three illness outcomes (Figure 1). Separate non-parametric regression lines were fit for adolescents based on academic year (9th/10th vs. 11th/12th) and based on sex. Due to the non-linear relationships between total sleep time and some of the illness outcomes, median splits were used to define long and short sleep groups. To calculate these, total sleep time was stratified by sex and academic year, given the known decline in total sleep time across high school in adolescents (Feinberg et al., 2012; Wolfson and Carskadon, 1998) Individuals whose sleep lengths fell above the median were considered longer sleepers, and those below the median were shorter sleepers. To classify values on the median, the midpoint of the two adjacent values was taken and the median value was compared to and classified as above (longer sleeper) or below (shorter sleeper) this midpoint. Median values for each sex and academic year group appear in Table 3. Multivariate analyses of variance (MANOVA) were used to test the association of total sleep time and illness outcomes, with sex, academic year, and sleep group being included as between subject factors. A full-factorial model was used which included interactions between factors as well as a higher order interaction term between all three factors. To analyze sleep before illness vs. wellness, mean actigraphically estimated total sleep times for the 6 nights before illness/wellness events were compared using 2-way repeated measures analysis of variance, with sex as a between-subjects factor.
Figure 1.
Mean illness bouts, mean duration of illness, and mean illness-related absences by sex and academic level, with mean weeknight total sleep time from January to May shown as a continuous variable. The left-hand graph shows 9th/10th graders, while the right-hand graph shows 11th/12th graders. Open circles represent males, and filled circles represent females. Dotted lines show a line of best fit for males, while solid lines show a line of best fit for females.
Table 3.
Means and Standard Deviations of Three Illness Outcomes Stratified by Sex, Sleep Group, and Academic Year
| SEX | Male | Male | Male | Male | Female | Female | Female | Female |
|---|---|---|---|---|---|---|---|---|
| SLEEP GROUP | Short | Long | Short | Long | Short | Long | Short | Long |
| ACADEMIC YEAR | 9/10 | 9/10 | 11/12 | 11/12 | 9/10 | 9/10 | 11/12 | 11/12 |
| Median Value (minutes) | 406.78 | 361.28 | 445.85 | 422.23 | ||||
| Mean Illness Bouts per Interview | .31 | .22 | .37 | .12 | .69 | .36 | .60 | .44 |
| SD | .11 | .16 | .29 | .19 | .30 | .20 | .29 | .20 |
| Mean Illness Duration per Interview | .24 | .14 | .17 | .17 | .22 | .21 | .17 | .19 |
| SD | .11 | .12 | .15 | .20 | .09 | .12 | .06 | .08 |
| Mean Absences per Interview | .37 | .04 | .14 | .09 | .34 | .20 | .28 | .29 |
| SD | .37 | .09 | .18 | .10 | .51 | .25 | .21 | .36 |
Adolescents who did not report illness were excluded from the matched analysis, as were adolescents who reported too many illness bouts, where it was not possible to find matched illness and non-illness bouts preceded by 6 well days. In order to incorporate adolescents at these extremes of illness, cases were selected and qualitative reports prepared by KMO. For these case reports, original interview notes and descriptive statistics about the sleep of individual participants were examined and two cases of short sleepers were selected, one of whom reported few illnesses and the other many. Notes were made on their week-by-week illness reports and sleep patterns, including notations about academic activities, social activities, and schedule changes (such as school vacation or a change in work schedule) that may have led to reduced or increased sleep. These notes were then synthesized into a brief case report that presents major factors affecting that student’s sleep and illness experience. All of the student names given in these case reports are pseudonyms.
RESULTS
Study participants were 16.6 years old on average and 39% were male. Eighty-four percent were white and 45% attended public schools in Rhode Island. See Table 1.
Participants reported a variety of common illnesses that were grouped post-hoc into illness categories that included cold, flu-like, gastroenteritis, menstrual complaints, pain and “other.” The number of ill-days captured by each category, and the number of participants reporting each illness category are reported in Table 2.
Table 2.
Illness Categories Reported by Participants (n=52)
| Illness Category | Description | Frequency | Number of participants mentioning |
|---|---|---|---|
| Cold | Clogged or running nose, sore throat, cough, slight or no fever | 290 | 40 |
| Flu-like | Fever, with 4 of 6: muscle pain, fatigue, sore throat, clogged or running nose, cough, headache | 34 | 9 |
| Gastroenteritis | Slight/no fever, gastritis, nausea, vomiting and/or diarrhea | 61 | 23 |
| Menstrual | Menstrual cramps or pain | 17 | 6 |
| Pain a | Pain, not menstrual or stomach | 131 | 28 |
| Other b | 140 | 29 | |
|
| |||
| Total | 681 | ||
Note: 52 ill adolescents, 18 M, 34 F
Most common Pain = headache (69), sore throat (37)
Most common Other = sinus infection/sinusitis (33), strep throat (24), allergies (13), general sick [e.g., didn’t feel well] (11).
Associations Between Sleep Group & January to May Illness
Omnibus tests for the interaction terms among the factors total sleep time, sex, and sleep group showed no significant interactions, nor was the omnibus test for academic year significant. On the other hand, the omnibus tests for sex (Wilks’ Λ = .72, F (3,46) = 5.92, p = .002), and sleep group (Wilks’ Λ = .81, F (3,46) = 3.55, p = .02) showed statistically significant effects. These findings are highlighted in Table 3, showing the means and standard deviations of the three illness outcomes (mean number of illness bouts per interview, mean duration of illness per interview, and mean absences per interview) stratified by sex, sleep group, and academic year. ANOVAs run on each outcome indicated significant differences on mean number of illness bouts per interview between males and females (F (1,48) = 17.70, p < .001, η2 = .27) and between longer and shorter sleepers (F (1,48) = 10.09, p =.003, η2 = .17), with males and longer sleepers reporting fewer illness bouts per interview. Figure 1 shows mean illness bouts, mean duration of illness, and mean illness-related absences in relation to sex, academic level, and mean weeknight total sleep time from January to May. Overall, reported bouts of illness per interview declined with longer sleep for males and females, with more pronounced effects for younger females. In younger participants, mean duration of illness per interview was shortest at approximately 7.5 hours of sleep per weeknight for males and females, while for older participants, mean weeknight sleep time had little apparent association with illness duration. In terms of illness-related absences, longer sleep was protective in all age/sex groups except for younger females.
Differences in Sleep Before Matched Periods of Illness and Wellness
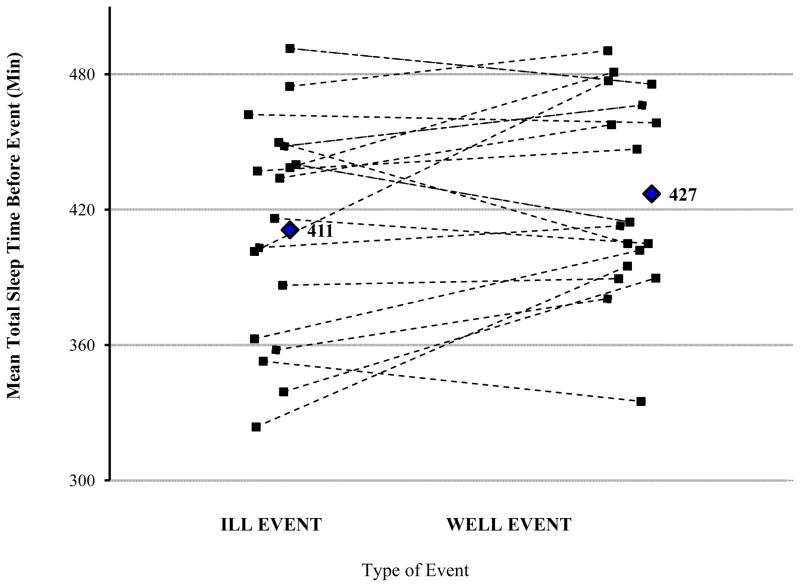
A trend was found for shorter total sleep time in the 6-day window before periods of illness. Before ill events, mean nightly total sleep time was 411 minutes (SD = 49 mins); before well events, mean nightly total sleep time was 427 minutes (SD = 43 mins), F (16) = 3.88, p = .066. No main effect of individual night (F (12) = 1.68, p = .21) nor interactions of event type and night (F (12) = 1.11, p = .41), event type and sex (F (16) = 0.45, p = .51), or sex and night (F (12) = 0.45, p = .80) were found. Figure 2 shows mean total sleep time before ill events vs. well events overall, and for each of the 18 participants included in this analysis.
Figure 2.
Mean sleep before ill and well events, by participant. Diamonds represent mean values across all participants (mean before ill events = 411 minutes of total sleep time, mean before well events = 427 minutes of total sleep time), and individual lines indicate participants’ mean total sleep time values for 6 nights before matched ill and well events.
Brief Case Reports
Adolescents who reported either no illness or illnesses so frequent to preclude a 6-day “well” window before illness were not included in the matched pairs assessment reported above. To incorporate such adolescents into our assessment of sleep and illness, we report qualitative impressions of sleep and alertness from the interviews of two participants. Both participants are 17-year-old males who are independent school students and classified as Shorter sleepers. One, Eric, reported 0 days ill, while the other, Bob, reported 35 days ill across the school semester.
ERIC: 12th grade, 17 years old.
Eric describes himself as “pretty much an A student.” His extracurricular activities include playing an instrument, participating in academic groups (i.e., Academic Decathalon), and involvement with a religious youth organization and an Arabic dancing group. He works on Sundays at a gas station.
Over the semester, Eric sleeps by actigraph estimate an average of 6 hours and 1 minute per night (SD: 73 mins) on weeknights [71 nights] and an average of 6 hours and 4 minutes per night (SD: 122 mins) on weekend nights [30 nights)].
Eric reports struggling to stay awake or dozing in several circumstances, including nodding off while watching TV after 11 pm and when watching a movie with friends after midnight. He reports wanting to do homework at 11:30 pm on a Saturday, but being too tired, even after taking a shower to wake himself up.
Eric manages his sleep by catching up over breaks and altering his work schedule. Over spring break he reports waking up “naturally” every day. Although he says he works every Sunday 9–5, from his weekly interviews he actually works only 4 of 13 possible Sundays. Some Sundays he engages in alternate activities like going to church or participating in an Academic Decathalon. On at least two days, however, he calls in “sick” to work to allow time for sleep. On both occasions, Eric reports conflicting feelings about calling in sick and getting much-needed sleep. On one “sick” day, he describes his mood as negative because he took the day off and didn’t fulfill his commitment, but positive because he caught up on sleep and got work done.
BOB: 12th grade, 17 years old.
Bob lives 35–40 minutes away from Providence, RI, where he attends high school. Because of the distance and his involvement in school activities, like the school play, Bob spends 1–2 nights per week with friends who live near school. Bob describes his work style as “nothing, punctuated by large intervals of work.” He reports starting projects at 5 or 6 pm and working through until 4 or 5 am, napping at school the next day to catch up. He is self-employed, repairing computers a few hours per week.
Over the semester, Bob sleeps by actigraph estimate an average of 5 hours and 30 minutes per night (SD: 91 mins) on weeknights [52 nights] and an average of 7 hours and 19 minutes per night (SD: 115 mins) on weekend nights [21 nights]. Bob’s reports of illness are dominated by colds and sinus infections.
Bob’s sleep patterns are shaped by extracurricular activities and his social life, including weeknight time at friends’ houses and a relationship he develops with a girl after several late-night on-line chats, including from 12 am to 7 am one Sunday night. Bob chooses to complete schoolwork at the last minute, which requires him to stay up late. He also works at night; he reports seven weeknight work bouts, none occurring earlier than 8 pm.
Bob manages his sleep in both intentional (like leaving social events to take naps), and unintentional ways. He reports falling asleep in school, always falling asleep as a passenger in a car, and struggling to stay awake or falling asleep when he is doing homework, sitting inactive in a public place, and even driving a car. He even reports sometimes falling asleep in the bathtub.
As the semester comes to a close, Bob’s sleep does not improve. His sleep schedule is still driven by his social life, by the onset of sleep problems related to a breakup and reunion with his on-line girlfriend, and a realization that he will begin college in a few months.
DISCUSSION
The negative consequences of shorter sleep in adolescents have been well documented in the literature, with investigated physical consequences including, for example, increased risk of obesity (Cappuccio et al., 2008), higher adiposity measures, and increased risk of high cholesterol, both of which were found to be more significant in females (Gangwisch et al., 2010; Yu et al., 2007). Known consequences also extend to psychiatric illness, substance abuse risk, and suicidality (Lee et al., 2012; Pasch et al., 2010).
Consistent with the literature examining incident illness in the context of shorter sleep (Cohen et al., 2009; Patel et al., 2012), and pertinent to the ongoing debate about how much sleep adolescents need (Matricciani et al., 2012), our study found that adolescents classified as Shorter sleepers based on an average of 91 days of actigraphy monitoring from January to May reported more frequent illness bouts than adolescents classified as Longer sleepers during the school year. Sex differences were apparent, with Males reporting significantly fewer illness bouts than Females. This finding of males reporting better outcomes, even in the face of similar sleep durations, is consistent with results from a large European cross-sectional study that showed a lower impact of shorter sleep on adiposity in male adolescents (Garaulet et al., 2011), although more research in this vein is needed. School absences per interview also differed between Longer and Shorter sleepers, although this finding did not achieve statistical significance (see Figure 1). Our work exploring the relationship between shorter sleep and common illness builds on the literature cited above. It also extends this work by examining the potential everyday correlates of shorter sleep, such as common illness and school absences, which do not require waiting months or years for a diagnosis of obesity, high cholesterol, depression, or suicidal tendencies. The significance of this analysis of the everyday is that it provides another brick in the wall of evidence that adolescents need more sleep than they are getting, and these findings should encourage individuals in families, schools, and policy-making positions to make additional sleep a priority for shorter-sleeping adolescents.
In addition to our main analysis of the relationship between shorter sleep and illness, analyzing matched ill/well bouts in this naturalistic sample represents an innovative approach. Few, if any, researchers have collected and analyzed sleep data in adolescents for up to 16 weeks across a school semester and paired that sleep data with detailed weekly interviews targeting such relatively low-incidence events as illness. These data demonstrated a trend that illness events in adolescents were associated with less sleep during the prior week than comparable periods before matched wellness episodes. This naturalistic approach lends modest support to findings from experimental studies showing that longer sleep is associated with better immune response and health outcomes (Faraut et al., 2012; Okun, 2011; Opp et al., 2007; Prather et al., 2012).
The two brief case reports contribute to understanding different ways that sleep and illness may be associated in the lives of individual adolescents. Researchers investigating sleep and illness rarely use qualitative methods such as interviews or analyses examining and coding interview notes. When they do use these methods, it is typically to collect general perceptions of sleep (Noland et al., 2009; Owens et al., 2006) and not to explore the sleep lives of individuals in detail (although see Henry and Rosenthal, 2013 for an example of close attention to sleep lives in individuals diagnosed with sleep apnea). In this study, although both Eric and Bob were defined as Shorter sleepers, Eric’s more consistent sleep timing across the weekday and weekend may have been protective (Owens and Mindell, 2011). He also may have been exposed to fewer illnesses than Bob, who reported spending significant amounts of time at friends’ houses. Bob’s preference for scheduling his social time and work time (both for school and his business) at night also limited his sleep to an even greater extent than Eric’s – while Eric reports trying (and failing) to do homework at 11:30 pm and struggling to stay awake during a movie past midnight, Bob reports successfully finishing computer work and chatting online with friends well into the overnight hours. Identifying specific issues in individual sleep patterns may be a useful way to begin to address factors that may be amenable to intervention in shorter sleeping adolescents.
Limitations
While collecting sleep and illness reports in a naturalistic sample represents an innovation of this work, it also presents limitations, notably the lack of control of factors beyond sleep that lead to illness in these adolescents. To address this limitation, we used several analyses and included two brief case reports designed to contextualize reports of sleep and illness in the lives of adolescents. Other factors associated with sleep and illness in previous studies were not measured in this study, including family functioning and stress. Stress has known effects on both sleep and the immune system in animal models (Toth, 1995). A recent study by Prather and colleagues, however, showed only a modest effect of perceived daily stress on their observed sleep-vaccination response link in humans (Prather et al., 2012). We were also not able to include extreme long sleepers in this study, due to our measurement of sleep among adolescents in school, although extant literature does address potential negative health consequences of longer sleep durations. In addition, the relationship between sleep duration, sex and age is complicated and we did not have full statistical power to model this relationship due both to our limited sample size and the complex relationship between sex and sleep duration, such that more males showed shorter sleep durations.
Conclusion
Examining concurrent sleep and illness data, we found that acute illnesses were more frequent in otherwise healthy adolescents with shorter sleep across a school semester. We also found a trend in this small naturalistic sample that illness events in adolescents were associated with less sleep during the prior week than comparable periods before matched wellness. Qualitative case reports highlighted regularity of sleep timing, exposure to friends, and preference for evening activity as potential contributors to illness; the latter may link to extroverted personality types and evening chronotypes. While many factors may affect incidence of common illnesses in adolescents, our findings indicate that further examination of the role of shorter total sleep time in the development of common illness may be a fruitful avenue for investigation.
Acknowledgments
Financial support for this study was provided by a grant from the National Institute of Mental Health (MH45945 to Dr. Carskadon) and analysis was supported by T32 training grants (MH19927 to Dr. Orzech and T32078788 to Dr. Barker). We thank Susan Labyak, Jennifer Maxwell, Bethany Quinn, and Bonnie Boyd for their assistance with this project.
Footnotes
Interview instrument available upon request from corresponding author.
Conflicts of Interest: Kathryn M. Orzech has no conflicts of interest to report.
Christine Acebo is an employee of Jazz Pharmaceuticals. Her involvement on this study preceded this employment and the study has no affiliation with Jazz Pharmaceuticals.
Ronald Seifer has no conflicts of interest to report.
David Barker has no conflicts of interest to report.
Mary A. Carskadon has no conflicts of interest to report.
Author Contributions:
Kathryn M. Orzech provided the impetus to analyze total sleep time and health in this existing data set, and performed initial statistical analyses to examine hypotheses about sleep and health. She also conducted the qualitative analysis to construct case studies, and was the primary author of the manuscript.
Christine Acebo was involved in the original design and implementation of the research project, specifically managing the actigraphy data collection and analysis. She was also instrumental in data reduction efforts.
Ronald Seifer was involved in the original design and implementation of the research project, including data reduction efforts, and also its revival, serving as a statistical consultant and a member of a consensus team to decide issues such as how to classify sets of symptoms into disease categories.
David Barker tutored Kathryn Orzech in basic statistics and provided higher level statistical analysis for the revised manuscript.
Mary A. Carskadon wrote and received the grant for the original research project and was instrumental in both its design and implementation. She supervised the original data collection and data reduction of the interviews. She also supervised the analysis of total sleep time and health in this data set, and was the final editor of the manuscript prepared by Kathryn Orzech.
REFERENCES CITED
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson AR, Hafer A, Carskadon MA. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- Bryant P, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nature Reviews Immunology. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-Analysis of Short Sleep Duration and Obesity in Children and Adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA. When worlds collide; adolescent need for sleep versus societal demands. Phi Delta Kappan. 1999;80:348–353. [Google Scholar]
- Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. Journal of the American Statistical Association. 1979;74:829–836. [Google Scholar]
- Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002;31:175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16:137–149. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Davis NM, De Bie E, Grimm KJ, Campbell IG. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am J Physiol Regul Integr Comp Physiol. 2012;302:R533–R540. doi: 10.1152/ajpregu.00532.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Babiss LA, Opler MG, Posner K, Shen S, Turner JB, Zammit GK, Ginsberg HN. Short Sleep Duration as a Risk Factor for Hypercholesterolemia: Analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33:956–961. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Ortega FB, Ruiz JR, Rey-López JP, Béghin L, Manios Y, Cuenca-García M, Plada M, Diethelm K, Kafatos A, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond) 2011;35:1308–1317. doi: 10.1038/ijo.2011.149. [DOI] [PubMed] [Google Scholar]
- Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–1561. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- Henry D, Rosenthal L. “Listening for his breath:” The significance of gender and partner reporting on the diagnosis, management, and treatment of obstructive sleep apnea. Social Science & Medicine. 2013;79:48–56. doi: 10.1016/j.socscimed.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to Hepatitis A vaccination. Psychosomatic Medicine. 2003;65:831–835. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Annals of the New York Academy of Sciences. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Bollinger T, Diekelmann S, Born J. Sleep after vaccination boosts immunological memory. J Immunol. 2011;187:283–290. doi: 10.4049/jimmunol.1100015. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Cho SJ, Cho IH, Kim SJ. Insufficient Sleep and Suicidality in Adolescents. Sleep. 2012;35:455–460. doi: 10.5665/sleep.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majde JA, Krueger JM. Links between the innate immune system and sleep. Journal of Allergy and Clinical Immunology. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Matricciani L, Olds T, Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev. 2012;16:203–211. doi: 10.1016/j.smrv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Noland H, Price JH, Dake J, Telljohann SK. Adolescents’ sleep behaviors and perceptions of sleep. J Sch Health. 2009;79:224–230. doi: 10.1111/j.1746-1561.2009.00402.x. [DOI] [PubMed] [Google Scholar]
- Okun ML. Biological consequences of disturbed sleep: Important mediators of health? Jpn Psychol Res. 2011;53:163–176. doi: 10.1111/j.1468-5884.2011.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp M, Born J, Irwin M. Sleep and the Immune System. In: Adler R, editor. Psychoneuroimmunology. Burlington, MA: Elsevier Academic Press; 2007. pp. 579–618. [Google Scholar]
- Owens J, Mindell JA. Sleep in Children and Adolescents, An Issue of Pediatric Clinics. Elsevier Health Sciences; 2011. [Google Scholar]
- Owens JA, Stahl J, Patton A, Reddy U, Crouch M. Sleep practices, attitudes, and beliefs in inner city middle school children: a mixed-methods study. Behav Sleep Med. 2006;4:114–134. doi: 10.1207/s15402010bsm0402_4. [DOI] [PubMed] [Google Scholar]
- Pasch KE, Laska MN, Lytle LA, Moe SG. Adolescent Sleep, Risk Behaviors, and Depressive Symptoms: Are They Linked? Am J Health Behav. 2010;34:237–248. doi: 10.5993/ajhb.34.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35:97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Hall M, Fury JM, Ross DC, Muldoon MF, Cohen S, Marsland AL. Sleep and Antibody Response to Hepatitis B Vaccination. Sleep. 2012;35:1063–1069. doi: 10.5665/sleep.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- Toth LA. Sleep, sleep deprivation and infectious disease: studies in animals. Adv Neuroimmunol. 1995;5:79–92. doi: 10.1016/0960-5428(94)00045-p. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, Martin JL. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–216. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- Yu Y, Lu BS, Wang B, Wang H, Yang J, Li Z, Wang L, Liu X, Tang G, Xing H, et al. Short Sleep Duration and Adiposity in Chinese Adolescents. Sleep. 2007;30:1688–1697. doi: 10.1093/sleep/30.12.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]