Abstract
A pattern photobleaching method has been used to measure the rates of lateral diffusion of fluorescent-labeled specific anti-nitroxide IgG bound with both combining sites to nitroxide-containing phospholipids in liposomal membranes composed of dimyristoyl phosphatidylcholine at 28 degrees C ("fluid"), dipalmitoyl phosphatidylcholine at 32 degrees C ("Solid"), and dipalmitoyl phosphatidylcholine containing 15 or 25 mol% cholesterol ("solid" or "fluid," respectively, at 32 degrees C). The diffusion coefficients of the bound immunoglobulin were found to be the same as those of fluorescent-labeled phospholipids in each case even though these diffusion coefficients range from 10(-11) to 10(-8) cm2/sec. Hapten-containing liposomal membranes of the type studied here have previously been shown to elicit a number of antibody-dependent immune responses. Therefore, this work indicates that membrane-bound but otherwise freely diffusing antibodies are sufficient for these reponses.
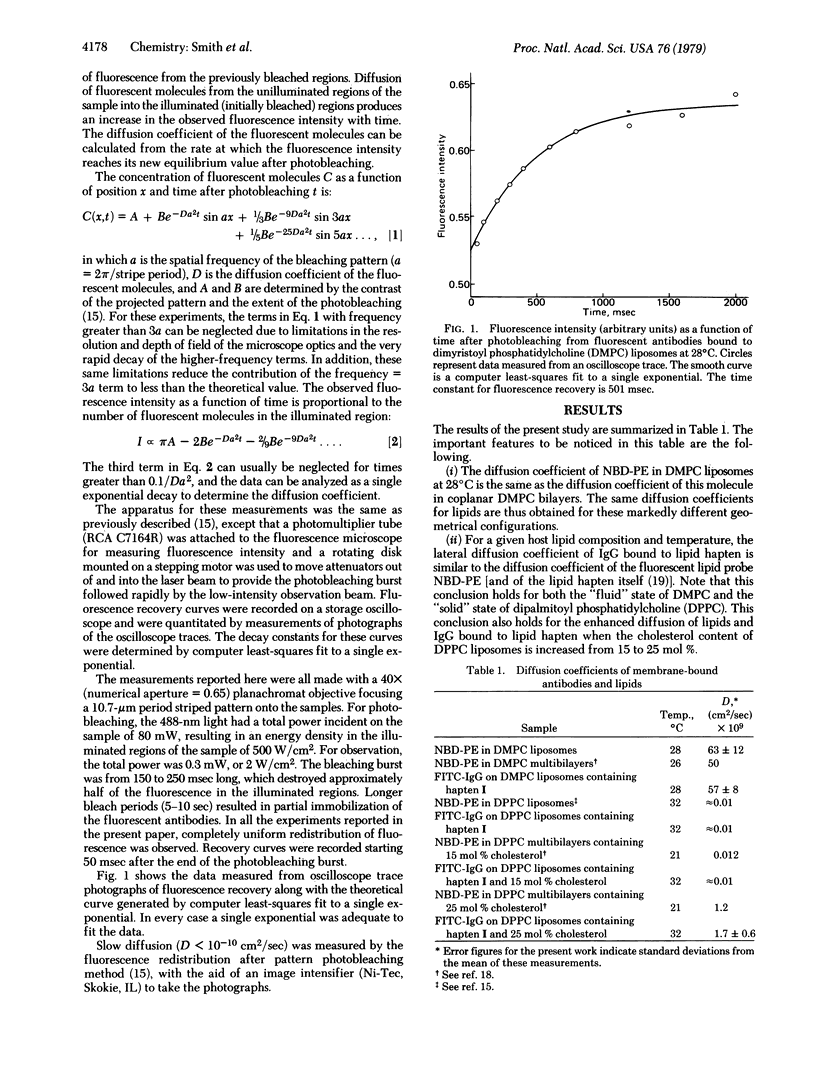
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brûlet P., McConnell H. M. Lateral hapten mobility and immunochemistry of model membranes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2977–2981. doi: 10.1073/pnas.73.9.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brûlet P., McConnell H. M. Structural and dynamical aspects of membrane immunochemistry using model membranes. Biochemistry. 1977 Mar 22;16(6):1209–1217. doi: 10.1021/bi00625a028. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Cuatrecasas P. Mobility of cholera toxin receptors on rat lymphocyte membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3844–3848. doi: 10.1073/pnas.72.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard V. H., Strominger J. L., Mescher M., Burakoff S. Induction of secondary cytotoxic T lymphocytes by purified HLA-A and HLA-B antigens reconstituted into phospholipid vesicles. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5688–5691. doi: 10.1073/pnas.75.11.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser A. F., Bartholomew R. M., Parce J. W., McConnell H. M. The physical state of membrane lipids modulates the activation of the first component of complement. J Biol Chem. 1979 Mar 25;254(6):1768–1770. [PubMed] [Google Scholar]
- Finberg R., Mescher M., Burakoff S. J. The induction of virus-specific cytotoxic T lymphocytes with solubilized viral and membrane proteins. J Exp Med. 1978 Dec 1;148(6):1620–1627. doi: 10.1084/jem.148.6.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D. G., Parce J. W., McConnell H. M. Specific antibody-dependent activation of neutrophils by liposomes containing spin-label lipid haptens. Biochem Biophys Res Commun. 1979 Feb 14;86(3):522–528. doi: 10.1016/0006-291x(79)91745-5. [DOI] [PubMed] [Google Scholar]
- Henry N., Parce J. W., McConnell H. M. Visualization of specific antibody and C1q binding to hapten-sensitized lipid vesicles. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3933–3937. doi: 10.1073/pnas.75.8.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander N., Mehdi S. Q., Weissman I. L., McConnell H. M., Kriss J. P. Allogeneic cytolysis of reconstituted membrane vesicles. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4042–4045. doi: 10.1073/pnas.76.8.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries G. M., McConnell H. M. Membrane-controlled depletion of complement activity by spin-label-specific IgM. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3537–3541. doi: 10.1073/pnas.74.8.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky S. C. Immunogenicity of liposomal model membranes. Ann N Y Acad Sci. 1978;308:111–123. doi: 10.1111/j.1749-6632.1978.tb22017.x. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C., Nicolotti R. A. Immunological properties of model membranes. Annu Rev Biochem. 1977;46:49–67. doi: 10.1146/annurev.bi.46.070177.000405. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. J., MacDonald R. C. Lipid vesicle-cell interactions. III. Introduction of a new antigenic determinant into erythrocyte membranes. J Cell Biol. 1976 Sep;70(3):515–526. doi: 10.1083/jcb.70.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINDERKNECHT H. Ultra-rapid fluorescent labelling of proteins. Nature. 1962 Jan 13;193:167–168. doi: 10.1038/193167b0. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroit A. J., Pagano R. E. Introduction of antigenic phospholipids into the plasma membrane of mammalian cells: organization and antibody-induced lipid redistribution. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5529–5533. doi: 10.1073/pnas.75.11.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlacek H. H., Stärk J., Seiler F. R., Ziegler W., Wiegandt H. Cholera toxin induced redistribution of sialoglycolipid receptor at the lymphocyte membrane. FEBS Lett. 1976 Jan 15;61(2):272–276. doi: 10.1016/0014-5793(76)81055-1. [DOI] [PubMed] [Google Scholar]
- Sheats J. R., McConnell H. M. A photochemical technique for measuring lateral diffusion of spin-labeled phospholipids in membranes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4661–4663. doi: 10.1073/pnas.75.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]



