Abstract
Background:
The beneficial effect of using nonacetylated salicylates such as salsalate on decreasing the speed of diabetes progression is a controversial issue. The aim of this study was to evaluate the effect of salsalate on metabolic-syndrome-associated parameters as well as the endothelial function of diabetic and impaired glucose tolerance patients.
Materials and Methods:
Patients were collected from Isfahan endocrinology research center referrals. Patients with impaired glucose tolerance diagnosis or newly diagnosed diabetes were enrolled in the study. Patients were randomized to receive 1.5 g salsalate (2 × 750 mg) BID or placebo twice a day for 3 months. After the mentioned period, all patients were recalled and complete examination was done; blood samples for biochemistry measurements were drawn (for measuring FBS, post prandial glucose, HbA1C, Total cholesterol, HDL, TG, LDL) and forearm flow-mediated dilation (FMD) was performed.
Results:
Forty patients were enrolled, 32 patients (80%) were female. Mean age of patients was 47.15 ± 6.67 years. FBS (fasting blood sugar) was shown to be significantly different between intervention and control subjects before or after treatment. FMD increased significantly in the intervention group (P = 0.004).
Conclusion:
The study showed that salsalate decreased FBS levels of patients. It may also improve endothelial function as FMD increased significantly in the intervention group.
Keywords: Diabetes, endothelial function, salsalate
INTRODUCTION
The prevalence of type-2 diabetes and metabolic syndrome is dramatically increasing all over the world; the main cause of which may lie in the changes in pattern of obesity, sedentary lifestyle, and population's aging.[1] Metabolic syndrome is composed of a group of prevalent risk factors comprising obesity, glucose intolerance, hypertension, and abnormal lipid levels.[2] Both metabolic syndrome and diabetes occur in the background of insulin resistance and lead to an increased risk of atherosclerosis and cardiovascular diseases (CVD).
Numerous studies have been conducted regarding various cardiovascular disorders and their increased risk in diabetic patients according to which abnormalities in vascular reactivity have been well recognized in type-2 diabetes.[3,4,5] As an important factor that mainly causes altered vascular reactivity, endothelial dysfunction plays a pivotal role in the initiation of micro- and macrovascular complications in diabetes and some other diseases that are hypothesized to be related to vascular changes like migraine.[6,7,8]
A growing bunch of studies have been conducted with the aim of suggesting ways, substances and medications for lowering down the speed of diabetes progression and its relevant vascular complications among which medications with anti-inflammatory effects have been reported to be positively effective. Despite the beneficial effects of aspirin as an acetylated salicylate in reduction of inflammatory responses, it has shown several unfavorable effects on coagulation pathways and may lead to gasterointestinal ulcers especially in high doses besides. However, nonacetylated salicylates such as Na salicylate and salsalate that tend to reduce mild inflammatory processes exist naturally in endothelial system of diabetic patients and can decrease the speed of diabetes process, with no unfavorable effect on platelets and coagulation pathways.[9]
This study intended to evaluate the effect of salsalate on metabolic syndrome parameters as well as endothelial function of prediabetic patients in an interventional setting. We aimed to test the hypothesis that using 3 mg salsalate daily would improve endothelial function of prediabetic patients according to FMD as a functional marker.
MATERIALS AND METHODS
Study populations
Patients were collected from Isfahan endocrinology research center referrals between February and June 2009. Patients enrolled in the study included prediabetic patients comprising of those with the diagnosis of impaired glucose tolerance or newly diagnosed impaired fasting glucose patients who had received no prior medications and those who did not need standard diabetes medications at the time of study and agreed to participate after being aware of risks and benefits of using salsalate. The subjects were required to have the following criteria for being eligible to enter the study: Between 20 and 60 years of age, FBS (fasting blood sugar) between 110 and 125 mg/dL (mentioned as impaired fasting glucose according to WHO criteria[10]) or BS (blood sugar) in OGTT (oral glucose tolerance test) between 140 and 199 mg/dL (mentioned as impaired glucose tolerance according to WHO criteria) and BS ≥ 250 mg/dL. Eligible participants had been also received life style modification recommendations previously and showed failure to response within several months. They were all previously recommended to exercise regularly, lose 5% to 7% of body weight, and limit the intake of (at least) sugar and highly processed carbohydrates.
According to studies in some cases for prediabetic patients with severe risk factors, prescribing medication may be appropriate especially in those for whom lifestyle therapy is not sustainable and who are at high-risk for developing type-2 diabetes.[11] As mentioned earlier such cases were not eligible to enter the survey. Additionally patients possessing any of the following features were not included in the study: Type-1 diabetes mellitus or history of DKA (diabetic ketoacidosis), pregnancy or lactation, use of corticosteroids or NSAIDS (nonsteroidal anti-inflammatory drugs) continuously, surgery within the past month, serum Cr (Creatinine) > 1.4 mg/dl in females and Cr > 1.5 mg/dl in males or GFR (glomerular filtration rate) < 60, Hb (hemoglobin) < 12 g/dL in males, Hb < 10 g/dL in females, PLT (Platelet) < 100,000 per mcL, TG (triglyceride) > 500 mg/dL, lbuminuria ≥ 300 mg/day, known diagnosed malignancy, confirmed peptic ulcer or GI bleeding, diagnosed heart failure, clinical ACS (acute coronary syndrome), CVA (cerebral vascular accident), TIA (transient ischemic attack), revascularization within the past 6 months, hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mmHg), previous allergy to aspirin or NSAIDs, continuous use of warfarin or clopidogrel and chronic tinnitus.
Exclusion criteria were hypersensitivity to salsalate or presenting salsalate complications (the flow diagram is shown in Figure 1.)
Figure 1.
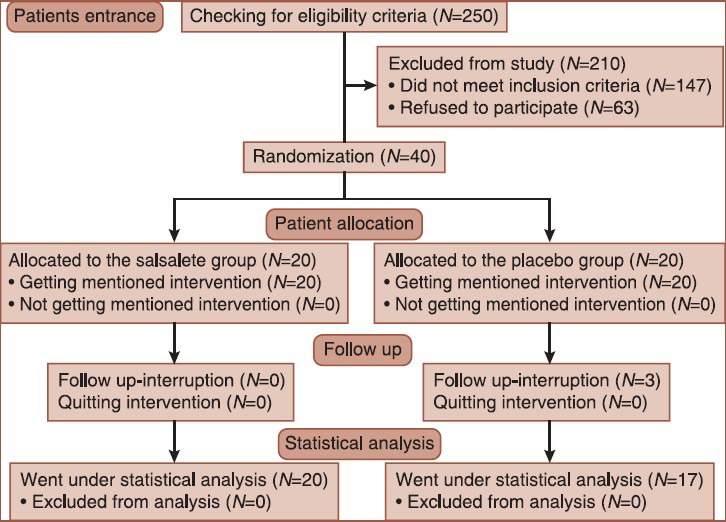
CONSORT flow diagram
Study design
In this randomized clinical trial, patients started the survey by a 1 month baseline period, in which no medication was used. After this period they underwent a complete examination including history taking and physical examination, blood sampling for biochemistry measurements (such as OGTT, HbA1C, Total cholesterol, HDL, TG, LDL), and forearm flow mediated dilation (FMD). The blood samples and FMD were evaluated after a period of fasting for 8 h and then the samples were kept in –70°C.
Patients were randomized to receive either 1.5 g salsalate (2 × 750 mg) BID or placebo twice a day for 3 months with the use of a computer generated randomization list with 10 consecutive balanced blocks of four patients (two salsalate, two placebo). Placebo was similar to salsalate tablet in shape, color, and size.
The number of prescribed tablets covered a 2-week treatment period, after which the patients were examined again and were asked about the existence of possible complications (such as heartburn, nausea, vomiting, melena, vertigo, tinnitus, diarrhea, and rashes) thereafter they received the drug for 2 more weeks. After these 3 months, all patients were recalled and an entire examination was done, blood samples for biochemistry measurements were drawn, and forearm FMD was performed, too. For testing the hypothesis that daily intake of salsalate would improve endothelial function of diabetic patients we used FMD as a functional marker in our study, details of which is followed. Patients’ weight and height were also measured and BMI index was calculated (height in centimeter divided by weight in kilogram) both in the first and the recall visit in order to evaluate BMI changes as an important relevant factor.
Standard protocol approvals, registrations, and patient consents
The study was approved by the ethical committee of the Isfahan University of Medical Sciences. Written informed consent was obtained from all patients participating in the study. This study is registered at Iranian randomized control trial (IRCT) database as: IRCT138709011465N1
Flow-mediated dilation
A high-resolution B-mode ultrasonographic system (ATL Ultrasound, HDI 5000, Bothell, Washington, USA) with a linear transducer mid-frequency of 7.5 MHz was used to determine flow-mediated dilation (FMD) of the brachial artery. An expert ultrasonographer blinded to intervention and placebo groups performed all FMDs. Patients rested 10 min prior to initiation. Then baseline brachial artery diameter was determined by locating probe on 4-5 cm above the antecubital fossa of the nondominant arm. A pneumatic tourniquet of a sphygmomanometer was inflated on the most proximal portion of the forearm to a pressure of 300 mmHg for 5 min. The cuff was then released and second scan was taken 30 s before and 90 s after cuff deflation. Artery diameters were determined with ultrasonic calipers from the leading edge of the anterior wall to the leading edge of posterior wall of the brachial artery at the end of diastolic period. Changes in diameter were computed as percentage based on the baseline diameter. FMD was not measured during the menstrual phase in female patients. All FMDs were performed at the same time of the day.
Statistical analysis
Differences in FMD, biochemistry, and BMI parameters between intervention and placebo groups were analyzed with independent-sample t-test and differences within the groups (before and after treatment) were determined by paired-sample t-test. P-value cut-off of 0.05 was considered to be significant. Statistical analyses were performed with SPSS16 software by an analyzer who was blind as to details of the research.
RESULTS
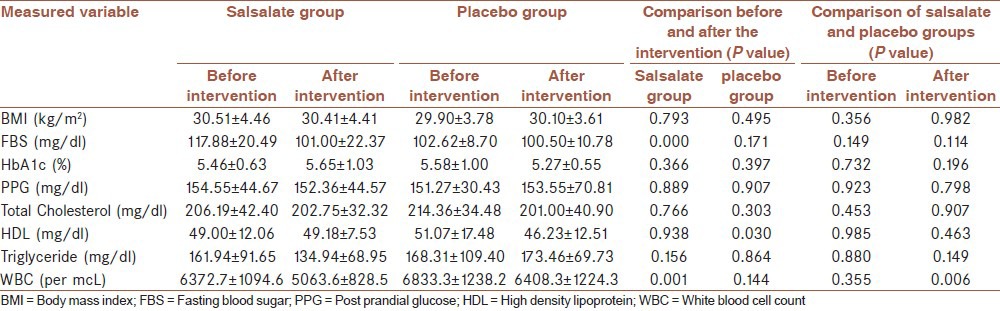
Forty patients were enrolled in this study, 8 (20%) of which were male and 32 (80%) were female. The patients’ average age was 47.15 ± 6.67 (with a range of 30-60 years). The patients were randomly divided to two 20 subjects including intervention and placebo groups, respectively. There was no meaningful difference between mean ages of the mentioned groups. Three individuals of intervention group refused to come for follow up sessions and were mentioned to be dropped out. They denied occurrence of any side effect as a cause of their refusal. Information about biochemistry parameters and data collected from physical examination are summarized in Table 1.
Table 1.
Comparison of biochemistry parameters and BMI measures of placebo and salsalate groups before and after intervention
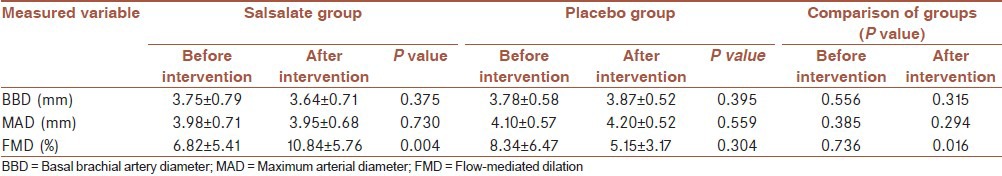
The primary outcome was the fact that FMD index was shown to be improved before and after 3 months of treatment. As illustrated in Table 2, there was no significant difference between two groups before the intervention. However, after that the intervention group had significantly higher FMD (P = 0.016). But the differences between FMDs were not significant before and after intervention in the placebo group (P = 0.304) while in the intervention group FMD increased significantly after treatment by salsalate (P = 0.004).
Table 2.
FMD associated variables
Moreover no significant correlations were found between FMD changes and HbA1C changes (Pearson Correlation: 0.425, P: 0.169), FBS changes (Pearson correlation: 0.111, P: 0.717) and BMI changes (Pearson correlation: 0.557, P: 0.151). The scattering diagrams of FMD and BMI, HbA1C and FBS changes are illustrated in Figures 2A, 2B, and 3.
Figure 2.
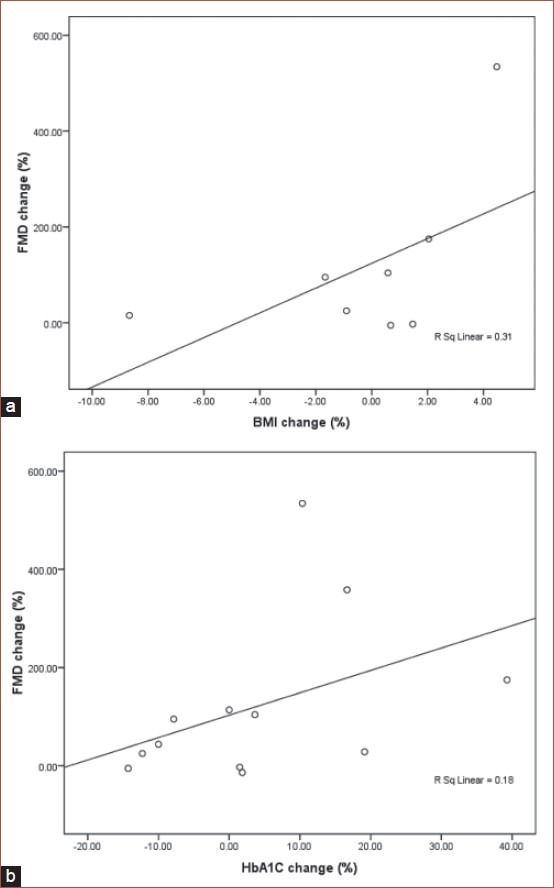
(a) Scattering diagram of flow-mediated dilation and body mass index in the intervention group. (b) Scattering diagram of flow-mediated dilation and HbA1C changes in the intervention group
Figure 3.
Scattering diagram of flow mediated dilation and fasting blood sugar in the intervention group.
Among all measured biochemical factors FBS was the only parameter which showed significant difference in the salsalate group after completing the intervention P-value = 0.000. There was no statistically significant change in other biochemical factors and BMI measures nor within groups before and after study neither between groups after taking the intervention except for HDL in the placebo group (P-value = 0.030). WBC count showed significant decrease in salsalate-treated patients after completing the intervention comparing with both their own status prior to the intervention (0.001) and WBC count changes of their counterparts in placebo group (0.006).
DISCUSSION
In the present placebo-controlled trial, which was planned to evaluate the safety and efficacy of salsalate for lowering blood glucose concentrations in patients with type-2 diabetes and improving of endothelial dysfunction, FBS was the only parameter lowered by salsalate and in contrast with some other studies.[12] parameters such as HBA1C did not show any significant decrease. Likewise, triglyceride and HDL concentration levels were improved but the changes were not significant enough.
In our study, except for FBS, none of the biochemical measured parameters showed dramatic changes.
Similarly, FMD, used as a functional assay for evaluating the function of endothelium, had significant improvement among the salsalate treated patients. To the best of our knowledge, there was no previous study which aimed to examine this parameter in salsalate intervention. This functional endothelial dysfunction improvement may be due to anti-inflammatory effects of salsalate or some other probable mechanisms such as lowering the blood glucose concentration.
Several studies[13,14,15,16] have shown that treatment with high-dose salicylate inhibits activity of the transcription factor NF-KB, which regulates the production of multiple inflammatory mediators.
Also the WBC count decreased significantly in salsalate treated group of patients while their counterparts experienced no dramatic change in WBC count. Some previous surveys have also shown this decline[17] and have reported it as a rare side effect of this drug.
The mechanisms through which the glucose-lowering effects of salicylates are inserted include inhibition of cellular kinases,[18] upregulation of the heat shock response,[19] and raising circulating insulin concentrations.[17,20,21] So, salsalate may lower glucose concentration in various ways and thus, the idea that salsalate decreases the glucose concentration by solely increasing the insulin concentration is not acceptable.[22]
Salsalate has been used for decades in treatment of arthralgia, without any serious safety concerns specifically for patients with diabetes. The privilege of using salsalate is the fact that it is an acidic PH insoluble prodrug which transits the stomach in suspension and causes less gastric irritation.
In contrast with nonsteroidal anti-inflammatory drugs (NSAIDs) like aspirin, salsalate and other nonacetylated salicylates are atypical NSAIDs main targets of which are not the cyclooxygenases. This is understood by the minimal effects of salsalate on circulating prostaglandin or rennin levels.[23] So its effects on gastrointestinal bleedings may be lower, nonetheless in our study we excluded the patients with peptic ulcer disease or GI bleeding history.
Despite some complains of patients about weight gaining and edema, there were no significant changes in BMI of patients after using salsalate and this result was verified by some previous studies as well. Our study showed that salsalate is well tolerated in patients with type-2 diabetes and improves FBS levels of patients but confirmation of long-term safety of the drug requires further investigations especially considering leukopenia as its probable side effect. Likewise several improvements in other biochemical parameters were seen in this study but the changes were not significant, a fact which may be the result of limited number of patients. Further studies with larger sample sizes are recommended to investigate salsalate efficacy and its long term effects and safety on diabetic patients.
ACKNOWLEDGMENTS
The authors would like to acknowledge Isfahan University of Medical Sciences for the financial support of this study.
Footnotes
Source of Support: The authors would like to acknowledge Isfahan University of Medical Sciences for the financial support of this studyl
Conflict of Interest: None declared.
REFERENCES
- 1.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–75. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Cheung JC, Stehouwer CD, Emeis JJ, Tong PC, Ko GT, et al. The central roles of obesity-associated dyslipidaemia, endothelial activation and cytokines in the metabolic syndrome — an analysis by structural equation modelling. Int J Obes Relat Metab Disord. 2002;26:994–1008. doi: 10.1038/sj.ijo.0802017. [DOI] [PubMed] [Google Scholar]
- 3.Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Diabetologia. 1995;38:1337–44. doi: 10.1007/BF00401767. [DOI] [PubMed] [Google Scholar]
- 4.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–74. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 5.Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali JR. Impairment of coronary vascular reserve and Ach-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993;42:1017–25. doi: 10.2337/diab.42.7.1017. [DOI] [PubMed] [Google Scholar]
- 6.Tooke JE. Microvascular function in human diabetes: A physiologic perspective. Diabetes. 1995;44:721–6. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]
- 7.Hsueh WA, Quinones M, Creager MA. Endothelium in insulin resistance and diabetes. Diabetes Rev. 1997;5:343–52. [Google Scholar]
- 8.Sonbolestan SA, Heshmat K, Javanmard SH, Saadatnia M. Efficacy of enalapril in migraine prophylaxis: Does it work through endothelial dysfunction recovery? Neurology. 2010;74:A574–A. [Google Scholar]
- 9.Ebstein W. Invited comment on W. Ebstein: On the therapy of diabetes mellitus, in particular on the application of sodium salicylate. J Mol Med (Berl) 2002;80:618. [PubMed] [Google Scholar]
- 10.World Health Organization. “Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus”. Retrieved. 2007 May 29; [Google Scholar]
- 11.Lilly M, Godwin M. Treating prediabetes with metformin: Systematic review and meta-analysis. Can Fam Physician. 2009;55:363–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. TINSAL-T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) Study Team. The effects of salsalate on glycemic control in patients with type 2 diabetes: A randomized trial. Ann Intern Med. 2010;152:346–57. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 14.Kopp E, Ghosh S. Inhibition of NF-_ B by sodium salicylate and aspirin. Science. 1994;265:956–9. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 15.Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit I _ B-_ phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol. 1996;156:3961–9. [PubMed] [Google Scholar]
- 16.Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996;274:1383–5. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 17.Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frantz B, O’Neill EA. The effect of sodium salicylate and aspirin on NFkappa B. Science. 1995;270:2017–9. doi: 10.1126/science.270.5244.2017. [DOI] [PubMed] [Google Scholar]
- 19.Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–5. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 20.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–6. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Real JM, López-Bermejo A, Ropero AB, Piquer S, Nadal A, Bassols J, et al. Salicylates increase insulin secretion in healthy obese subjects. J Clin Endocrinol Metab. 2008;93:2523–30. doi: 10.1210/jc.2007-1212. [DOI] [PubMed] [Google Scholar]
- 22.Koska J, Ortega E, Bunt JC, Gasser A, Impson J, Hanson RL, et al. The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: Results of a randomised double-blind placebo-controlled study. Diabetologia. 2009;52:385–93. doi: 10.1007/s00125-008-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris HG, Sherman NA, McQuain C, Goldlust MB, Chang SF, Harrison LI. Effects of salsalate (nonacetylated salicylate) and aspirin on serum prostaglandins in humans. Ther Drug Monit. 1985;7:435–8. doi: 10.1097/00007691-198512000-00012. [DOI] [PubMed] [Google Scholar]


