Abstract
Asthma has long been characterized as a disease of dysregulated TH2 immune responses to environmental allergens. However, clinical studies suggest that asthma is a heterogeneous disorder with distinct types of inflammatory processes. Accumulating evidence suggests that aberrant IL-17 production is a key determinant of severe forms of asthma. However, the identity of IL-17-producing cells and the factors regulating IL-17 production during the course of allergic inflammation remain elusive. In this report, we will summarize the potential IL-17-producing cells and their involvement in the inflammatory responses that mediate distinct features of asthma. The role of proinflammatory cytokines and the complement pathway in regulating the generation of IL-17-producing T cells will also be discussed. Understanding the biology of IL-17 in the context of allergic inflammation may inform into the development of novel approaches for the diagnosis and treatment of asthma.
Keywords: IL-17, T cells, asthma
Introduction
Allergic asthma is a chronic and heterogeneous disorder of airways that affects 5–10% of the population in the United States. The cardinal features of asthma include chronic airway inflammation, mucus hypersecretion, airway hyperresponsiveness (AHR) to inhaled allergens, and subepithelial fibrosis [1]. Studies from patients and animal models have demonstrated that upon allergen exposure, lung resident T helper 2 (TH2) cells release TH2 cytokines to initiate a cascade of immunological events, resulting in the pathogenesis of asthma [2]. The TH2 cytokine IL-4 is critical for the generation of TH2 cells and mediating allergen-specific B cells to undergo isotype class switching to IgE [3]. IL-5 promotes airway eosinophilia, and IL-13 mediates the induction of AHR, goblet cell hyperplasia, and mucin production [2]. Although TH2 cells are thought to drive asthma in patients with eosinophilic inflammation, asthma patients often appear to manifest different patterns of airway inflammation that cannot be explained by the TH2 immune response alone. Specifically recent cluster analyses have led to the classification of asthma into subgroups depending on the nature of the inflammatory response including: (1) eosinophilic; (2) neutrophilic; (3) mixed (both neutrophils and eosinophils found); and (4) paucigranulocytic (few or no granulocytes) [4, 5]. Patients with asthma who have elevations in both eosinophils as well as neutrophils in their airways have the lowest lung function, worse asthma control, and increased exacerbations [5, 6]. Given that Th17-derived cytokines, such as IL-17A and IL-17F are important regulators of neutrophilic inflammation, it has been postulated that aberrant IL-17A/F production may drive severe forms of the disease. Indeed, recent studies demonstrate that individuals with severe asthma display not only severe AHR, but also robust neutrophilia and increased IL-17A/F production [6, 7]. These discoveries have led to the revision of the TH1/TH2 paradigm and provide new perspectives on the immunopathogenesis of allergic asthma and highlight the importance of understanding the regulation of IL-17 production and the identity of IL-17-producing cells in the lung during the course of allergic inflammation. Herein we discuss recent advances in our understanding of the role of IL-17A in asthma.
IL-17 and its signaling
IL-17 (IL-17A) was originally identified from activated T cell clones and named CTLA-8 [8–10]. Subsequently, genomic approaches led to the discovery of five additional family members, designated as IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F [11–13]. Among IL-17 family members, the expression patterns and function of IL-17, IL-17F, and IL-17E (IL-25) are better characterized. Alignment of the predicted amino acid sequence of IL-17 and the other family members revealed that IL-17F shares the greatest similarity with IL-17 (55% identity) whereas IL17E (IL-25) shares the least (17%) [14]. IL-17 is a disulfide-linked homodimeric glycoprotein with a molecular weight of 35 kD whose C-terminus possesses a cysteine knot structure similar to that found in TGF-β and nerve growth factor [15]. Recent reports demonstrate that IL-17 can also form heterodimers with IL-17F, termed IL-17A/F [16, 17]. Studies of the IL-17 receptor family (IL-17RA-E) showed that the cognate receptor for IL-17 is IL-17RA, which is ubiquitously expressed [18]. However, the biological activity of IL-17 is dependent on the heterodimeric receptor complex composed of IL-17RA and IL-17RC [19]. Bioinformatic analyses identified a conserved cytoplasmic motif of IL-17R family members, termed SEFIR (similar expression to FGF receptor and IL-17R), that is homologous to the Toll/IL-1R (TIR) domains common to Toll-like receptors (TLR) and IL-1 receptors [20]. The signaling adaptor ACT1, which also contains the SEFIR domain, is essential for mediating IL-17R signaling [21]. The homotypic interaction between IL-17RA and ACT1 through the SEFIR domain recruits TNFR-associated factor 6 (TRAF6), which activates downstream canonical nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways [18].
IL-17-producing cells in asthma
IL-17 was first shown to be produced by activated CD4+ T cells. The analysis of IL-23-mediated immune pathogenesis later led to the delineation of a distinct CD4+ T helper cell subset, termed the TH17 cell lineage [22–25]. TH17 cells produce IL-17, IL-17F, IL-22, and, to a lesser extent, tumor necrosis factor (TNF) and IL-6 [26]. The retinoid acid-related orphan receptor (RORγt) was later identified as the master transcription factor controlling the development of the TH17 cell lineage [27–31]. Although the importance of TH17 cells was originally described in driving inflammatory autoimmune disorders [22], accumulating evidence now suggests that TH17 cells and their related cytokines are also involved in the pathophysiology of allergic asthma. IL-17 expression is increased in the lung, sputum, bronchoalveolar lavage fluid (BALF), and sera in patients with asthma, and the severity of AHR is positively correlated with IL-17 expression levels [32, 33]. IL-17 and IL-17F can induce lung structural cells to secrete proinflammatory cytokines (e.g. TNF, IL-1β, G-CSF, and IL-6) and chemokines (e.g. CXCL1/Gro-α, CXCL2, and CXCL8/IL-8), thereby triggering neutrophil infiltration [34–36]. In a mouse model of allergic lung disease, mice lacking IL-17A exhibited a reduced TH2 response to antigen sensitization, and mice lacking IL-17RA exhibited reduced neutrophil and eosinophil recruitment [37, 38]. While these studies demonstrate the importance of IL-17 in driving the immunopathogenesis of asthma, the identity of IL-17-producing cells during the course of allergic inflammation remains elusive.
In addition to TH17 cells, other innate-like T cells (e.g. γδ T cells and invariant natural killer T [iNKT] cells) are also capable of producing IL-17 in the context of allergic asthma [39]. γδ T cells are in close contact with lung epithelium and have served an immunosurveillance function. In mice, IL-17-producing γδ T cells have been shown to play important roles in the pathogenesis of inflammatory granulomatous diseases and in lung tissue damage during pulmonary aspergillosis [40]. These cells can produce IL-17 rapidly, drive the recruitment and activation of neutrophils at the sites of inflammation, and contribute to AHR [41]. In humans, the frequency of γδT cells in BALF from patients with asthma is higher than that of healthy controls [42]. However, the function of lung resident γδ T cells in the immunopathogenesis of human asthma remains unclear [43]. iNKT cells express restricted T cell receptor (TCR) that recognizes lipid antigens in the context of the MHC class I-like molecule CD1d. After activation with synthetic α-galactosylceramide, these cells rapidly produce high levels of IL-17 in vitro. Administration of α-galactosylceramide intranasally in mice activates iNKT cells to produce IL-17, leading to airway neutrophilia [44]. Furthermore, in a mouse model of allergic lung disease, mice deficient in iNKT cells failed to develop AHR, despite of attenuated eosinophilc inflammation [45]. In humans, iNKT cells were found in the lungs of asthmatic patients and the frequency of iNKT cells are increased after allergen challenges [46, 47]. These studies suggest a role of iNKT cells in the development of AHR and of asthma with neutrophilic inflammation.
Recently, a subset of human TH2 memory cells that are capable of producing TH17 and TH2 cytokines concurrently has been identified [48]. These IL-17-producing TH2 cells express both GATA3 and RORγt transcription factors, which are known to be required for the development of the TH2 and TH17 cell lineage, respectively. Notably, the frequency of circulating IL-17-producing TH2 cells within the total CD4+ TH2 memory/effector cell pool is significantly elevated in the blood of patients with atopic asthma. Mirroring these findings in humans, IL-17-producing TH2 cells are found primarily in the inflamed lungs, but not other lymphoid organs, in a mouse model of allergic lung disease. Notably, these lung resident IL-17-producing TH2 cells persist in inflamed lungs as the dominant IL-17-producing T cells during the chronic stage of allergic airway inflammation. These intriguing findings suggest that TH2 memory cells are a dominant cellular source of IL-17 in the chronic stage of asthma.
Regulation of IL-17 production
Since IL-17-producing cells play important roles in the inflammatory response at mucosal sites, understanding the regulation of IL-17 production is a key subject for investigations. Although the specific requirements for individual IL-17-producing cell types may vary, extensive studies have identified the key factors or inflammatory cytokines that are crucial for the induction of IL-17 production, summarized as follows. (1) Studies in vitro have shown that the absence of IL-4 and IFN-γ is the primary prerequisite for TH17 cell differentiation from naïve T cells [49]. (2) Transforming growth factor-β (TGF-β) is the essential cytokine that induces transcription factor RORγt expression in naïve T cells during TH17 cell development [49–51], although the role of TGF-β in human TH17 cell lineage commitment has been controversial [52–54]. While the role of TGF-β in regulating IL-17 production by γδ T cells and iNKT cells remains to be determined, a recent study demonstrates that TGF-β is not required for the induction of IL-17 production by TH2 memory cells [48]. (3) Amidst the accumulating evidence, the proinflammatory cytokine IL-1β has emerged as the critical factor to trigger IL-17 production [55]. IL-1β can induce TH17 polarization from CD4+ naïve T cells and trigger IL-17 production by classical TH2 memory cells, as well as iNKT cells, via upregulating the transcription factor interferon regulator factor 4 (IRF4) expression [56]. (4) Although IL-23 was originally identified to be the key cytokine for the development of TH17 cell lineage [22], recent studies suggest that IL-23 is critical for the expansion and maintenance of TH17 cells [57, 58] and functions to promote IL-17 production by γδT cells and iNKT cells [44]. Notably, IL-23 is not required for the induction of IL-17 production by classical TH2 memory cells due to their lack of IL-23R [48]. (5) IL-6 can synergize with TGF-β to induce surface IL-23R expression on TH17 cells, thereby promoting TH17 development in the presence of IL-23. While IL-6 can also enhance the effect of IL-1β on the induction of IL-17 production by TH2 memory cells, IL-6 is dispensable for IL-17 production by γδ T cells and iNKT cells [27, 56]. (6) The cytokine IL-21 has pleiotropic effects on the proliferation, differentiation, and effector function of B, T, and natural killer (NK) cells [59]. Recent studies suggest that IL-21 secreted by TH17 or TH2 cells can also synergize with IL-1β and/or IL-6 to drive IL-17 production in an autocrine manner [48, 60, 61]. Although the proinflammatory cytokines described above are now known to have the potential to induce IL-17 production, the factors that regulate their temporal and combinatorial expression during the course of allergic inflammation remain to be investigated.
One example of an innate immune pathway shown to regulate the expression of proinflammatory cytokines, which in turn induces IL-17 production, are the anaphylatoxins C5a and C3a. A recent study designed to decipher the differences in susceptibility to severe asthma between strains of mice that are highly susceptible to the development of allergen-driven severe airway hyperresponsiveness (A/J) and strains that are relatively resistant (C3H/HeJ) has shown a role of the complement components C3a and C5a in regulating IL-17 production [62, 63]. Using microarray analysis and single-nucleotide polymorphism-based genotyping, a deletion in the coding sequence of the C5 gene in A/J mice was identified and shown to be responsible for the susceptibility to severe AHR in a mouse model of allergic asthma [63]. Furthermore, A/J mice that produced TH2 cytokines and elevated IL-17 cytokines developed more severe AHR than C3H/HeJ mice that produced only TH2 cytokines after intratracheal challenge with house dust mite (HDM) [62]. Indeed in vivo blockade of IL-17A in susceptible A/J mice reduced their airway responses to the level of the resistant C3H/HeJ mice. Taken together these findings suggested that the lack of C5a in A/J mice might contribute to their aberrant IL-17 production, leading to severe allergic asthma. The authors go on to show that the differences in IL-17A production were due to differences in bone marrow-derived dendritic cell (DCs) cytokine production in that C5a-deficient A/J mice produced elevated levels of IL-23 compared to those from C5a-proficient C3H/HeJ mice; however, no differences were observed in their IL-1β or IL-6 production, suggesting that the presence of C5a signaling in C3H/HeJ mice controls IL-17 production by limiting IL-23 secretion from DCs during allergic inflammation. In addition to C5a, C3a also has an effect on regulating allergic inflammation [64]. Mice deficient in complement receptor C3a (C3aR) had less IL-23 production and fewer detectable IL-17-producing cells in the lung, and thus less AHR induction, after HDM challenge [62]. HDM-treated bone marrow–derived DCs from C3ar1-deficient mice produced less IL-23 compared to those from wild type mice, suggesting that C3aR signaling promotes IL-23 production by DCs [62]. Furthermore, HDM challenges induced enhanced C3a and C3aR mRNA expression, which mediates the elevated IL-23 production in A/J mice and, to a lesser extent, in C3H/HeJ mice. These interesting results show that C5a and C3a have reciprocal roles in the immunopathogenesis of allergic asthma by regulating the IL-23/IL-17 pathway and point to an additional layer of regulation of IL-17 production during allergic inflammation. As complement activation is important in the clearance of infections and has specifically been shown to play an important role in respiratory syncytial virus (RSV)-induced pulmonary inflammation [65], these studies suggest that aberrant IL-17 production in the lungs of severe asthmatics may be driven by viral infection induction of complement and subsequent activation of the IL-23/IL-17 axis. Thus understanding the involvement of anaphylatoxin control of the IL-23/IL-17 axis in virally-induced exacerbations of asthma may be an important subject for future investigation.
IL-17 and severity of asthma
Although asthma symptoms in the majority of patients with mild asthma are well-controlled with current therapies, approximately 10% of the asthmatic population with severe asthma remains poorly controlled despite high-dose inhaled therapy [66]. This subpopulation of asthma patients were found to express elevated IL-17 protein in their airways, which correlated with increased neutrophil infiltration, chemokine IL-8 production, and the degree of airway hyperresponsiveness [6, 32, 67]. Using an immunohistochemical approach, a recent study further demonstrated that the number of detected IL-17-producing cells in lung tissue of patients with severe asthma is significantly higher compared to other groups of asthma patients [6]. Notably, these immunoreactive IL-17+ cells were exclusively mononuclear cells and located within clusters of inflammatory cells in the subepithelial tissues [6]. These studies provide evidence that the presence of IL-17A+ cells is positively correlated with the severity of human asthma. However, it remains to be determined which IL-17-producing cell type contributes to acute exacerbation asthma in response to various airway insults in humans.
The direct role of IL-17 in contributing to the severity of asthma was further substantiated in mouse studies. Mice deficient in IL-17RA or IL-17A have markedly diminished recruitment of neutrophils into the lung in response to a challenge with gram-negative bacteria or allergens [37, 38]. The identification of a novel IL-17-producing TH2 memory cell type may provide a plausible explanation for the cause of severe asthma with a mixed airway inflammation during the chronic phase [48]. IL-17-producing TH2 cells secrete both TH2 and TH17 cytokines that have profound synergistic effects on the induction of various chemokine genes in primary human lung bronchial epithelial cells, including MIP-1β, MCP-1, Gro-α, IL-8, and particularly eotaxin-3, thereby promoting the recruitment of a heterogeneous pattern of inflammatory leukocytes [48]. In animal models of asthma, transfer of antigen-specific IL-17-producing TH2 cells triggered influx of heterogeneous leukocytes, including neutrophils, eosinophils, macrophages, and lymphocytes, resulting in distinct pathophysiological features of severe asthma [48], similar to those of the combined TH2 and TH17 cells [48, 68, 69]. Importantly, IL-17-producing TH2 cells may represent the key pathogenic TH2 cell that may have additional inflammatory properties capable of promoting exacerbations of allergic asthma. To examine whether increased IL-17 contributes directly to the severity of AHR in response to allergen, several studies utilized intranasal IL-17 administration or neutralization in mouse models of allergic lung disease. Treatment of A/J mice that have aberrant IL-17 production with IL-17-specific antibody results in significantly less AHR and fewer neutrophils in the BALF [62]. Conversely, administration of recombinant IL-17 to C3H/HeJ mice that have little IL-17 production during allergen challenge increased their susceptibility to AHR [62]. In another study, a causative link between TH17 cells and glucocorticoids (GC)-insensitive allergic airway disease in mice was demonstrated [70]. GC treatments did not abrogate IL-17 and IL-22 production by TH17 cells in vitro and TH17-induced neutrophilic inflammation in airway by transferred TH17 cells in vivo, whereas GC treatments was effective in inhibiting TH2-driven diseases [70]. While most studies provide similar findings, one study suggested that IL-17 may act as a negative regulator during the effector phase of the allergic response [71]. These discrepancies may be due to differences in the experimental design (e.g. timing of IL-17 administrations/neutralizations and allergen challenges) or the experimental system used to assess AHR. Overall, the findings of most of these human and mouse studies provide direct evidence to suggest a causative role of IL-17 in inducing severe asthma.
Conclusion
In summary, emerging evidence suggest that activation of the IL-17-producing cells may be associated with the neutrophilic inflammatory response and the development of severe forms of asthma. In response to exposure to irritants, infectious agents, or allergens, injured airway epithelial cells and altered innate immune responses initiate distinct inflammatory processes that may determine the identity of the initial IL-17-producing cells during the acute phase of asthma. The inflammatory response may be propagated in the airway and regulate the maintenance of TH2 and TH17 cell subsets during the chronic phase. The interplay between innate and adaptive immunity may thus have an important role in the induction and maintenance of IL-17 production. Environmental insults and intrinsic genetic factors, such as complement genes, may also be involved in the development of IL-17-producing cells during allergic inflammation. Future studies to explore further the factors that regulate IL-17 induction and the generation of IL-17-producing cells in the context of allergic inflammation will provide the basis to elucidate the distinct pathophysiological mechanisms that mediate the severity of asthma. As severe forms of asthma have proven difficult to treat with existing therapies, modulation of this pathway may hold promise for the treatment of this ever-increasing disease.
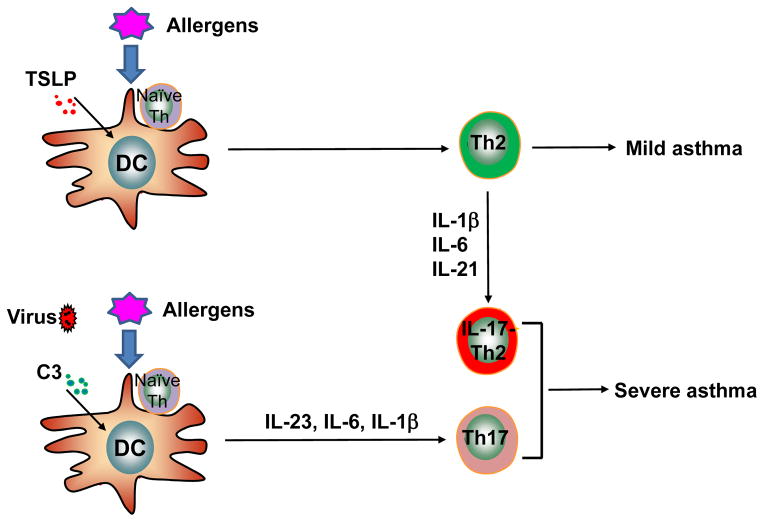
Fig. 1. The development of IL-17-producing T cells and the severity of asthma.
Upon exposure to allergens and infectious agents, dendritic cells activated by TSLP (thymic stroma lymphopoietin) or anaphylatoxin C3a can induce naïve T cells to differentiate into TH2 or TH17 cells, respectively. Depending on the inflammatory signals in the microenvironment, local TH2 memory/effector cells may acquire distinct inflammatory properties to become IL-17-producing cells that promote severity of asthma.
Acknowledgments
We thank Shawna Hottinger for editorial assistance; and NIAID (Y.-H. Wang: R01 AI090129-01) and the ALAI/AAAAI Foundation (Y.-H. Wang) and (MWK: 2RO1HL067736, RO1AIO83315) for research support.
Footnotes
The authors have no conflicting financial interests.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Epstein MM. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol Ther. 2006;109:107–136. doi: 10.1016/j.pharmthera.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 5.Hastie AT, Moore WC, Meyers DA, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036. e1013. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Al-Ramli W, Prefontaine D, Chouiali F, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. This article demonstrated that the number of IL-17-producing cells in lung tissue of patients with severe asthma is significantly higher than those of patients with mild asthma. These immunoreactive IL-17+ cells were exclusively mononuclear cells and located within clusters of inflammatory cells in the subepithelial tissues. [DOI] [PubMed] [Google Scholar]
- 7.Chakir J, Shannon J, Molet S, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 8.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Z, Maraskovsky E, Spriggs MK, Cohen JI, Armitage RJ, Alderson MR. Herpesvirus saimiri open reading frame 14, a protein encoded by T lymphotropic herpesvirus, binds to MHC class II molecules and stimulates T cell proliferation. J Immunol. 1996;156:3260–3266. [PubMed] [Google Scholar]
- 10.Yao Z, Painter SL, Fanslow WC, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 11.Lee J, Ho WH, Maruoka M, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Chen J, Huang A, et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starnes T, Robertson MJ, Sledge G, et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 14.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 15.Hymowitz SG, Filvaroff EH, Yin JP, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell research. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 17.Wright JF, Guo Y, Quazi A, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 18.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright JF, Bennett F, Li B, et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol. 2008;181:2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 20.Maitra A, Shen F, Hanel W, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 22.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 23.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 31.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakir J, Shannon J, Molet S, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 33.Molet S, Hamid Q, Davoine F, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 34.Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 35.Laan M, Cui ZH, Hoshino H, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 36.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 37.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 39.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–994. doi: 10.1016/j.jaci.2009.03.033. quiz 995–986. [DOI] [PubMed] [Google Scholar]
- 40.Romani L, Fallarino F, De Luca A, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura R, Shibata K, Yamada H, Shimoda K, Nakayama K, Yoshikai Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J Immunol. 2008;181:2071–2075. doi: 10.4049/jimmunol.181.3.2071. [DOI] [PubMed] [Google Scholar]
- 42.Spinozzi F, Agea E, Bistoni O, et al. Increased allergen-specific, steroid-sensitive gamma delta T cells in bronchoalveolar lavage fluid from patients with asthma. Annals of internal medicine. 1996;124:223–227. doi: 10.7326/0003-4819-124-2-199601150-00005. [DOI] [PubMed] [Google Scholar]
- 43.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel ML, Keller AC, Paget C, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 46.Matangkasombut P, Marigowda G, Ervine A, et al. Natural killer T cells in the lungs of patients with asthma. J Allergy Clin Immunol. 2009;123:1181–1185. doi: 10.1016/j.jaci.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynolds C, Barkans J, Clark P, et al. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J Allergy Clin Immunol. 2009;124:860–862. doi: 10.1016/j.jaci.2009.07.022. author reply 862. [DOI] [PubMed] [Google Scholar]
- 48••.Wang YH, Voo KS, Liu B, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. This article described a novel subset of TH2 memory/effector cells that feature concurrent TH17 and TH2 cytokine production and contribute to the exacerbation of allergic asthma at chronic stage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 51.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 52.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 53.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 55.Dinarello CA. Blocking interleukin-1beta in acute and chronic autoinflammatory diseases. J Int Med. 2011;269:16–28. doi: 10.1111/j.1365-2796.2010.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doisne JM, Soulard V, Becourt C, et al. Cutting edge: crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J Immunol. 2011;186:662–666. doi: 10.4049/jimmunol.1002725. [DOI] [PubMed] [Google Scholar]
- 57.McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Leonard WJ, Zeng R, Spolski R. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol. 2008;84:348–356. doi: 10.1189/jlb.0308149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou L, Ivanov, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 62••.Lajoie S, Lewkowich IP, Suzuki Y, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. This article describe the reciprocal role of complement C3a and C5a in regulating the IL-23-TH17 axis that controls the severity of asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karp CL, Grupe A, Schadt E, et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol. 2000;1:221–226. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Lewkowich IP, Kohl G, Clark JR, Wills-Karp M, Kohl J. A protective role for C5a in the development of allergic asthma associated with altered levels of B7-H1 and B7-DC on plasmacytoid dendritic cells. J Immunol. 2009;182:5123–5130. doi: 10.4049/jimmunol.0804276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polack FP, Teng MN, Collins PL, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196:859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. This review highlights the concept that distinct inflammatory pathways contributing to the heterogeneity of asthma. [DOI] [PubMed] [Google Scholar]
- 67.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respiratory medicine. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 68.Wakashin H, Hirose K, Maezawa Y, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 69.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKinley L, Alcorn JF, Peterson A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schnyder-Candrian S, Togbe D, Couillin I, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]