Abstract
Background:
Headache is a common complaint for emergency visits. Common drugs used in relief of headache are opioids and their agonists and antagonists, ergot alkaloids, and nonsteroidal anti-inflammatory drugs (NSAIDs). Lack of appropriate medications or serious side effects of available drugs, motivated us to perform the study for evaluating the efficacy of intranasal lidocaine on different types of headache.
Materials and Methods:
A double-blind, randomized clinical trial (RCT) was performed among 90 adult patients with acute headache in Shahid Rahnemoon Emergency Center of Yazd city of Iran (45 patients in lidocaine group and 45 patients in placebo group). Patients with history of epilepsy, allergy to lidocaine, signs of skull base fracture, Glasgow Coma Scale (GCS) < 15, patients younger than 14 years and patients who had received any medication in previous 2 h were excluded. After checking vital signs and taking the demographic data, one puff of 10% lidocaine or normal saline (placebo) was sprayed into each nostril. Patients’ headache severity measured by visual analog scale (VAS) before drug administration and at 1, 5, 15, and 30 min after intervention. Data were analyzed by Statistical Package for Social Sciences (SPSS) version 17 and statistical tests including t-test, repeated measures analysis of variance (ANOVA), Fisher's exact test, and Mann-Whitney test were performed. Descriptive variables were expressed by mean ± standard deviation (SD) and quantitative variables reported by frequency and percentages. P-values less than 0.05 were considered significant.
Results:
57.8% of patients were female. The mean age of patients was 35.32 years. According to sex and age, there was no significant difference between groups (P-values were 0.83 and 0.21; respectively). The mean base pain score was 6.97 in lidocaine group and 6.42 in placebo group which was not significantly different (P-value = 0.198). After intervention, the mean scores were significantly lower in lidocaine group than placebo group in all mentioned times (P-value < 0.001). The primary and secondary headaches had no significant difference in mean pain relief score in lidocaine group (P = 0.602).
Conclusion:
Intranasal lidocaine is an efficient method for pain reduction in patients with headache. Regarding easy administration and little side effects, we recommend this method in patients referred to emergency department (ED) with headache.
Keywords: Headache, intranasal, lidocaine
INTRODUCTION
Headache is one of the most common causes of seeking medical help.[1] Life prevalence of headache is 93% in men and 99% in women.[2] For practical purposes, headaches generally are divided into primary headache syndromes including migraine, tension-type, and cluster headaches, and secondary headache causes.[3] Headache as a primary complaint represents between 3 and 5% of all emergency department (ED) visits;[4] these headaches are usually benign and more than 90% of patients who visit ED for headache suffer from migraine or tension headache; tension headache is the most common type of primary headaches.[5,6] The brain parenchyma is largely insensible to pain. Pain may originate from large cranial vessels, proximal intracranial vessels, and the dura mater.[3]
Common drugs using for headaches include opioids and their partial agonists, ergot alkaloids, antiemetics, serotonin inhibitors, and nonsteroidal anti-inflammatory drugs (NSAIDs). Chlorpromazine is now used as the first line of headache treatment in most of EDs in the USA.[5]
Local anesthetics such as lidocaine inhibit conduction of neural impulses by decreasing permeability of neuron membrane to sodium. Probable mechanism of action of intranasal lidocaine is local anesthesia by blocking neural transfer from Vidian nerve, sphenopalatine ganglion (SPG) or maxillary branch of trigeminal nerve.[5]
Previous studies on efficacy of intranasal lidocaine in headache relief were done on specific type of headache; some of these studies reported paradoxical results.
In the randomized clinical trial (RCT) of Maizels and Geiger in 1999, 4% intranasal lidocaine was efficient in relief of migraine headache.[5] In the study of Mills and Scoggin on the efficacy of intranasal lidocaine on cluster and migraine headaches, this medication mentioned as a secondary drug of choice and in special patients.[7] In an uncontrolled trial in a headache clinic in southern California by Kudrow et al., in 1995, 4% intranasal lidocaine was efficient in immediate headache relief, but further controlled trials were recommended.[8] Chae et al., in 2006, reported the efficacy of 2% intranasal lidocaine in pain relief of two cases of posttraumatic headache.[9] Kanai et al., in 2006, performed a randomized, double-blind study and reported 8% intranasal lidocaine spray produced prompt, but temporary analgesia in patients with second-division trigeminal neuralgia.[10] In a case report by Maizels, 4% intranasal lidocaine has been shown to consistently prevent developing migraine symptoms following aura in a 15-year-old boy fulfilling the criteria for migraine with aura.[11]
In the other hand Blanda et al., (2001) did not support the rapid efficacy of 4% intranasal lidocaine in pain relief of migraine headache.[12] Robbins in USA reported that 4% intranasal lidocaine has little effect of immediate relief in patients with cluster headache, but it is a valuable medication due to its easy administration and absence of side effects.[13]
Regarding to importance of headache as a common complaint in medical practice and lack of appropriate medications or serious side effects of available drugs in our country and previous studies investigated treatment for only some types of headache, we performed this study to evaluate efficacy of intranasal lidocaine on different types of headache.
MATERIALS AND METHODS
Our study was double blind RCT; and all adults referred to ED of Shahid Rahnemoon Medical Center of Yazd city of Iran with chief complaint of headache, stable hemodynamic status in the triage were enrolled the study, except who met our exclusion criteria:
Patients under 14-years-old;
Loss of consciousness;
Signs of skull base fracture (raccoon eyes, battle sign, rhinorrhage, rhinorrhea, otorrhage, otorrhea);
Instability of hemodynamic status;
Previous history of allergy to lidocaine;
Patients that received any medication in the past 2 h for omitting the confounding effect of previous drug using;
Epileptic patients;
Penetrating head trauma.
Patients divided into primary headaches (migraine, tension-type, and cluster) and secondary headaches. Because our hospital was a trauma center, we divided secondary type headaches into traumatic and nontraumatic subgroups. Nontraumatic secondary type headaches were divided into etiologic types by history, physical examination, neuroimaging, and laboratory data, based on the International Headache Society (IHS) classification (some of them initially and others retrospectively after intervention). Patients who diagnosed with migraine and tension type headache met the diagnostic criteria of IHS, as well.
Ninety cases were selected consecutively among patients referred to ED with headache and they were randomly divided equally into case group (intranasal lidocaine) and control group (placebo). After getting informed consent, full examination was done and data were collected from each patient by triage physician. Patients in each group were asked to rank their pain on the basis of visual analog scale (VAS).[14] One puff of 10% lidocaine (Iran Daru Company-Iran) contained of 10 mg lidocaine or normal saline (placebo) was sprayed into each nostril. Medication was packaged, so the patients were unaware of the contents; and after 1, 5, 15, and 30 min, pain severity were reevaluated by VAS score.
After information collection, data were analyzed by Statistical Package for Social Sciences (SPSS) version 17 and statistical tests including t-test, repeated measures analysis of variance (ANOVA), Fisher's exact test, and Mann-Whitney test were performed. Descriptive variables were expressed as mean ± standard deviation (SD) and quantitative variables reported by frequency and percentages. P-value less than 0.05 was considered as significant.
RESULTS
Ninety patients were enrolled in the study (45 as case group and 45 as control group) [Tables 1 and 2]. Patients were 15-72-years-old (mean 35.3 years); including 57.8% female and 42.2% male.
Table 1.
Distribution of patients enrolled to study according to headache types
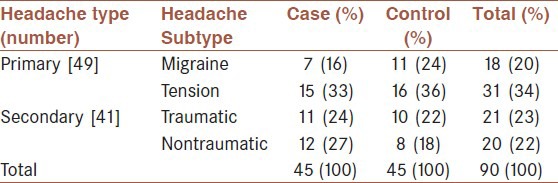
Table 2.
Distribution of subgroups of nontraumatic-secondary headache in case and control groups

In lidocaine (case) group, 60% were female and in control group 55.6% were female. Based on Fisher's exact test, there is no significant difference in gender distribution between the two groups (P = 0.83).
Mean age of patients in case group was 33.48 ± 13.33 and 37.17 ± 14.58 years in control group. Based on t-test there was no significant difference in age distribution between the two groups (P = 0.21).
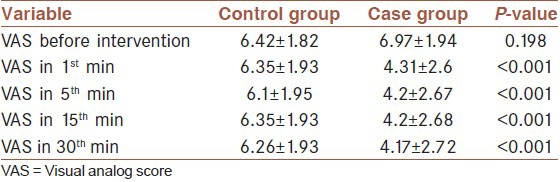
Mean VAS score before intervention was 6.97 ± 1.94 and 6.42 ± 1.82 in case and control group, respectively; which unified between the two groups as 6.7 ± 1.89 [Figure 1 and Table 3]. Thirty minutes after intervention mean VAS score was 4.17 ± 2.72 and 6.26 ± 1.93 in case and control group, respectively. In repeated measure test this difference was significant (P < 0.001), mean VAS score was 4.31 ± 2.6 in 1st min after intervention in the case group, in the other words, most of pain reduction happened in the first minute after lidocaine intake in the case group [Figure 1 and Table 3].
Figure 1.
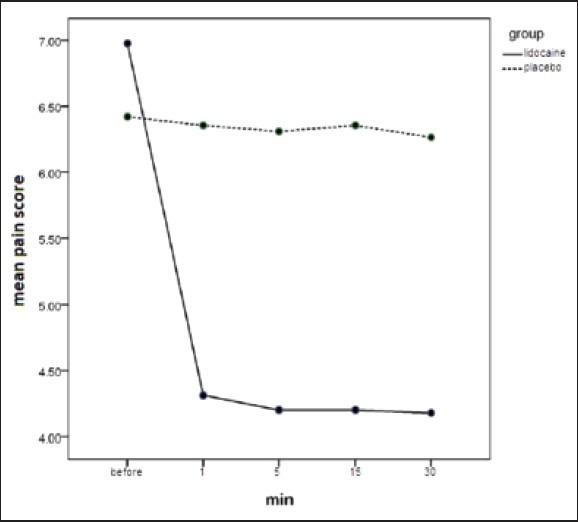
Comparison of VAS score changes between the two groups
Table 3.
Comparison of pain (VAS) score before and 1, 5, 15, and 30 min after intervention between the two groups
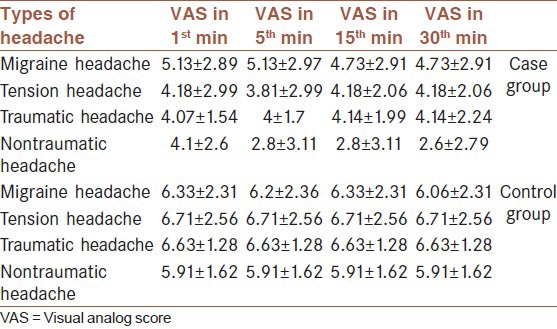
Repeated measures analysis of variance (ANOVA) test revealed no significant difference in VAS score between the four subtypes of headache in the mentioned times in case and control groups (P = 0.87 and 0.602, respectively) [Table 4].
Table 4.
Comparison of VAS score changes in four types of headache in case and control groups
DISCUSSION
Our results showed the effectiveness of 10% intranasal lidocaine spray on pain relief in patients with headache. This effect had no difference in primary and secondary headaches and their subgroups. The maximum pain reduction happened in the 1st min after treatment.
Lidocaine provides its anesthetic effect as a sodium pump inhibitor. Used intranasally its action occurs at the SPG which resides just posterior and immediately above the posterior tip of the middle turbinate, beneath the nasal mucosa at a depth of 1-9 mm. This ganglion along with the internal carotid and cavernous sinus ganglion provide parasympathetic innervation of cerebral blood vessels. It also releases neuropeptides, which can induce headache. The rapid onset of intranasal lidocaine suggests interruption or blockage of nerves or neurons.[5]
Systemic lidocaine has been shown to relieve neuropathic pain with a significant plasma concentration-dependent decrease in pain intensity starting at 1.5 mg/ml. Scott[15] and colleagues measured plasma concentration of lidocaine in patients after spraying of the trachea and larynx with 50 mg of lidocaine. Mean maximum plasma concentration of lidocaine in the patients was 0.6 mg/ml.[10] Therefore, itcan be considered that the basic mechanism for the rapid effect of intranasal spray with 20 mg of lidocaine in our patients is the anesthetic effect on the SPG.
Our study evaluated the efficacy of 10% intranasal lidocaine on different types of headache and confirmed it. Other studies evaluated this matter only on one or two types of headache, and in this regard this study was unique.
Maizels and Geiger (1999) studied the efficacy of intranasal lidocaine for the treatment of migraine. They used injection of 4% lidocaine by syringe into nostrils and reported that intranasal lidocaine 4% provided rapid relief of migraine symptoms. They followed-up the patients over a 6-month period; and found that 57.6% of headaches were relieved within 30 min, with a relapse rate of 20%. The rate of response did not diminish over time. They assessed the patients 5, 15 and 30 min after intervention and none after 1 min. They mentioned the method of administration as their study limitation.[5] We used lidocaine in spray form, thus had not their limitation. We assessed the patients 1 min after intervention, additionally. Our study verified this effect of lidocaine on different types of headache, but we did not follow-up the patients and it was our study limitation. According to these two studies we conclude 10% intranasal lidocaine spray is effective in headache relief with acceptable rate of relapse.
Chae et al., (2006) evaluated the use of intranasal SPG blockade with administration of intranasal 2% viscous lidocaine for the treatment of posttraumatic headache in two cases. Their patients had suffered from headache for a long period of time after trauma. They concluded, while a RCT was needed, these two cases supported the role of intranasal SPG block as a safe, noninvasive technique for treating this condition.[9] Our study confirmed Chae et al.'s report, although we used lidocaine in spray form, and our traumatic patients were acutely injured.
Kanai et al., (2006) assessed the efficacy of intranasal lidocaine 8% spray for second-division trigeminal neuralgia. They reported that intranasal lidocaine 8% administered by a metered-dose spray produced prompt but temporary analgesia without serious adverse reactions in patients with second-division trigeminal neuralgia. The VAS in each patient was assessed before and 15 min after the treatment. Patients were asked to rate whether the pain returned and how long after therapy it recurred. In this study the analgesic effect lasted for a median period of 4.3 h.[10] Their findings were nearly parallel to our results, although we did not have any case of trigeminal neuralgia.
Buckley et al., administered 15 mg lidocaine spray in migraine patients and recommended this method.[16] They evaluated efficacy of this spray in only six patients, we compared efficacy of intranasal lidocaine spray and placebo in different types of headache on 90 patients in two groups.
Blanda et al., (2001) performed a study about this matter. They compared administration of 4% intranasal lidocaine and intravenous prochlorperazine against intranasal normal saline and intravenous prochlorperazine and concluded that lidocaine was inefficient in headache relief.[12] This result is in contrast with our study. It could be due to these reasons: The confounding effect of prochlorperazine and the form and concentration of lidocaine. We used 10% lidocaine in spray form and did not use any concurrent drug.
In our study, the maximum pain reduction in the case group was obtained in the 1st min after intervention and no significant change was seen in VAS score in the next 29 min. Thus, we concluded that maximum effect of intranasal lidocaine was in the 1st min. It can be explained by the mechanism of lidocaine action mentioned above. Other studies did not investigate the VAS 1 min after intervention.
In comparison with other current therapies for headache, intranasal lidocaine spray had some advantages. The first advantage was the fast action. In our study, the maximum effect of intranasal lidocaine was obtained in the 1st min. The second advantage was noninvasive therapy. The third advantage was therapy with a portable device. The patient can carry a metered-dose spray bottle and use it whenever pain appears.
Our study evaluated the efficacy of 10% lidocaine spray for 30 min, and did not follow the patients for longer times. It was our study limitation and we recommend performing further studies with patient's follow-up for longer times.
CONCLUSION
We concluded that intranasal lidocaine is an efficient method for pain reduction in any type of headache and regarding to its easy prescription and little side effects, it is recommended in patients referred to ED with headache. Nevertheless, we recommend performing further studies with longer follow-up and evaluation of headache relapse.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension-type headache. JAMA. 1998;279:381–3. doi: 10.1001/jama.279.5.381. [DOI] [PubMed] [Google Scholar]
- 2.Kernick D. An introduction to the basic principles of health economics for those involved in the development and delivery of headache care. Cephalalgia. 2005;25:709–14. doi: 10.1111/j.1468-2982.2005.00946.x. [DOI] [PubMed] [Google Scholar]
- 3.Christopher JD, Schall MJ. Headache and facial pain. In: Tintinalli J, Stapczynski J, Ma O, Cline D, Cydulka R, Meckler G, editors. Tintinalli's Emergency Medicine. 7th ed. MCGrawHill; 2011. p. 1113. [Google Scholar]
- 4.Christopher SR. Rosen's Emergency Medicine. In: Marx J, Hockberger R, Walls R, editors. 7th ed. Mosby; 2010. p. 118. [Google Scholar]
- 5.Maizels M, Geiger AM. Intranasal lidocaine for migraine: A randomized trial open label follow up. Headache. 1999;39:543–51. doi: 10.1046/j.1526-4610.1999.3908543.x. [DOI] [PubMed] [Google Scholar]
- 6.Welch MA, Aurora TK, Suri P, Arnold G. Headaches in emergency room. In: Olesen J, Tfelt-Hansen P, Welch MA, editors. The Headaches. 2th ed. Lippincott Williams Wilkins; 2000. p. 993. 1000. [Google Scholar]
- 7.Mills TM, Scoggin JA. Intra-nasal lidocaine for migraine and cluster headaches. Ann Pharmacother. 1997;31:914–5. [PubMed] [Google Scholar]
- 8.Kudrow L, Kudrow DB, Sandweiss JH. Rapid and sustained relief of migraine attacks with intranasal lidocaine: Preliminary findings. Headache. 1995;35:79–82. doi: 10.1111/j.1526-4610.1995.hed3502079.x. [DOI] [PubMed] [Google Scholar]
- 9.Chae H, Jonas Matthew S, Mark N, Arthur L. The use of intranasal sphenopalatine ganglion blockade for the treatment of post-traumatic headache: A case series. Arch Phys Med Rehabil. 2006;87:E39. [Google Scholar]
- 10.Kanai A, Suzuki A, Kobayashi M, Hoka S. Intranasal lidocaine 8% spray for second-division trigeminal neuralgia. Br J Anaesth. 2006;97:559–63. doi: 10.1093/bja/ael180. [DOI] [PubMed] [Google Scholar]
- 11.Morris M. Intranasal lidocaine to prevent headache following migraine aura. Headache. 1999;39:439–42. doi: 10.1046/j.1526-4610.1999.3906439.x. [DOI] [PubMed] [Google Scholar]
- 12.Blanda M, Rench T, Gerson L, Weigand JV. Intranasal lidocaine for treatment of migraine headache: A randomized control trial. Acad Emerg Med. 2001;8:337–42. doi: 10.1111/j.1553-2712.2001.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 13.Robbins L. Intra nasal lidocaine for cluster headache. Headache. 1995;35:83–4. doi: 10.1111/j.1526-4610.1995.hed3502083.x. [DOI] [PubMed] [Google Scholar]
- 14.Melzack R, Katz J. Pain assessment in adult patient. In: Stephen BM, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. 5th ed. Elsevier; 2006. p. 291. [Google Scholar]
- 15.Scott DB, Littlewood DG, Covino BG, Drummond GB. Plasma lignocaine concentrations following endotracheal spraying with an aerosol. Br J Anaesth. 1976;48:899–902. doi: 10.1093/bja/48.9.899. [DOI] [PubMed] [Google Scholar]
- 16.Buckley R, McCurry T, Gera J. Intranasal lidocaine for migraine using a metered-dose spray. Headache. 2000;40:498. doi: 10.1046/j.1526-4610.2000.00077.x. [DOI] [PubMed] [Google Scholar]


