Abstract
Hypertension is a major risk factor for myocardial infarction, heart failure, stroke, peripheral arterial disease, and aortic aneurysm, and is a cause of chronic kidney disease. Hypertension is often associated with metabolic abnormalities such as diabetes and dyslipidemia, and the rate of these diseases is increasing nowadays. Recently it has been hypothesized that oxidative stress is a key player in the pathogenesis of hypertension. A reduction in superoxide dismutase and glutathione peroxidase activity has been observed in newly diagnosed and untreated hypertensive subjects, which are inversely correlated with blood pressure. Hydrogen peroxide production is also higher in hypertensive subjects. Furthermore, hypertensive patients have higher lipid hydroperoxide production. Oxidative stress is also markedly increased in hypertensive patients with renovascular disease. If oxidative stress is indeed a cause of hypertension, then, antioxidants should have beneficial effects on hypertension control and reduction of oxidative damage should result in a reduction in blood pressure. Although dietary antioxidants may have beneficial effects on hypertension and cardiovascular risk factors, however, antioxidant supplementation has not been shown consistently to be effective and improvement is not usually seen in blood pressure after treatment with single or combination antioxidant therapy in subjects thought to be at high risk of cardiovascular disease. This matter is the main focus of this paper. A list of medicinal plants that have been reported to be effective in hypertension is also presented.
Keywords: Antioxidant, hypertension, oxidative stress
INTRODUCTION
Hypertension (HTN) is a chronic medical condition that is rarely accompanied by any symptoms. It is usually identified through screening or when the patient is seeking for an unrelated problem. Hypertension is a major risk factor for myocardial infarction, heart failure, stroke, peripheral arterial disease, aortic aneurysm, and is a cause of chronic kidney disease.[1,2,3,4] Elevation of arterial blood pressure is associated with a shortened life expectancy.[1,5,6]
In 2000, nearly one billion people of the adult world population had hypertension. The rate of blood pressure is increasing, so that in 1995, 43 million people (22%) in the United States suffered hypertension or were taking antihypertensive medication,[7] in 2006, 76 million US adults (34% of the population) had hypertension.
Hypertension is often associated with metabolic abnormalities such as diabetes and dyslipidemia and the rate of these diseases is increasing too. It is estimated that 30-60% of diabetic patients have associated hypertension.[8]
Recently it has been hypothesized that oxidative stress is a key player in the pathogenesis of hypertension.[9,10] Endothelial cells play a major role in arterial relaxation. Nitric oxide is released by the endothelium and causes vascular relaxation.[11] Nitric oxide is rapidly degraded by the oxygen-derived free radical superoxide anion. Superoxide anion acts as a vasoconstrictor and is a major determinant of nitric oxide (NO) biosynthesis and bioavailability. It, therefore, can modify the endothelial function. Human hypertension is associated in a decrease in NO bioavailability and an increase in oxidative stress.[12] Decreased catalase and/or superoxide dismutase and reduced levels of reactive oxygen species (ROS) or reactive nitrogen species (RNS) scavengers such as glutathione, and vitamins C and E also contribute to oxidative stress.[13,14] Activation of reduction-oxidation (redox)-dependent signaling cascades and NADPH oxidase-driven generation of ROS are involved in the role of angiotensin-II-induced hypertension.[15] Angiotensin II stimulates nonphagocytic NADPH oxidase, causing the accumulation of hydrogen peroxide, superoxide, and peroxynitrite.[16]
In obesity and diabetes that are usually associated with hypertension, chronic oxidative stress is present.[17,18,19,20] In this regard, caloric restriction in fasting and obese subjects leads to a marked reduction in ROS generation and other indices of oxidative stress.[17]
A reduction in superoxide dismutase and glutathione peroxidase activity have been observed in newly diagnosed and untreated hypertensive subjects, which are inversely correlated with blood pressure. Hydrogen peroxide production is also higher in hypertensive subjects.[21] Furthermore, hypertensive patients have higher lipid hydroperoxide production. Oxidative stress is also markedly increased in hypertensive patients with renovascular disease.[22]
Furthermore, there is a correlation between renin activation and elevated oxidative stress which may suggest that angiotensin II is a stimulus for oxidant stress in renovascular disease.[23] The role of oxidative stress in hypertension and the possible mechanisms involved are well documented in a couple of recently published original and review papers[9,10] and are further discussed in this paper. If oxidative stress is indeed a cause of hypertension, then, these all suggest that antioxidants should have beneficial effect on hypertension control. Hence, reduction of oxidative damage may result in a reduction in blood pressure. This matter is the main focus of this paper. Other than further discussing about oxidative stress and its importance in hypertension, a list of medicinal plants which have been reported to be effective in hypertension is also presented.
Oxidative stress and its importance in hypertension
Oxidative stress (OS) is believed to be involved in many types of disease processes. Oxidative stress occurs when there is an imbalance between the generation of ROS and the antioxidant defense systems [Figure 1]. The ROS comprises many molecules with different effects on the cellular function. ROS and RNS are regularly formed as the result of excess oxidative stress or even normal organ functions. In any biological system there should be an important balance between the formation of ROS and RNS, and their removal.[11] The reactive species including superoxide (O2-), hydroxyl radical (HO•), hydrogen peroxide (H2O2), peroxynitrite (ONOO-), nitrogen oxide (NO•), and hypochlorous acid (HOCl) are produced in normal metabolic pathways and scavenged by antioxidants, however, in excess amounts, they can exert harmful reactions. In the vascular system, superoxide and hydrogen peroxide are particularly important. Superoxide, one of the most important sources of initiating radicals in the body, is produced in mitochondria and leaks outside of the mitochondria. To maintain an oxido/redox balance, organs protect themselves from the toxicity of excess ROS/RNS in different ways, including the use of endogenous and exogenous antioxidants [Figure 1].[12]
Figure 1.
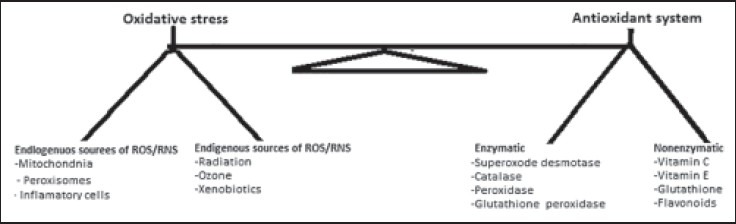
The balance between oxidative stress and antioxidant defence system
As was mentioned earlier, there is a strong association between blood pressure and some oxidative stress-related parameters. Genetic deficiencies in ROS-generating enzymes have also been shown to be lower in blood pressure compared with their wild-type counterparts.[24] Furthermore, in isolated arteries from hypertensive humans and animals, antioxidant bioactivity is reduced, redox-dependent signaling is amplified and ROS production is enhanced.[25] The beneficial effects of antihypertensive agents, such as angiotensin I converting enzyme inhibitors, angiotensin-II receptor antagonists, b-adrenergic, and calcium channel blockers might be mediated, at least in part, by decreasing vascular oxidative stress.[26,27,28,29,30]
Different sources of ROS might exist in blood vessels. One of the best characterized sources of ROS is NADPH oxidase. Several other enzymes including NO synthase, xanthine oxidase, and mitochondrial enzymes may also contribute to ROS generation. The vasculature and kidney are the rich sources of NADPH oxidase-derived ROS, having important role in vascular damage and renal dysfunction under.[31] This system functions as an electron donor and catalyses the reduction of oxygen by NADPH which increases the generation of superoxide upregulation of NADPH oxidase in hypertensive patients.[31]
The function of NADPH oxidase-derived superoxide is inactivation of NO in the reaction that forms peroxynitrite, leading to impaired endothelium dependent vasodilation. The activation of NADPH oxidase has been strongly associated with hypertension.[32] Oxidation or deficiency of tetrahydrobiopterin (BH4) and L-arginine which are two cofactors for endothelium-derived NO synthase (eNOS) action are associated with the uncoupling of the L-arginine-NO pathway that results in increased eNOS-mediated generation of superoxide and decreased formation of NO.
NADPH oxidase is the initial source of ROS. Superoxide combines with NO, which is synthesized by eNOS, to form peroxynitrite. In turn, peroxynitrite oxidizes and destabilizes eNOS to produce additional superoxide.[33] BH4 is highly sensitive to oxidation and upper oxide leads to BH4 oxidation, which promotes eNOS uncoupling and further ROS production. Xanthine oxidase that is believed to be involved in hypertension is another important source of ROS in the vascular endothelium. It catalyzes the last two steps of purine metabolism and reduction of oxygen to superoxide.[32] Hypertensive rats have demonstrated increased ROS production and elevated levels of endothelial xanthine oxidase, which is associated with increased arteriolar tone.[31] Xanthine oxidase may also have a function in end-organ injury in hypertensive patients.[34]
The endothelium is also greatly involved in ROS production and furthermore releases agents that regulate vasomotor function. Under some pathophysiological circumstances, endothelium-derived vasoconstricting factors, such as angiotensin-1 and -II, vasoconstrictor prostaglandins and thromboxane A2, urotensin II, and superoxide anions, can be released and contribute to the paradoxical vasoconstrictor effects.[34]
As was described, NO has an important role in vascular tone. The decrease in bioavailability of NO in the vasculature reduces hypertension by increasing in vasodilation capacity. The NO is synthesized by NOS from oxygen and arginine. NO, in addition to its antiproliferative and vasorelaxing roles, has an important role in antagonizing the effects of endothelins, AT-II, and ROS.[35]
Reduced NO levels can be attributed to elevated levels of ROS. Superoxide combines with NO to form peroxynitrite that oxidizes BH4 and destabilizes eNOS to produce more superoxide and enhancing the development of oxidative stress.[33]
Angiotensin-II also, as a potent activator of NADPH oxidase, contributes to the production of ROS. Angiotensin-II not only increases NADPH oxidase activity but also upregulates the activity of superoxide dismutase, possibly to compensate the increased levels of ROS.[36]
Captopril and enalapril prevented increases in blood pressure in young, spontaneously hypertensive rats by inhibiting ACE. The ability of angiotensin-II to induce endothelial dysfunction is also due to its ability to downregulate soluble guanylyl cyclase, thereby leading to impaired NO/cGMP signaling. The decrease in NO will also lead to reduced acetylcholine-mediated vasodilation, and an overall increase in ROS is associated in the reduction in the bioavailability of NO.[37]
Urotensin-II which is the most potent identified vasoconstrictor also acts through the activation of NADPH oxidase.[31] In addition, overexpression of inducible NOS increases blood pressure via central activation of the sympathetic nervous system, which is mediated by an increase in oxidative stress.[38]
In general, although there are multiple sources producing ROS, the most potential sources of excessive ROS in hypertension are as follows: NADPH oxidase, eNOS, mitochondria, xanthine oxidase, cytochrome P450 epoxygenase, cycloxygenase 1 and 2, transition metals (e.g. Iron).[38,39,40]
Effects of antioxidants on hypertension
Antioxidants are compounds that are able to trap ROS and thus may be capable of reducing oxidative damage and possibly blood pressure.[26,27,28,29,30,31,32,33] Antioxidants terminate the chain reactions of ROS by removing free radical intermediates, and inhibit other oxidation reactions. They do this by being oxidized themselves, so antioxidants are often reducing agents such as ascorbic acid, vitamin E or polyphenols that act by different mechanisms [Table 1].[41,42,43]
Table 1.
Exogenous antioxidants and their mechanisms in hypertension[34]
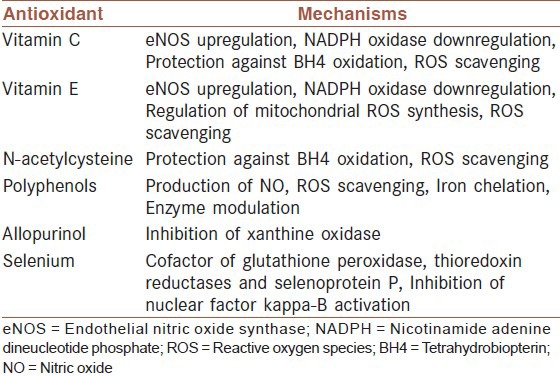
As was mentioned earlier, there might be a correlation between stress oxidative and arterial hypertension[9,10] This matter has led to the use of exogenous antioxidants to reduce blood pressure.[44]
Polyphenols that are widespread antioxidants in vegetables and fruits in some studies have demonstrated their beneficial role in prevention and therapy of hypertension. Polyphenols inhibit ROS-producing enzymes such as NADPH and xanthine oxidases, activate, and enhance eNOS expression and increase glutathione. These have improved endothelial function, subsequent normalization of vascular tone, and an overall antihypertensive effect.[45]
The antioxidants vitamins C and E and other antioxidants have been considered as possible therapy for decreasing oxidative stress and thereby lowering blood pressure. Additionally vitamins C and E down-regulate NADPH oxidase, a major source of ROS in the vascular wall, and up-regulate eNOS, both of these effects lower blood pressure.[46] In this regard, a randomized double-blind clinical trial other than demonstrating a specific association between oxidative-stress-related parameters and blood pressure, documented enhancement of antioxidant status by supplementation with antioxidants vitamins C and E and their hypotensive properties.[47]
Combination supplement with vitamin C, vitamin E, beta-carotene, and zinc also resulted in a significant reduction in systolic blood pressure and a nonsignificant reduction in diastolic blood pressure.[48]
The diets rich in fruits and vegetables reduce blood pressure in hypertensive and normotensive patients.[49] The modification in diet results in an increase in serum antioxidant capacity and a decrease in malondialdehyde, an in-vitro marker of lipid peroxidation, suggestive of a reduction in oxidative stress.[49,50,51,52,53] In a study, increase of fruit and vegetable intake for a period of 6 months to the diet of hypertensive subjects, increased blood antioxidant capacity and decreased in systolic and diastolic blood pressure.[49]
Large clinical trials examining the effects of antioxidants specifically on blood pressure are rare. Moreover, the majority of large clinical trials did not find any antihypertensive effects of antioxidants.[53] A large study reported no improvement in blood pressure after treatment with a combination of vitamin C, vitamin E, and beta carotene versus placebo after 5 years in subjects thought to be at high risk of cardiovascular disease.[54] Furthermore, a meta-analysis has revealed no clear benefit after antioxidant supplementation in cardiovascular mortality.[55]
In general, long-term clinical trials have failed to consistently support the antihypertensive effects of antioxidants. Results of studies showing that supplemental antioxidants intake lowers blood pressure in short-term trials are inconsistent. Most of such studies have looked at all-cause or cardiovascular mortality, rarely focusing on blood pressure as a primary end point.[49,50,56]
Possible reasons for these disappointing outcomes might also be relate to 1) the trial design, 2) the type of antioxidants used, and 3) Patient cohorts included in trials. With respect to antioxidants, it is possible that dosing regimens and duration of therapy were insufficient or agents examined were ineffective and nonspecific. It is also possible that the antioxidants administered are inaccessible to the source of free radicals, particularly if ROS are generated in intracellular organelles and compartments.[9]
Furthermore, antioxidants do not inhibit ROS production and antioxidant vitamins do not scavenge H2O2, which may be more important than •O2− in cardiovascular diseases.[9]
Regarding cohorts included in large trials, most subjects had significant cardiovascular disease, in which case damaging effects of oxidative stress might be irreversible. Moreover, not all hypertensions are related to oxidative stress and there are not any large clinical trials in which patients be recruited based on evidence of elevated ROS formation. Also, none of the large clinical trials were designed to examine effects of antioxidants specifically on blood pressure.
However, most therapeutic guidelines suggest that the general population consumes a diet emphasizing antioxidant-rich fruits and vegetables and whole grains.[46]
From the literature review we may conclude that the diets high in antioxidants (fruits and vegetables) are capable of reducing blood pressure and cardiovascular diseases, but this is not the case for diet supplementations.[56,57,58,59]
The possible explanation is that, in the diet, there is a mix of antioxidants and it is well recognized that they work as a continuous chain, while supplementation is usually given using one or two substances. Therefore, the antioxidant chain is not completely available.
Moreover, it is well known that after scavenging free radicals, if an antioxidant is not restored by the following antioxidant in the chain, it begins to be a pro-oxidant. In this situation, the final effect of such supplementations would be no effect or a damaging effect.[60,61,62,63]
Other than beneficial effects of fruits and vegetables, there are some medicinal plants that are believed to ameliorate blood pressure. Some of these plants are presented in the following.
Medicinal plants effective in blood pressure
Clinical trials of the plant extracts in human being have shown reliable evidence of antihypertensive effects. Even small diet changes such as cooking with garlic and savory herbs instead of salt can have beneficial effects in reduction of hypertension.[64]
When considering medicinal plant therapy for hypertension, although medicinal products have advantages over synthetic drugs, however, lessons from prior disappointing attempts to reduce blood pressure and cardiovascular risks with antioxidants and these products should be considered. Some advantages of natural antioxidants and medicinal products are as follows:[65]
Good safety profile with limited side effects, good oral bioavailability, patient-friendly dosing regimen (once or twice daily dosing, concentrating in relevant tissues (brain, kidney, and/or vasculature), limited potential for pro-oxidative activity and secondary cell signaling that may limit their effectiveness, mostly inhibition of ROS production rather than quenching ROS post-production, efficacious for hypertension originating from disparate etiologies, pleiotropic effects that go beyond blood pressure lowering and translate into reversal or prevention in progression of end organ damage, and finally limited interactions with the metabolism of concomitant antihypertensive therapies.[65]
It seems that antioxidant activity is not the sole cause of these plants in hypertension reduction. Because most of medicinal plants have antioxidant activity but it does not seem to be a correlation between antioxidant activity and their capacity in reduction of hypertension.
Daily consumption of green tea or hibiscus, a delicious red tea, has been shown in studies to reduce high blood pressure. Herbal diuretics such as Buchu and Cornsilk tea can also help by eliminating water. Angelica contains compounds that act much like calcium channel blockers, which are often prescribed for high blood pressure and heart health.
Some medicinal plants with antihypertension activity are discussed in the following.
Garlic or Allium sativum
Garlic is from Alliaceae family or Liliaceae. Garlic has been shown to have antioxidant activity[66] and antihypertension effects. It has long been used for a variety of diseases including hypertension and hyperlipidemia. It increases nitric oxide production that results in smooth muscle relaxation and vasodilatation. Meta-analysis of data has demonstrated that garlic decreases BP in patients with increased systolic pressure.[11]
Annona muricata or Prickly custard apple
Annona muricata is a member of the family of Annonaceae and a species of the genus Annona, known mostly for its edible fruits Annona. The leaf extract of the plant has been reported to lower an elevated BP by decreasing the peripheral vascular resistance.[70]
Apium graveolens or Celery
Celery is from Apiaceae family, effective against hypertension. It acts on liver and one type of hypertension is associated with liver. In a study in which the juice was mixed with equal amount of honey and about 8 ounces were taken orally three times each day for up to 1 week was useful in reducing HTN in 14 of 16 patients.[69] It has also been reported to reduce systolic and diastolic BP. The difference of BP in human beings before and after treatment has been found to be significant. It is also administered in HTN associated with pregnancy and climacteric.[70]
Avena sativa or Green Oat
Avena sativa is from Poaceae/Gramineae family. It contains soluble fibers that can control hypertension. It improves blood lipid and glucose, too.[71] The addition of oat cereals to the normal diet of patients with HTN has been found to significantly reduce both systolic and diastolic BP.[72]
Blond psyllium or Indian plantago
Indian plantago is from Plantaginaceae family. It has been shown that daily consumption of 15 g Indian plantago can lower both systolic and diastolic blood pressure.[73]
Cassia absus or Chaksu
Cassia absus is from Caesalpiniaceae family. This plant is found in the tropical region of India. It has been shown that 1-30 mg/kg of crude extract of C. absus dose dependently decreases the BP. Repeated injections of the same dose of the crude extract have been seen to produce tachyphylaxis.[74]
Cassia occidentalis or Coffee weed
Coffee weed is from Caesalpiniaceae family. In-vitro studies of the leaf extract of coffee weed have been shown to have a relaxant effect on the aortic rings. It is used in local folk medicine as an antihypertensive agent. The studies revealed that cassia extract may be relaxing smooth muscle and reducing BP by inhibiting Ca2+ influx through receptor-operated channel and voltage-sensitive channel, showing its nonselectivity on these Ca2+ channels.[75]
Camellia sinensis or Tea
Camellia sinensis is from Theaceae family. Population research links consumption of green tea and oolong tea with a decreased risk of developing HTN. Research on black tea shows no effect on BP in people with HTN. It has also good effects in a wide range of other complications. Black bean is also from Fabaceae family. Crude extract of C. australe (1-100 mg/kg) has been shown to reduce both systolic and diastolic blood pressure in a dose-dependent manner. This fall in BP has been attributed to the saponin fraction and medicagenic acid glucoside present in the crude extract.[76,77]
Commelina virginica or Virginia dayflower
Virginia dayflower is from Commelinaceae family; it is native to the mideastern and southeastern United States. Whole plant extract has been reported to decrease the tension of phenylephrine-stimulated isolated guinea pig aorta rings by 15% to 35%; however, its antihypertension has not been investigated, yet.[78]
Daucus carota or Carrot
Carrot is from Umbelliferae family. It has been used in traditional medicine to treat HTN. Isolation of two coumarin glycosides coded as DC-2 and DC-3. Intravenous administration of these compounds indicate that DC-2 and DC-3 may be acting through blockade of calcium channels, and this effect may be responsible for the BP-lowering effect of the compounds observed in the in vivo studies.[79]
Glycine max or Soybean
Soybean is from Fabaceae family. Soybean has been found to be effective as a hypotensive agent.[80]
Gossypium barbadense or Pima cotton
Pima cotton is from Malvaceae family. It has been shown that the leaf extract of Pima cotton decreases the tension of phenylephrine-stimulated isolated guinea pig aorta rings by 15% to 35%. In Suriname's traditional medicine, the leaves of the plant are used to treat HTN and delayed/irregular menstruation.[68,78]
Hibiscus sabdariffa or Roselle
Roselle is from Malvaceae family. The leaves of Roselle have been used traditionally as antihypertensive agent. Its antihypertensive has been reported in a couple of studies.[78,79] The antihypertensive effects of the crude extract of the HS have been attributed to mediation through acetylcholine and histamine like dependent mechanism through direct vasorelaxant effects.[79] The chronic administration of aqueous extract of HS has been reported to reverse cardiac hypertrophy in renovascular hypertensive rats.[80]
Clinical trials of the plant extract in human being have shown reliable evidence of antihypertensive effects.[81]
Lavandula stoechas or French Lavender
French Lavender is from Lamiaceae family. Crude extract of L. stoechas has been reported to produce a fall in BP and HR in anesthetized rats. Pretreatment of atropine abolished the cardiovascular responses, suggesting that the antihypertensive and bradycardia effects of the crude extract may be mediated through mechanism(s) similar to that of acetylcholine.[82]
Linum usitatissimum or Linseed, Flaxseed
Linseed, Flaxseed is from Linaceae family. Flaxseed oil is rich in α-linolenic acid, an essential fatty acid that that is belonged to a group of substances called omega-3 fatty acids. Several studies suggest that diets rich in omega-3 fatty acids lower BP significantly in people with HTN. Daily consumption of 15 to 50 g/day of ground flaxseed can modestly reduce total cholesterol and low-density lipoprotein concentrations without altering triglycerides or high-density lipoprotein cholesterol. However, the exact mechanism is unclear.[82]
Lycopersicon esculentum or Tomato
Tomato is from Solanaceae family. Its extract contains carotenoids, such as lycopene, beta carotene, and vitamin E, which are known as effective antioxidants, to inactivate free radicals and to slow the progress of atherosclerosis. The tomato extract of tomato has been shown to reduce BP in patients with mild HTN.[83] Tomato extract when added to patients treated with low doses of ACE inhibition, calcium channel blockers, or their combination with low-dose diuretics had a clinically significant effect-reduction of BP by more than 10 mmHg systolic and more than 5 mmHg diastolic pressure.[84]
Ocimum basilicum or Basil
Basil is from Lamiaceae family. Crude extract of Basil has been shown to reduce systolic, diastolic, and mean BP in a dose-dependent manner with median effective dose of 30 mg/kg. The cardiovascular effect of the extract has been attributed to eugenol, which exerts its effect by blocking the calcium channels.[85]
Peganum harmala or Harmal
Harmal is from Nitrariaceae family. The crude extract fraction and all pure compounds: Harmine, harmaline, tetrahydroharmine, harmol, and harmaloi from Harmal produced antihypertensive effects in anesthetized rats in a dose-dependent manner.[86,87]
Punica granatum or Pomegranate
Pomegranate is from Lythraceae family. Researches have shown pomegranate juice reduces the activity of angiotensin converting enzymes (ACE) by about 36%. One study shows modest reduction in systolic BP after drinking 50 ml/day of pomegranate juice for a year. Another study shows no benefit after drinking 240 ml/day of the juice for 3 months.[88]
Rauwolfia serpentina or Rauwolfia
Rauwolfia is from Apocynaceae family. Extracts of its different parts considered to be the most powerful hypotensive plant medicine. Reserpine, the purified alkaloid of R. serpentina, was the first potent drug widely used in the long-term treatment of HTN. Only a small dose is required to achieve results and to avoid side effects. Nasal congestion is the most common side effect. In 1952, reserpine was introduced under the name Serpasil in the treatment of HTN, tachycardia, and thyrotoxicosis. The combination of reserpine, dihydroergocristine, and a diuretic is still on the market. However, I may cause depression.[89]
Sesamum indicum or Sesame
Sesame is from Pedaliaceae family. Alcoholic extract of seeds (1-30 mg/kg) in a dose-dependent manner decreased blood pressure. Atropine (2 mg/kg) was reported to abolish the cardiovascular responses, indicating the presence of acetylcholine-like substance in the seeds. Sesamin and sesaminol are the major phenolic constituents of sesame oil. A study in hypertensive patients indicated that sesame oil consumption remarkably reduced oxidative stress and simultaneously increased glutathione peroxidase, superoxidase dismutase, and catalase activities. These results support the hypothesis that sesame oil consumption may help to enhance antioxidant defense system in human beings. The investigators suggested that sesamin is a useful prophylactic treatment in HTN and cardiovascular hypertrophy.[90,91]
Solanum sisymbriifolium or Wild Tomato
Wild Tomato is from Solanaceae family. The root of S. sisymbriifolium has been used as a traditional medicine possessing diuretic and antihypertensive properties. The intravenous administration of the extract (50 and 100 mg/kg) produced a significant decrease in BP in anesthetized hypertensive (adrenal regeneration HTN + deoxycorticosterone acetate) rats. Oral administration of the extract (10, 50, 100, and 250 mg/kg) also produced a dose-dependent hypotensive effect in conscious hypertensive animals. The results suggested that nuatigenosido may play an important role in the therapeutic effects of this herb.[92]
Theobroma cacao or Cocoa Butter
Cocoa Butter is from Malvaceae family. Cocoa powder, enriched with flavonoid constituents, is used for preventing cardiovascular disease. Flavonoids, contained in chocolate, stimulate formation of nitric oxide, increase vasodilatation, and reduce endothelial dysfunction. A growing body of clinical research also shows that daily consumption of dark or milk chocolate (T. cacao), 46 to 105 g daily, providing 213 to 500 mg of cocoa polyphenols, can lower systolic BP by about 5 mmHg and diastolic by about 3 mmHg.[93]
Uncaria rhynchophylla or Cat's Claw herb
Cat's Claw herb is from Rubiaceae family. It has been used to lower BP and to relieve various neurological symptoms. The hypotensive activity has been attributed to an indole alkaloid called hirsutine[85] that reduces intracellular Ca2+ level through its effect on the Ca2+ store as well as through its effect on the voltage-dependent Ca2+ channel.[94] Alteration in HR, possibly involves sympathetic mechanism, too.[95]
Vitex doniana Black plum
Black plum is from Verbenaceae family. The extract was found to exert hypotensive effect. Both the systolic and diastolic BPs were significantly reduced within 45 min after oral administration of the extract. The BP began to return to normal after 2 h.[96]
Zingiber officinale or Ginger
Ginger is from Zingiberaceae family. Ginger root dose-dependently (0.3-3 mg/kg) induced a fall in the arterial BP of anesthetized rats. Human trials for hypotensive effect of ginger have been few and generally used a low dose with inconclusive results.[97,98]
Cautions in the use of medicinal plants
The renewed interest in the search for new drugs from natural sources, especially from plant sources, has gained global attention during the last two decades. This attention is primarily due to the rich biodiversity, which promises a high diversity of chemicals with the potential novel structures and promising effects.[99,100,101,102,103] However, of this rich biodiversity, only a small portion has been studied for its medicinal potential.[104,105] Thus, natural plants and herbs can be our source of drugs, with fewer side effects and better bioavailability for treatment of HTN in future. However, it should be noted that the use of medicinal plants is not guaranteed to be nontoxic. For example ginseng is contraindicated for those in whom high blood pressure is dangerous, because high blood pressure has been reported in some ginseng consumers. This is because conflicting reports are about the effect of this medicinal plant on hypertension. It is not known yet whether ginseng is a cure for high blood pressure, or worsens it. Maintaining electrolyte balance is also important; for instance licorice (Glycyrrhiza glabra) can decrease potassium levels, cause sodium and water retention, and increase blood pressure.[106,107]
CONCLUSIONS
There is a proposed hypothesis relating the pathogenesis of hypertension and cardiovascular diseases to oxidative stress of arterial wall. However, antioxidant therapy has not been shown to be consistently beneficial. It seems that fruits and vegetables due to having a large variety of antioxidants are more effective than supplementation therapy with a limited number of antioxidants. Nevertheless, large clinical trials are needed to document the role of oxidative stress in hypertension and the possible treatment of hypertension with antioxidants.
ACKNOWLEDGEMENT
We would like to thank Research Deputy of Shahrekord University of Medical Sciences, Shahrekord, Iran for valuable support.
Footnotes
Source of Support: Research Deputy of Shahrekord University of Medical Sciences, Shahrekord, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Behradmanesh S, Nasri P. Serum cholesterol and LDL-C in association with level of diastolic blood pressure in type 2 diabetic patients. J Renal Inj Prev. 2012;1:23–6. doi: 10.12861/jrip.2012.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasri H. Comment on: Serum cholesterol and LDL-C in association with level of diastolic blood pressure in type 2 diabetic patients. J Renal Inj Prev. 2012;1:13–4. doi: 10.12861/jrip.2012.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafieian-Kopaei M, Nasri H. Ginger and diabetic nephropathy. J Renal Inj Prev. 2012;2:9–10. doi: 10.12861/jrip.2013.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasri H. Antiphospholipid syndrome-associated nephropathy: Current concepts. J Renal Inj Prev. 2012;2:1–2. doi: 10.12861/jrip.2013.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behradmanesh S, Nasri H. Association of serum calcium with level of blood pressure in type 2 diabetic patients. J Nephropathol. 2013;2:254–7. doi: 10.12860/JNP.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Behradmanesh S, Derees F, Rafieian-kopaei M. Effect of Salvia officinalis on diabetic patients. J Renal Inj Prev. 2013;2:55–9. doi: 10.12861/jrip.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann Med. 2012;44(Suppl 1):S2–16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–40. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- 11.Rafieian-Kopaei M, Baradaran A, Rafieian M. Plants antioxidants: From laboratory to clinic. J Nephropathol. 2013;2:152–3. doi: 10.12860/JNP.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension. 2004;44:248–52. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 13.Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41:1096–101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 14.Nasri H, Behradmanesh S, Ahmadi A, Rafieian-Kopaei M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J Nephropathol. 2014;3:29–32. doi: 10.12860/jnp.2014.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells: Implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263–73. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 16.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, et al. Expression of a gp91phox-containing leu-kocyte-type NADPH oxidase in human vascular smooth muscle cells: Modulation by Ang II. Circ Res. 2002;90:1205–13. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 17.Dandona P, Mohanty P, Ghanim H, Al-jada A, Browne R, Hamouda W, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid per-oxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–62. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 18.Nayer A, Ortega LM. Catastrophic antiphospholipid syndrome: A clinical review. J Nephropathol. 2014;3:9–17. doi: 10.12860/jnp.2014.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering JW, Endre ZH. The definition and detection of acute kidney injury. J Renal Inj Prev. 2014;3:19–23. doi: 10.12861/jrip.2014.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nasri H. Hypertension and renal failure with right arm pulse weakness in a 65 years old man. J Nephropathol. 2012;1:130–3. doi: 10.5812/nephropathol.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedro-Botet J, Covas MI, Martin S, Rubies-Prat J. Decreased endogenous anti-oxidant enzymatic status in essential hypertension. J Hum Hypertens. 2000;14:343–5. doi: 10.1038/sj.jhh.1001034. [DOI] [PubMed] [Google Scholar]
- 22.Lip GY, Edmunds E, Nuttall SL, Landray MJ, Blann AD, Beevers DG. Oxidative stress in malignant and non-malignant phase hypertension. J Hum Hypertens. 2002;16:333–6. doi: 10.1038/sj.jhh.1001386. [DOI] [PubMed] [Google Scholar]
- 23.Pickering TG. Diagnosis and evaluation of renovascular hypertension: Indications for therapy. Circulation. 1997;83:I147–54. [PubMed] [Google Scholar]
- 24.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, et al. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 25.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: Role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens. 2001;19:1245–54. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishikawa N, Nishio M, et al. AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failure. Hypertension. 2004;43:686–91. doi: 10.1161/01.HYP.0000118017.02160.fa. [DOI] [PubMed] [Google Scholar]
- 27.Ghorbani A, Rafieian-Kopaei M, Nasri H. Lipoprotein (a): More than a bystander in the etiology of hypertension? A study on essential hypertensive patients not yet on treatment. J Nephropathol. 2013;2:67–70. doi: 10.5812/nephropathol.9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez GT, Nasri H. World Kidney Day 2014: Increasing awareness of chronic kidney disease and aging. J Renal Inj Prev. 2014;3:3–4. doi: 10.12861/jrip.2014.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardalan MR, Vahedi A. Antiphospholipid syndrome: A disease of protean face. J Nephropathol. 2013;2:81–4. doi: 10.5812/nephropathol.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasri H, Behradmanesh S, Maghsoudi AR, Ahmadi A, Nasri P, Rafieian-Kopaei M. Efficacy of supplementary vitamin D on improvement of glycemic parameters in patients with type 2 diabetes mellitus; a randomized double blind clinical trial. J Renal Inj Prev. 2014;3:31–4. doi: 10.12861/jrip.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feairheller DL, Brown MD, Park JY, Brinkley TE, Basu S, Hagberg JM, et al. Exercise training, NADPH oxidase p22phox gene polymorphisms, and hypertension. Med Sci Sports Exerc. 2009;41:1421–8. doi: 10.1249/MSS.0b013e318199cee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:277–97. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 33.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, et al. Endothelial regulation of vasomotion in apoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–8. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 34.Laakso JT, Teravainen TL, Martelin E, Vaskonen T, Lapatto R. Renal xanthine oxidoreductase activity during development of hypertension in spontaneously hyper-tensive rats. J Hypertens. 2004;22:1333–40. doi: 10.1097/01.hjh.0000125441.28861.9f. [DOI] [PubMed] [Google Scholar]
- 35.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem. 1997;272:25907–12. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 36.Taniyama Y, Griendling K. Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension. 2003;42:1075–81. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 37.Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol. 2005;511:53–64. doi: 10.1016/j.ejphar.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Jegou S, Cartier D, Dubessy C, Gonzalez BJ, Chatenet D, Tostivint H, et al. Localization of the urotensin II receptor in the rat central nervous system. J Comp Neurol. 2006;495:21–36. doi: 10.1002/cne.20845. [DOI] [PubMed] [Google Scholar]
- 39.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: Comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–44. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 40.Hool LC, Corry B. Redox control of calcium channels: From mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2007;9:409–35. doi: 10.1089/ars.2006.1446. [DOI] [PubMed] [Google Scholar]
- 41.Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc Ther. 2010;28:e20–32. doi: 10.1111/j.1755-5922.2010.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadkhodaee M, Sedaghat Z. Novel renoprotection methods by local and remote conditioning. J Renal Inj Prev. 2014;3:37–38. doi: 10.12861/jrip.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavafi M. Diabetic nephropathy and antioxidants. J Nephropathol. 2013;2:20–7. doi: 10.5812/nephropathol.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasri H. Antiphospholipid syndrome-associated nephropathy: Current concepts. J Renal Inj Prev. 2013;2:1–2. doi: 10.12861/jrip.2013.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward NC, Hodgson JM, Croft KD, Burke V, Beilin LJ, Puddey IB. The combination of vitamin C and grape-seed polyphenols increases blood pressure: A randomized, double-blind, placebo-controlled trial. J Hypertens. 2005;23:427–34. doi: 10.1097/00004872-200502000-00026. [DOI] [PubMed] [Google Scholar]
- 46.Briones AM, Touyz RM. Oxidative stress and hypertension: Current concepts. Curr Hypertens Rep. 2010;12:135–42. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigo R, Prat H, Passalacqua W, Araya J, Bächler JP. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin Sci (Lond) 2008;114:625–34. doi: 10.1042/CS20070343. [DOI] [PubMed] [Google Scholar]
- 48.Ardalan MR, Rafieian-Kopaie M. Antioxidant supplementation in hypertension. J Renal Inj Prev. 2014;3:39–40. doi: 10.12861/jrip.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamadon MR, Baradaran A, Rafieian-Kopaei M. Antioxidant and kidney protection; differential impacts of single and whole natural antioxidants. J Renal Inj Prev. 2014;3:41–42. doi: 10.12861/jrip.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baradaran A. Antiphospholipid syndrome-associated nephropathy: A nephropathy needs classification. J Nephropharmacol. 2012;1:7–9. [PMC free article] [PubMed] [Google Scholar]
- 51.Nasri H. On the occasion of the world diabetes day 2013; diabetes education and prevention; a nephrology point of view. J Renal Inj Prev. 2013;2:31–2. doi: 10.12861/jrip.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rafieian-Kopaei M, Nasri H. Serum lipoprotein (a) and atherosclerotic changes in hemodialysis patients. J Renal Inj Prev. 2013;2:47–50. doi: 10.12861/jrip.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller ER, III, Appel LJ, Risby TH. Effect of dietary patterns on measures of lipid per-oxidation: Results from a randomized clinical trial. Circulation. 1998;98:2390–5. doi: 10.1161/01.cir.98.22.2390. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishikawa N, Nishio M, et al. AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failure. Hypertension. 2004;43:686–91. doi: 10.1161/01.HYP.0000118017.02160.fa. [DOI] [PubMed] [Google Scholar]
- 55.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vita-mins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet. 2003;361:2017–23. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 56.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, et al. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 57.Ward NC, Hodgson JM, Croft KD, Burke V, Beilin LJ, Puddey IB. The combination of vitamin C and grape-seed polyphenols increases blood pressure: A randomized, double-blind, placebo-controlled trial. J Hypertens. 2005;23:427–34. doi: 10.1097/00004872-200502000-00026. [DOI] [PubMed] [Google Scholar]
- 58.Nasri H, Ahmadi A, Baradaran A, Nasri P, Hajian S, Pour-Arian A, et al. A biochemical study on ameliorative effect of green tea (Camellia sinensis) extract against contrast media induced acute kidney injury. J Renal Inj Prev. 2014;3:47–49. doi: 10.12861/jrip.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rafieian-Kopaei M, Asgary S, Adelnia A, Setorki M, Khazaei M, Kazemi S, et al. The effects of cornelian cherry on atherosclerosis and atherogenic factors in hypercholesterolemic rabbits. J Med Plants Res. 2011;5:2670–6. [Google Scholar]
- 60.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose chal-lenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–3. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 61.Rafieian-Kopaie M. Medicinal plants for renal injury prevention. J Renal Inj Prev. 2013;2:63–5. doi: 10.12861/jrip.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nasri H, Rafieian-Kopaei M. Herbal medicine and diabetic kidney disease. J Nephropharmacol. 2013;2:1–2. [PMC free article] [PubMed] [Google Scholar]
- 63.Rafieian-Kopaei M, Baradaran A. Combination of metformin with other antioxidants may increase its renoprotective efficacy. J Renal Inj Prev. 2013;2:35–6. doi: 10.12861/jrip.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc Ther. 2010;28:e20–32. doi: 10.1111/j.1755-5922.2010.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shirzad H, Taji F, Pourgheysari B, Raisi S, Rafieian-Kopaei M. Comparison of antitumour activities of heated and raw garlic extracts on fibrosarcoma in Mice. J Babol Univ Med Sci Nov. 2012;14:77–83. [Google Scholar]
- 66.Shirzad H, Taji F, Pourgheysari B, Raisi S, Rafieian-Kopaei M. Comparison of antitumour activities of heated and raw garlic extracts on fibrosarcoma in Mice. J Babol Univ Med Sci Nov. 2012;14:77–83. [Google Scholar]
- 67.Reinhart KM, Coleman CI, Teevan C, Vachhani P, White CM. Effects of garlic on blood pressure in patients with and without systolic hypertension: A meta-analysis. Ann Pharmacother. 2008;42:1766–71. doi: 10.1345/aph.1L319. [DOI] [PubMed] [Google Scholar]
- 68.Hasrat JA, Pieters L, Vlietinck AJ. Medicinal plants in Suriname. J Pharm Pharmacol. 2004;56:381–7. doi: 10.1211/0022357022917. [DOI] [PubMed] [Google Scholar]
- 69.Somanadhan B, Varughese G, Palpu P, Sreedharan R, Gudiksen L, Smitt UW, et al. An ethnopharmacological survey for potential angiotensin converting enzyme inhibitors from Indian medicinal plants. J Ethnopharmacol. 1999;65:103–12. doi: 10.1016/s0378-8741(98)00201-3. [DOI] [PubMed] [Google Scholar]
- 70.Simpson D. Buchu--South Africa's amazing herbal remedy. Scott Med J. 1998;43:189–9. doi: 10.1177/003693309804300610. [DOI] [PubMed] [Google Scholar]
- 71.John JH, Ziebland S, Yudkin P, Roe LS, Neil HA. Oxford Fruit and Vegetable Study Group: Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: A randomised controlled trial. Lancet. 2002;359:1969–74. doi: 10.1016/s0140-6736(02)98858-6. [DOI] [PubMed] [Google Scholar]
- 72.Keenan JM, Pins JJ, Frazel C, Moran A, Turnquist L. Oat ingestion reduces systolic and diastolic blood pressure in patients with mild or borderline hypertension: A pilot trial. J Fam Pract. 2002;51:369. [PubMed] [Google Scholar]
- 73.Burke V, Hodgson JM, Beilin LJ, Giangiulioi N, Rogers P, Puddey IB. Dietary protein and soluble fiber reduce ambulatory blood pressure in treated hypertensives. Hypertension. 2001;38:821–6. doi: 10.1161/hy1001.092614. [DOI] [PubMed] [Google Scholar]
- 74.Tabassum N, Ahmad F. Role of natural herbs in the treatment of hypertension. Pharmacogn Rev. 2011;5:30–40. doi: 10.4103/0973-7847.79097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ajagbonna OP, Mojiminiyi FB, Sofola OA. Relaxant effects of the aqueous leaf extract of Cassia occidentalis on rat aortic rings. Afr J Biomed Res. 2001;4:127–9. [Google Scholar]
- 76.Yang YC, Lu FH, Wu JS, Wu CH, Chang CJ. The protective effect of habitual tea consumption on hypertension. Arch Intern Med. 2004;164:1534–40. doi: 10.1001/archinte.164.14.1534. [DOI] [PubMed] [Google Scholar]
- 77.Asadi SY, Parsaei P, Karimi M, Ezzati S, Zamiri A, Mohammadizadeh F, et al. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int J Surg. 2013;11:332–7. doi: 10.1016/j.ijsu.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 78.Burns JH, Faden RB, Steppan SJ. Phylogenetic studies in the commelinaceae subfamily commelinoideae inferred from nuclear ribosomal and chloroplast DNA sequences. Syst Bot. 2011;36:268–76. [Google Scholar]
- 79.Gilani AH, Shaheen E, Saeed SA, Bibi S, Irfanullah, Sadiq M, Faizi S. Hypotensive action of coumarin glycosides from Daucus carota. Phytomedicine. 2000;7:423–6. doi: 10.1016/s0944-7113(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 80.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: A summary of a statement for professionals from the american heart association nutrition committee. Arterioscler Thromb Vasc Biol. 2006;26:1689–92. doi: 10.1161/01.ATV.0000227471.00284.ef. [DOI] [PubMed] [Google Scholar]
- 81.Odigie IP, Ettarh RR, Adigun SA. Chronic administration of aqueous extract of Hibiscus sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2K-1C hypertensive rats. J Ethnopharmacol. 2003;86:181–5. doi: 10.1016/s0378-8741(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 82.Herrera-Arellano A, Flores-Romero S, Chávez-Soto MA, Tortoriello J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffain patients with mild to moderate hypertension: A controlled and randomized clinical trial. Phytomedicine. 2004;11:375–82. doi: 10.1016/j.phymed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 83.Bloedon LT, Szapary PO. Flaxseed and cardiovascular risk. Nutr Rev. 2004;62:18–27. doi: 10.1111/j.1753-4887.2004.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 84.Engelhard YN, Gazer B, Paran E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am Heart J. 2006;151:100. doi: 10.1016/j.ahj.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 85.Paran E, Novack V, Engelhard YN, Hazan-Halevy I. The effects of natural antioxidants from tomato extract in treated but uncontrolled hypertension patients. Cardiovasc Drugs Ther. 2009;23:145–51. doi: 10.1007/s10557-008-6155-2. [DOI] [PubMed] [Google Scholar]
- 86.Rafieian-Kopaei M, Hosseini-asl K. Effects of Ocimum basilicum on functional dyspepsia. Iran J Med Sci. 2005;30:134–7. [Google Scholar]
- 87.Bahmani M, Rafieian-kopaei M, Parsaei P, Mohsenzadegan A. The anti-leech effect of Peganum harmala L. extract and some anti-parasite drugs on Limnatis Nilotica. Afr J Microbiol Res. 2012;6:2586–90. [Google Scholar]
- 88.Gilani AH, Aftab K, Saeed SA, Suria A. Effect of harmalol on blood pressure in anaesthetized rats. Biochem Soc Trans. 1992;20:359S. doi: 10.1042/bst020359s. [DOI] [PubMed] [Google Scholar]
- 89.Jerie P. Milestones of cardiovascular therapy: IV, Reserpine. Cas Lak Cesk. 2007;146:573–7. [PubMed] [Google Scholar]
- 90.Nakano D, Itoh C, Takaoka M, Kiso Y, Tanaka T, Matsumura Y. Antihypertensive effect of sesamin IV Inhibition of vascular superoxide production by sesamin. Biol Pharm Bull. 2002;25:1247–9. doi: 10.1248/bpb.25.1247. [DOI] [PubMed] [Google Scholar]
- 91.Asgari S, Setorki M, Rafieian-kopaei M, Heidarian E, Shahinfard N, Ansari R, et al. Postprandial hypolipidemic and hypoglycemic effects of Allium hertifolium and Sesamum indicum on hypercholesterolemic rabbits. Afr J Pharm Pharmacol. 2012;6:1131–5. [Google Scholar]
- 92.Ibarrola DA, Montalbetti Y, Heinichen O, Alvarenga N, Figueredo A, Ferro EA. Isolation of hypotensive compounds from Solanum sisymbriifolium L. Am J Ethnopharmacol. 2003;70:301–7. doi: 10.1016/s0378-8741(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 93.Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290:1029–30. doi: 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
- 94.Horie S, Yano S, Aimi N, Sakai S, Watanabe K. Effects of hirsutine, an antihypertensive indole alkaloid from Uncaria rhynchophylla, on intracellular calcium in rat thoracic aorta. Life Sci. 1992;50:491–8. doi: 10.1016/0024-3205(92)90388-6. [DOI] [PubMed] [Google Scholar]
- 95.Ben EE, Eno AE, Ofem OE, Aidem U, Itam EH. Increased plasma total cholesterol and high density lipoprotein levels produced by the crude extract from the leaves of Viscum album(mistletoe) Niger J Physiol Sci. 2006;21:55–60. doi: 10.4314/njps.v21i1-2.53932. [DOI] [PubMed] [Google Scholar]
- 96.Ladeji O, Udoh FV, Okoye ZS. Activity of aqueous extract of the bark of Vitex donianaon uterine muscle response to drugs. Phytother Res. 2005;19:804–6. doi: 10.1002/ptr.1588. [DOI] [PubMed] [Google Scholar]
- 97.Baradaran A, Mahmoud Rafieian-kopaei M. Histopathological study of the combination of metformin and garlic juice for the attenuation of gentamicin renal toxicity in rats. J Renal Inj Prev. 2012;2:15–21. doi: 10.12861/jrip.2013.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fugh-Berman A. Herbs and dietary supplements in the prevention and treatment of cardiovascular disease. Prev Cardiol. 2000;3:24–32. doi: 10.1111/j.1520-037x.2000.80355.x. [DOI] [PubMed] [Google Scholar]
- 99.Shirzad H, Kiani M, Shirzad M. Impacts of tomato extract on the mice fibrosarcoma cells. J HerbMed Pharmacol. 2013;2:13–6. [Google Scholar]
- 100.Tavafi M. Complexity of diabetic nephropathy pathogenesis and design of investigations. J Renal Inj Prev. 2013;2:59–62. doi: 10.12861/jrip.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nasri H. Renoprotective effects of garlic. J Renal Inj Prev. 2012;2:27–8. doi: 10.12861/jrip.2013.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rafieian-Kopaei M. Medicinal plants and the human needs. J HerbMed Plarmacol. 2012;1:1–2. [Google Scholar]
- 103.Nasri H, Shirzad H. Toxicity and safety of medicinal plants. J HerbMed Plarmacol. 2013;2:21–2. [Google Scholar]
- 104.Nasri H, Ardalan MR, Rafieian-Kopaei R. On the occasion of world hypertension day 2014. J Parathyr Dis. 2014;2:19–20. [PMC free article] [PubMed] [Google Scholar]
- 105.Shahbazian N, Shahbazian H, Mohammadjafari R, Mousavi M. Ambulatory monitoring of blood pressure and pregnancy outcome in pregnant women with white coat hypertension in the third trimester of pregnancy: A prospective cohort study. J Nephropharmacol. 2013;2:5–9. [PMC free article] [PubMed] [Google Scholar]
- 106.Nam KY. Clinical applications and efficacy of Korean ginseng (Panax ginseng C.A. Meyer) J Ginseng Res. 2002;26:111–31. [Google Scholar]
- 107.Tamadon MR, Ardalan MR, Nasri H. World Kidney Day 2013; acute renal injury; a global health warning. J Parathyr Dis. 2013;1:27–8. [Google Scholar]


