Abstract
Background
The development of acute kidney injury (AKI) after cardiac surgery is associated with significant mortality, morbidity, and cost. The last decade has seen major changes in the complexity of cardiac surgical candidates and in the number and type of cardiac surgical procedures being performed.
Methods
Using data from the Nationwide Inpatient Sample, we determined the annual rates of AKI, AKI requiring dialysis (AKI-D), and inpatient mortality after cardiac surgery in the United States in the years 1999 through 2008.
Results
Inpatient mortality with AKI and AKI-D decreased from 27.9% and 45.9%, respectively, in 1999 to 12.8% and 35.3%, respectively, in 2008. Compared with 1999, the odds of AKI and AKI-D in 2008, adjusted for demographic and clinical factors, were 3.30 (95% confidence interval [CI]: 2.89 to 3.77) and 2.23 (95% CI: 1.78 to 2.80), respectively. Corresponding adjusted odds of death associated with AKI and AKI-D were 0.31 (95% CI: 0.26 to 0.36) and 0.47 (95% CI: 0.34 to 0.65.) Taken together, the attributable risks for death after cardiac surgery associated with AKI and AKI-D increased from 30% and 5%, respectively, in 1999 to 47% and 14%, respectively, in 2008.
Conclusions
In sum, despite improvements in individual patient outcomes over the decade 1999 to 2008, the population contribution of AKI and AKI-D to inpatient mortality after surgery increased over the same period.
Acute kidney injury (AKI) is a serious complication of cardiac surgery with cardiopulmonary bypass and occurs with a reported frequency of 6% to 8%. Acute kidney injury is severe enough to necessitate dialysis in approximately 1% of patients [1–5]. Postoperative AKI is associated with significantly higher patient mortality, morbidity, length of stay, and cost [2, 3, 6]; and even minor postoperative increases in serum creatinine are associated with an increase in long-term mortality [7].
Ischemia-reperfusion injury is the most common cause of AKI in the cardiac surgery population and is associated with the pathologic features of acute tubular necrosis [8]. In renal ischemia-reperfusion injury, the initial ischemic injury is compounded by reperfusion, associated reactive oxygen species formation, profound inflammation, and further tissue injury [9].
Unfortunately, there are no efficacious treatments to prevent or treat established AKI after cardiac surgery. Management consists of preoperative risk stratification, avoidance of nephrotoxins, optimization of renal perfusion, and when required to prevent complications, such as hyperkalemia, metabolic acidosis, and refractory volume overload, initiation of dialysis [10–14].
Surgical coronary artery bypass graft surgery (CABG) and open valve repair or replacement remain the treatments of choice for many patients with ischemic and valvular heart disease [15–17]. However, advances in medical therapy and percutaneous coronary interventions over the last decade have led to a significant decline in the number of cardiac surgeries performed annually and a trend toward more complex patients coming to surgery [18–23]. The changes in the cardiac surgical demographic combined with increased recognition of AKI as an important adverse outcome have likely altered the epidemiology and approach to AKI in the cardiac surgery population [24].
We conducted the present study of a representative sample of patients undergoing coronary artery bypass grafting and open-heart valve surgery in the United States. We specifically determined trends in the incidence and the adverse outcomes associated with AKI in the cardiac surgery population from 1999 to 2008.
Patients and Methods
Study Population
We extracted data from the Nationwide Inpatient Sample (NIS) for the years 1999 to 2008. The NIS is a stratified random sample of approximately 20% of all US community hospitals in states that participate in the Healthcare Cost and Utilization Project. Twenty-four states participated in this effort in 1999, increasing to 42 states in 2008. The NIS contains all discharge records for the sampled hospitals and, for each year, holds approximately 8 million records from approximately 1,000 hospitals. Sample weights are provided to allow the generation of national estimates. Because NIS data are deidentified, the Stanford University School of Medicine Committee on Human Subjects in Medical Research waived approval of this retrospective study.
The study population demographics, procedures, co-morbid illnesses and outcomes were identified through NIS administrative data and the appropriate International Classification of Diseases, Ninth Revision (ICD-9) codes (Table 1). The study cohort consisted of all patients 18 years of age and older who underwent an on-pump CABG or an open-chamber valve procedure (valve repair or replacement), or both. For each year of the study period, we identified the outcomes of AKI, AKI-D, and in-hospital death in the study cohort. The definitions of AKI and AKI-D were based on ICD-9 coding rather than on laboratory or clinical data. The ICD-9 codes for AKI and AKI-D have previously been shown to have positive predictive values of 47.9% and 94.0% and negative predictive values and of 96.1% and 90.0% for their respective diagnoses identified on medical record review [25].
Table 1.
Procedure and Diagnosis Codes Used to Define Study Population, Outcomes, and Covariates
| Disease/Procedure | ICD-9 Code |
|---|---|
| Acute kidney injury | 584.5, 584.6, 584.7, 584.8, 584.9 |
| Hemodialysis/hemofiltration | V45.1, V56.0, 39.95, V56.1 |
| Peritoneal Dialysis | 54.98 |
| Cardiopulmonary bypass | 39.61 |
| Coronary artery bypass graft | 361.0–9 |
| Valve surgery | 351.0–4, 352.0–8 |
| Diabetes mellitus | 250.x, 357.2, 362.0, 362.07 |
| Hypertension | 401, 402, 405 |
| Heart Failure | 428.x |
| Obstructive airways disease | 490.x–496.x, 500.x–505.x, 516.x, V813 |
| Cerebrovascular disease | 430.x–438.x, 00.61, 00.63, 38.10 |
| Peripheral vascular disease | 440.20–440.24, 424.3x, 424.4, 39.50, 39.90, 00.55, 39.25, 39.29 |
| Obesity | 278.00, 278.01 |
| Intraaortic balloon pump | 37.61 |
| Mechanical ventilation | 93.90, 93.92, 96.01, 96.04, 96.05, 96.70, 96.71, 96.72 |
| Sepsis | 038.x, 112.5, 112.81, 020.2, 790.7, 785.59 |
We excluded hospitalizations with an ICD-9 procedure code for dialysis but without a diagnosis code for AKI (n = 6,389), assuming that these patients were treated with dialysis for end-stage renal disease. Additionally, we excluded patients for whom information on demographic or clinical characteristics were unavailable (n = 978).
In adjusted analyses, we considered age, sex, surgery type (CABG or open-heart valve repair or replacement, or both), seven comorbid conditions associated with the risk of AKI after cardiac surgery (heart failure, hypertension, chronic obstructive pulmonary disease, diabetes mellitus, cerebrovascular disease, obesity, and peripheral arterial disease); indicators of noncardiac morbidity (mechanical ventilation and sepsis), and severity of myocardial dysfunction (intraaortic balloon pump placement.) All comorbid conditions and procedures were defined by the presence of a corresponding ICD-9 code (Table 1). To account for changing hospitalization utilization practices during the study period, we extracted the median hospital length of stay and the fraction of patients discharged to skilled nursing facilities for each year.
Statistical Analysis
We compared the odds of AKI and AKI-D and associated odds of in-hospital mortality over time by fitting a series of logistic regression models. We fitted unadjusted and multivariable adjusted models to estimate the odds for each outcome for patients discharged in each year relative to 1999. We graphically displayed unadjusted and adjusted odds ratios (OR), with 95% confidence intervals (CI), for each outcome over time. We tested linear trends using multivariable adjusted weight least square regression.
Odds ratios describe the relative effects of the covariates but they do not give us any sense of the absolute size of those effects and can be deceptive when the fraction of events is high. We therefore generated predicted marginal percents (model adjusted risks or adjusted percents) for mortality among patients with AKI and AKI-D for each year. The predicted marginal percent for a particular year represents the average predicted response assuming that all covariates in the given year are distributed similarly to the whole population [26, 27].
Finally, we computed the population attributable risk of death associated with AKI and AKI-D [28], indicating the proportion of deaths that could potentially be avoided if AKI and its associated complications were abrogated. To examine whether the main results differed by type of surgery (CABG alone versus valve repair alone versus combined), all analyses were subjected to tests for interaction with year, while adjusting for multiple comparisons using the Bonferroni approach. None of the statistical tests for interaction was statistically significant; as a result, surgery specific results are not reported herein (these data are available from the authors upon request.) All estimates presented account for the complex survey design (weighting and stratification) and subpopulation estimation. We created the cohort using the Statistical Analysis System software, version 9.2 (SAS Institute, Cary, NC), and conducted the analyses using StataMP, version 11 (StataCorp, College Station, TX).
Results
Population Characteristics and Trends in Cardiac Surgery
We identified 602,134 discharges with procedure codes for cardiopulmonary bypass with coronary artery bypass graft surgery or open valve repair or replacement from the NIS between 1999 and 2008, which extrapolates to a total of 2,952,528 surgeries for the entire US adult population. The demographic, clinical, and surgical characteristics for the entire study population and for each individual calendar year are shown in Table 2.
Table 2.
Characteristics of Patients Undergoing On-Pump Open-Heart Surgery in the United States, 1999 to 2008
| Characteristics | All Years | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac surgeries, n | 2,952,528 | 327,515 | 352,151 | 337,489 | 320,169 | 313,925 | 280,737 | 256,484 | 272,720 | 245,708 | 245,630 | |
| Age, median (IQR) | 67 (57–74) | 67 (58–74) | 67 (57–74) | 67 (57–74) | 67 (57–75) | 67 (57–74) | 67 (57–75) | 67 (57–75) | 67 (58–75) | 67 (58–75) | 67 (58–75) | 0.44 |
| Female, % | 31.8 | 31.7 | 31.9 | 32.0 | 32.4 | 31.7 | 31.7 | 31.2 | 31.7 | 31.4 | 31.8 | 0.23 |
| Surgery type | ||||||||||||
| CABG, % | 72.4 | 79.5 | 79.3 | 75.7 | 75.1 | 72.8 | 71.3 | 67.5 | 66.8 | 65.7 | 63.4 | <0.001 |
| Valve, % | 16.2 | 11.2 | 11.6 | 13.8 | 13.9 | 15.9 | 16.4 | 19.9 | 19.8 | 21.2 | 22.6 | <0.001 |
| CABG and valve, % | 11.5 | 9.3 | 9.1 | 10.6 | 11.0 | 11.3 | 12.3 | 12.6 | 13.4 | 13.1 | 14.0 | <0.001 |
| Heart failure, % | 22.0 | 19.7 | 19.5 | 20.5 | 21.4 | 22.6 | 23.5 | 23.9 | 23.8 | 24.1 | 22.4 | <0.001 |
| Diabetes mellitus, % | 29.6 | 27.1 | 28.1 | 28.1 | 29.6 | 29.5 | 30.2 | 29.4 | 31.0 | 33.1 | 31.8 | <0.001 |
| Hypertension, % | 58.4 | 53.6 | 55.2 | 56.4 | 59.0 | 59.2 | 61.1 | 60.8 | 60.3 | 60.4 | 60.2 | <0.001 |
| Pulmonary disease, % | 19.4 | 17.0 | 17.4 | 17.6 | 19.9 | 19.5 | 19.7 | 21.6 | 22.3 | 21.1 | 19.7 | <0.001 |
| PVD, % | 2.0 | 1.9 | 2.0 | 2.1 | 1.9 | 1.8 | 1.9 | 2.1 | 2.2 | 2.4 | 2.2 | 0.01 |
| CVD, % | 7.1 | 7.4 | 6.6 | 6.7 | 6.9 | 6.6 | 6.9 | 7.0 | 7.3 | 7.7 | 8.3 | <0.001 |
| Obesity, % | 9.2 | 6.6 | 7.0 | 7.3 | 8.4 | 9.3 | 9.6 | 9.9 | 10.6 | 12.2 | 13.0 | <0.001 |
| IABP, % | 7.2 | 7.3 | 7.0 | 6.8 | 7.0 | 6.6 | 7.2 | 8.2 | 7.2 | 7.6 | 7.9 | 0.03 |
| Mechanical ventilation, % | 7.9 | 8.5 | 5.2 | 5.4 | 7.0 | 7.6 | 8.6 | 7.9 | 8.6 | 10.1 | 11.5 | <0.001 |
| Sepsis, % | 2.2 | 1.6 | 1.5 | 1.8 | 2.0 | 2.1 | 2.2 | 2.6 | 2.5 | 2.8 | 3.3 | <0.001 |
| LOS, days, median (IQR) | 8 (6–11) | 7 (5–10) | 7 (6–11) | 8 (6–11) | 8 (6–11) | 8 (6–11) | 8 (6–11) | 8 (6–11) | 8 (6–11) | 8 (6–11) | 8 (6–11) | <0.001 |
| Discharge to skilled nursing facility, % | 15.3 | 12.6 | 12.6 | 14.4 | 14.8 | 14.7 | 15.7 | 16.2 | 16.2 | 19.1 | 19.1 | <0.001 |
Frequencies, medians, and percents reflect national estimates. The p values were derived from tests for linear trend of means using weighted least squares regression for complex survey.
CABG = coronary artery bypass graft surgery; CVD = cerebrovascular disease; IABP = intraaortic balloon pump; IQR = interquartile range; LOS = length of stay; PVD = peripheral vascular disease.
We observed a marked decrease in the number of cardiac surgeries with cardiopulmonary bypass performed annually in our representative sample of US hospital admissions during the study period, with an estimated 327,515 procedures performed in 1999 compared with 245,630 in 2008. This decrease was driven by declines in CABG without valve surgery, as the opposite was true of open-heart valve surgery (either isolated or combined with CABG), which increased annually from 67,064 surgeries (20.5%) in 1999 to 89,847 (36.6%) in 2008. The patients’ mean age was 65.5 years, and 31.7% were women; both characteristics remained essentially unchanged over the decade of observation. There were significant increases in the proportion of coded comorbidities and severity indicators (Table 2).
Incidence of AKI, AKI-D, and Mortality
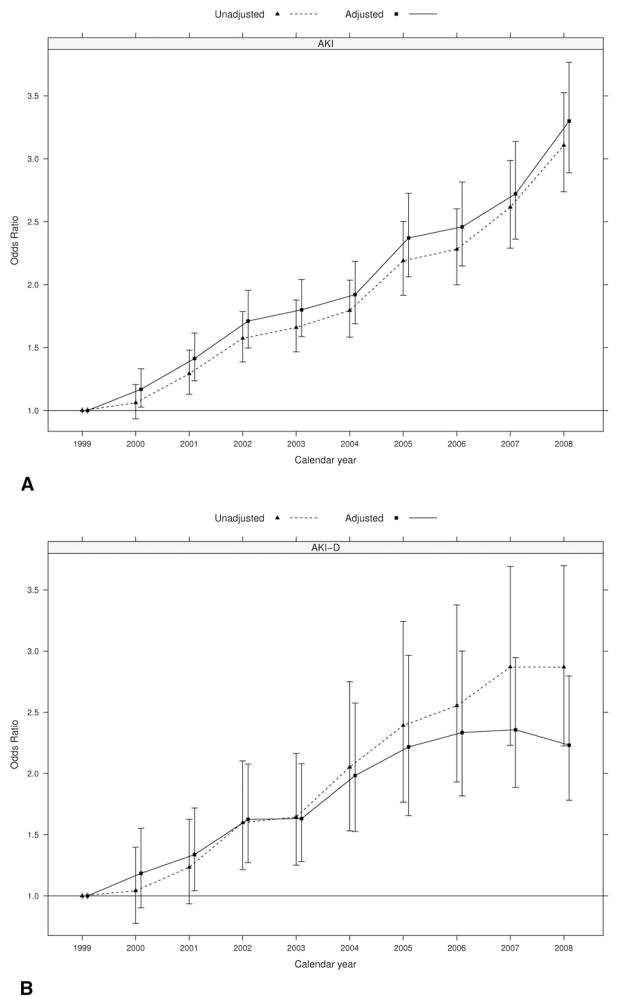
For the entire cohort, the proportion of patients having the complications of AKI and AKI-D were 7.7% and 0.8%, respectively. However, examination of annual AKI rates demonstrated a tripling of diagnosed AKI from 4.5% in 1999 to 12.8% in 2008 (OR 3.11, 95% CI: 2.74 to 3.52). We observed a similar trend after adjusting for demographic and clinical factors; the adjusted OR of AKI in 2008 versus 1999 was 3.30 (95% CI: 2.89 to 3.77; Fig 1A). The proportion of patients with AKI who underwent dialysis (AKI-D) after cardiac surgery similarly increased from 0.45% in 1999 to 1.28% in 2008 (OR 2.87, 95% CI: 2.23 to 3.7). When adjusted for demographic and clinical factors, the odds for AKI-D in 2008 were 2.23 times those in 1999 (95% CI: 1.78 to 2.80; Fig 1B).
Fig. 1.
Temporal trends in (A) diagnosed acute kidney injury (AKI), (B) acute kidney injury requiring dialysis (AKI-D), and (C) in-hospital mortality, based on 601,328 discharges identified with ICD-9 coding for cardiopulmonary bypass and coronary artery bypass graft surgery or open-heart valve repair or replacement. Adjusted models included covariates on age, sex, surgery type, heart failure, diabetes mellitus, hypertension, pulmonary disease, peripheral vascular disease, cerebrovascular disease, obesity, sepsis, use of intraaortic balloon pump, and mechanical ventilation. (Solid line = adjusted; dotted line = unadjusted.)
Risk of Mortality Associated With AKI and AKI-D
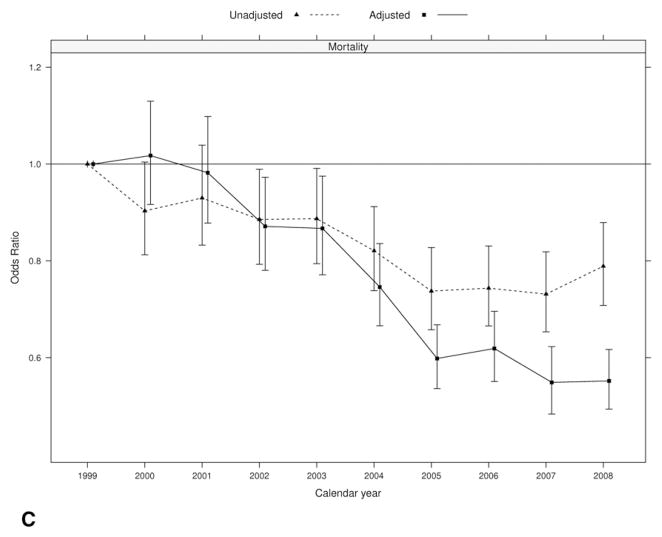
We observed a decrease in inpatient mortality after cardiac surgery during the study period, from 3.8% in 1999 to 3.3% in 2008 (unadjusted OR 0.79, 95% CI: 0.71 to 0.88; and multivariable adjusted OR 0.55, 95% CI: 0.49 to 0.62; Fig 1C).
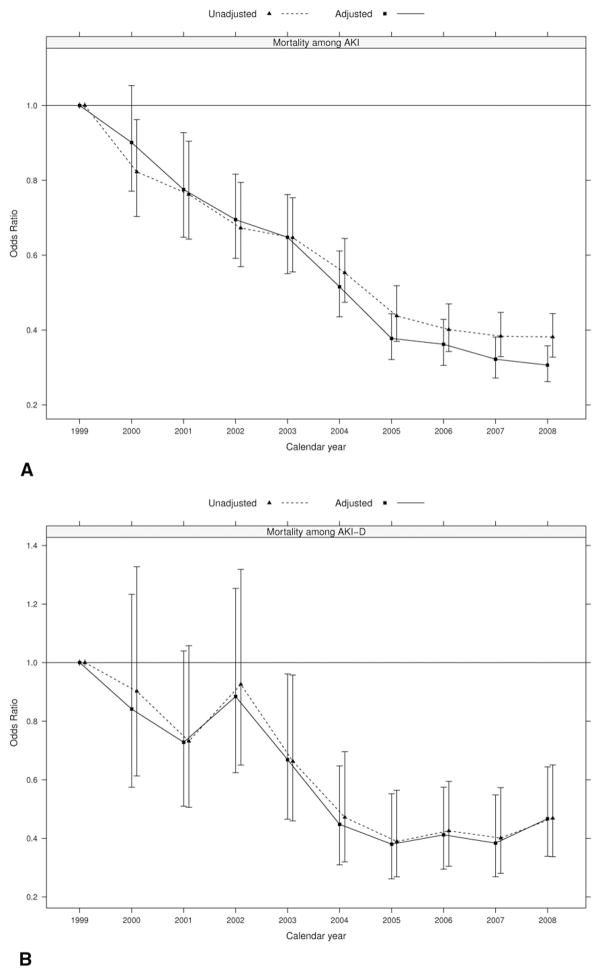
Mortality in cardiac surgery patients with AKI and AKI-D declined significantly over the study period from a baseline of 27.9% and 45.9%, respectively, in 1999 to 12.8% and 35.3% respectively in 2008 (Fig 2). Adjusted for demographics, surgery type, comorbidities, and severity of illness, the likelihood of death with AKI remained lower in 2008 compared with 1999. We observed an absolute decrease in adjusted mortality of 14.6% and 14.4% in patients with AKI and AKI-D, respectively, in the period 1999 to 2008 (Tables 3 and 4).
Fig. 2.
Mortality trends among patients undergoing open-heart surgery complicated by acute kidney injury (AKI) and acute kidney injury requiring dialysis (AKI-D), based on (A) 46,195 discharges identified with ICD-9 coding for AKI and (B) 4,923 discharges identified with ICD-9 coding for AKI-D. Adjusted models included covariates on age, sex, surgery type, heart failure, diabetes mellitus, hypertension, pulmonary disease, peripheral vascular disease, cerebrovascular disease, obesity, sepsis, use of intraaortic balloon pump, and mechanical ventilation. (Solid line = adjusted; dotted line = unadjusted.)
Table 3.
Predicted Marginal Percents for Mortality Among Patients Undergoing Open-Heart Surgery Complicated by Acute Kidney Injury
| Year | Mortality % (SE)
|
||
|---|---|---|---|
| Unadjusteda | Adjustedb | Adjusted Differenceb | |
| 1999 | 27.9 (1.1) | 27.6 (1.0) | Referent |
| 2000 | 24.1 (1.1) | 25.1 (0.8) | −1.6 (1.2) |
| 2001 | 22.8 (1.2) | 22.9 (1.0) | −3.8 (1.4) |
| 2002 | 20.6 (1.1) | 21.4 (0.8) | −5.3 (1.2) |
| 2003 | 20.0 (0.9) | 20.4 (0.8) | −6.3 (1.2) |
| 2004 | 17.6 (0.8) | 17.6 (0.7) | −9.1 (1.2) |
| 2005 | 14.5 (0.8) | 14.1 (0.6) | −12.6 (1.1) |
| 2006 | 13.4 (0.7) | 13.7 (0.6) | −13.0 (1.1) |
| 2007 | 12.9 (0.6) | 12.6 (0.6) | −14.1 (1.1) |
| 2008 | 12.8 (0.6) | 12.2 (0.5) | −14.6 (1.1) |
Results were unadjusted for model covariates but accounted for complex survey design.
Results were adjusted for model covariates (age, sex, surgery type, heart failure, diabetes, hypertension, pulmonary disease, peripheral vascular disease, cerebrovascular disease, obesity, sepsis, use of intraaortic balloon pump, mechanical ventilation) and accounted for complex survey design.
Table 4.
Predicted Marginal Percents for Mortality Among Patients Undergoing Open-Heart Surgery Complicated by Acute Kidney Injury Requiring Dialysis
| Year | Mortality % (SE)
|
||
|---|---|---|---|
| Unadjusteda | Adjustedb | Adjusted Differenceb | |
| 1999 | 45.9 (3.3) | 45.9 (2.8) | Referent |
| 2000 | 38.9 (3.5) | 43.9 (2.9) | −2.0 (3.9) |
| 2001 | 38.9 (3.0) | 39.8 (2.5) | −6.1 (3.7) |
| 2002 | 43.5 (2.8) | 44.4 (2.4) | −1.5 (3.6) |
| 2003 | 37.4 (3.0) | 37.9 (2.4) | −8.0 (3.6) |
| 2004 | 31.2 (2.6) | 31.7 (2.5) | −14.3 (3.7) |
| 2005 | 28.2 (3.3) | 28.4 (2.3) | −17.6 (3.5) |
| 2006 | 29.5 (2.4) | 29.9 (1.8) | −16.0 (3.2) |
| 2007 | 30.1 (2.5) | 28.9 (2.1) | −17.1 (3.4) |
| 2008 | 35.3 (2.1) | 31.5 (1.7) | −14.4 (3.2) |
Results were unadjusted for model covariates but accounted for complex survey design.
Results were adjusted for model covariates (age, sex, surgery type, heart failure, diabetes, hypertension, pulmonary disease, peripheral vascular disease, cerebrovascular disease, obesity, sepsis, use of intraaortic balloon pump, mechanical ventilation) and accounted for complex survey design.
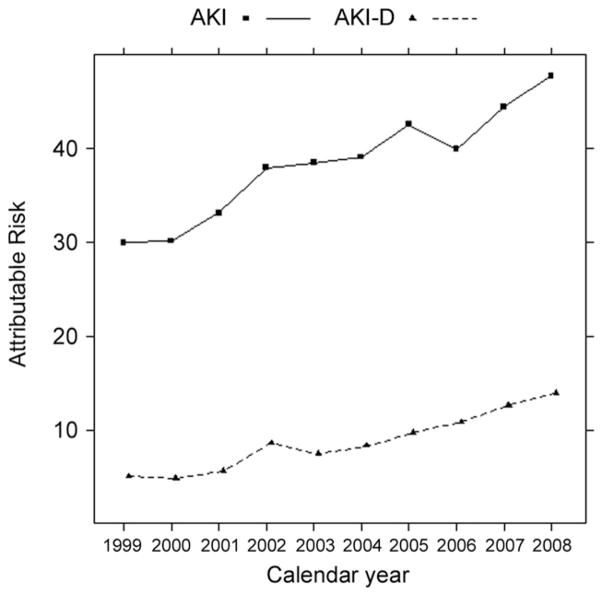
However, the temporal reductions in mortality among patients with AKI and AKI-D were countered by marked increases in their incidences. As a consequence, the overall association of AKI and AKI-D with inpatient mortality after cardiac surgery increased over the decade. The attributable risk percent of mortality associated with AKI was 30.0% in 1999 and 47.8% in 2008. The attributable risk percent associated with AKI-D was 5.0% in 1999 and 13.9% in 2008 (Fig 3).
Fig 3.
Trends in the attributable risk percent of death from acute kidney injury (AKI), based on 95,950 deaths after cardiopulmonary bypass and coronary artery bypass graft surgery or open-heart valve repair or replacement. (Solid line = AKI; dotted line = AKI with dialysis [AKI-D].)
Finally, while hospital length of stay after cardiac surgery has essentially remained static, we did observe an increase in the proportion of patients discharged to skilled nursing facilities from 12.6% in 1999 to 19.1% in 2008 (Table 1). This trend may partly contribute to the observed decrease in mortality during the study period as our data do not capture deaths after hospital discharge.
All analyses were subjected to tests for effect modification by surgery type; however, no significant interactions with time were detected. Stratified results by surgery type are available in the Appendix (see Appendix in Auxiliary Annals section of the STS website: http://www.sts.org/auxiliaryannals/Lenihan-2013-95-1-20-Appendix.pdf).
Comment
In this comprehensive analysis of cardiac surgical procedures with cardiopulmonary bypass performed in the United States, we describe a significant increase in the rates of postoperative AKI and AKI-D over the period 1999 to 2008. While mortality among patients with AKI or AKI-D has decreased, the risk of death attributable to AKI has increased substantially: in 2008, nearly half of the estimated 7,406 patients who died in hospital after cardiac surgery had a diagnosis of AKI.
There are a number of potential explanations for our findings. During the decade of observation, there has been a shift toward more complex and higher risk open heart procedures; the rates of open valve surgery and combined CABG and open valve surgery have increased, in contrast to the decline in isolated CABG surgery. In addition, we observed an increasing burden of comorbid acute and chronic conditions that have previously been linked to the risk of AKI after cardiac surgery [5, 14]. However, even with adjustment for the higher burden of comorbid conditions and complications, rates of AKI and AKI-D were higher over time.
It is possible that increasing awareness of AKI related to the development of diagnostic criteria (including RIFLE [risk, injury, failure, loss, end-stage renal disease), and numerous publications highlighting risk associated with relatively modest increases in serum creatinine, has resulted in increased diagnosis of AKI [2–6, 23]. It is also possible that there was no real difference in AKI incidence but rather, changing coding practices, sometimes referred to as “code creep.” However, we were reassured that the higher incidence of AKI was real, given that procedural coding for dialysis has increased in proportion to that for AKI as a whole.
Two observational US studies and the Canadian Blood Conservation Using Antifibrinolytics (BART) trial, published in 2006 and 2008, respectively, demonstrated an increase in renal dysfunction associated with the antifibrinolytic drug aprotinin in patients undergoing cardiac surgery with cardiopulmonary bypass [29–31]. Interestingly, although we do not have access to drug administration data, it is notable that the withdrawal of aprotinin from the US market in 2007 corresponds temporally with a relative stabilization of AKI-D rates in those years. However, aprotinin does greatly reduce perioperative transfusion requirements (and its associated risks), and several retrospective studies suggest that patients at the greatest risk of perioperative bleeding may still benefit from its use [32, 33]. After extensive review, the drug has been reintroduced in Canada and Europe and is being considered for reintroduction in the United States.
We observed lower mortality associated with AKI over time. There have been few specific therapeutic developments for the treatment of ischemic AKI; however, cardiac surgical patients have benefited from numerous advances and quality improvements in intensive care over the period studied [34, 35]. In addition, some studies have suggested that earlier nephrology consultation may be associated with lower mortality after AKI; other studies have suggested that nephrology consultation after AKI is more likely now than in past years [36, 37].
While the assumption of the attributable risk percent is overreaching, it does enable us to illustrate that the association between AKI and AKI-D and inpatient mortality has strengthened for the cardiac surgical population as a whole, where the increased survival after AKI has been “outpaced” by a threefold increase in the incidence of the complication.
Our study compliments a number of older studies involving patients undergoing CABG alone that have observed comparable trends in the rates and outcomes of AKI and AKI-D and demonstrated that postoperative AKI is associated with increased levels of discharge to long-term facilities, short-term hospital, or home health care [38–40]. Another study observed that heart transplantation, excluded from our study, has had a greater rise in AKI incidence when compared with other open-heart surgeries [41].
The strengths of our study include the use of a large nationally representative dataset and a focus on the current era of cardiac surgery. Our use of attributable risk allows us to demonstrate the net burden of AKI on the cardiac surgical population as a whole and has implications for health care resource utilization.
There are several important limitations as well. First, we relied on diagnosis codes for AKI, rather than medical records or laboratory data that are routinely used in clinical practice [5]. Previous studies have suggested that diagnosis codes for AKI are reasonably specific but not sensitive; we believe that milder cases of AKI are often missed in studies using administrative data [25]. As such, we are likely underestimating the fraction of surgical cases (including deaths) associated with AKI. Second, we limited our study to on-pump cardiac surgery as it remains the most prevalent mode of surgical revascularization, and recent evidence has cast doubt on off-pump surgical outcomes [42, 43], although a recent randomized trial showed no significant difference between off-pump and on-pump CABG with respect to the 30-day rate of death, myocardial infarction, stroke, or AKI-D [44]. Additionally, off-pump surgery prevalence, patient selection, and outcome are far more center- and surgeon-dependent than on-pump procedures are, making it difficult to draw conclusions from our nationwide sample [45]. Third, we made the assumption that cardiac surgery temporally preceded AKI on the basis that it would be a relatively rare for a patient in the midst of an episode of AKI to undergo cardiac surgery.
In summary, we demonstrated that the rates of AKI and AKI-D after cardiac surgery with cardiopulmonary bypass increased significantly between 1999 and 2008, on a background of major changes in the clinical characteristics of patients undergoing cardiac surgery and changes in the type and frequency of procedures being performed. Based on these findings, roughly half of all deaths after on-pump cardiac surgery are seen in conjunction with AKI. The design and implementation of new strategies to prevent or ameliorate AKI after cardiac surgery are desperately needed.
References
- 1.Conlon PJ, Stafford-Smith M, White WD, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158–62. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 2.Cooper WA, O’Brien SM, Thourani VH, et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: results from The Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006;113:1063–70. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 3.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–8. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 4.Boyle JM, Moualla S, Arrigain S, et al. Risks and outcomes of acute kidney injury requiring dialysis after cardiac transplantation. Am J Kidney Dis. 2006;48:787–96. doi: 10.1053/j.ajkd.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194–203. doi: 10.7326/0003-4819-128-3-199802010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–4. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 7.Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 8.Yeboah ED, Petrie A, Pead JL. Acute renal failure and open heart surgery. Br Med J. 1972;1:415–8. doi: 10.1136/bmj.1.5797.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–5. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–84. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 11.Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 12.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72:624–31. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 13.Stafford-Smith M, Shaw A, Swaminathan M. Cardiac surgery and acute kidney injury: emerging concepts. Curr Opin Crit Care. 2009;15:498–502. doi: 10.1097/MCC.0b013e328332f753. [DOI] [PubMed] [Google Scholar]
- 14.Englberger L, Suri RM, Li Z, et al. Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. Am J Kidney Dis. 2010;56:623–31. doi: 10.1053/j.ajkd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 16.Hannan EL, Wu C, Walford G, et al. Drug-eluting stents versus coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331–41. doi: 10.1056/NEJMoa071804. [DOI] [PubMed] [Google Scholar]
- 17.Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–7. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 18.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305:1769–76. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SJ, Kim YH, Park DW, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718–27. doi: 10.1056/NEJMoa1100452. [DOI] [PubMed] [Google Scholar]
- 20.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–16. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 22.Pierri MD, Capestro F, Zingaro C, Torracca L. The changing face of cardiac surgery patients: an insight into a Mediterranean region. Eur J Cardiothorac Surg. 2010;38:407–13. doi: 10.1016/j.ejcts.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Dinh DT, Lee GA, Billah B, Smith JA, Shardey GC, Reid CM. Trends in coronary artery bypass graft surgery in Victoria, 2001–2006: findings from the Australasian Society of Cardiac and Thoracic Surgeons database project. Med J Aust. 2008;188:214–7. doi: 10.5694/j.1326-5377.2008.tb01587.x. [DOI] [PubMed] [Google Scholar]
- 24.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification, codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–94. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 26.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol. 2010;171:618–23. doi: 10.1093/aje/kwp440. [DOI] [PubMed] [Google Scholar]
- 27.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–9. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 28.Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3. Hoboken, NJ: Wiley-Interscience; 2003. pp. 125–9. [Google Scholar]
- 29.Mangano DT, Tudor IC, Dietzel C. Multicenter study of perioperative ischemia research group: Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 30.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358:771–83. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 31.Fergusson DA, Hébert PC, Mazer CD, et al. for the BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–31. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 32.Mora Mangano CT, Neville MJ, Hsu PH, Mignea I, King J, Miller DC. Aprotinin, blood loss, and renal dysfunction in deep hypothermic circulatory arrest. Circulation. 2001;104(Suppl 1):276–81. doi: 10.1161/hc37t1.094702. [DOI] [PubMed] [Google Scholar]
- 33.Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Tait G, Beattie WS. The risk-benefit profile of aprotinin versus tranexamic acid in cardiac surgery. Anesth Analg. 2010;110:21–9. doi: 10.1213/ANE.0b013e3181c0ea6d. [DOI] [PubMed] [Google Scholar]
- 34.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patient. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 35.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 36.Ponce D, de Zorzenon CP, dos Santos NY, Balbi AL. Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol Dial Transplant. 2011;26:3202–6. doi: 10.1093/ndt/gfr359. [DOI] [PubMed] [Google Scholar]
- 37.Ali T, Tachibana A, Khan I, et al. The changing pattern of referral in acute kidney injury. QJM. 2011;104:497–503. doi: 10.1093/qjmed/hcq250. [DOI] [PubMed] [Google Scholar]
- 38.Nicoara A, Patel UD, Phillips-Bute BG, et al. Mortality trends associated with acute renal failure requiring dialysis after CABG surgery in the United States. Blood Purif. 2009;28:359–63. doi: 10.1159/000235856. [DOI] [PubMed] [Google Scholar]
- 39.Swaminathan M, Phillips-Bute BG, Patel UD, et al. Increasing healthcare resource utilization after coronary artery bypass graft surgery in the United States. Circ Cardiovasc Qual Outcomes. 2009;2:305–12. doi: 10.1161/CIRCOUTCOMES.108.831016. [DOI] [PubMed] [Google Scholar]
- 40.Swaminathan M, Shaw AD, Phillips-Bute BG, et al. Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med. 2007;35:2286–91. doi: 10.1097/01.ccm.0000282079.05994.57. [DOI] [PubMed] [Google Scholar]
- 41.Martinelli SM, Patel UD, Phillips-Bute BG, et al. Trends in cardiac surgery-associated acute renal failure in the United States: a disproportionate increase after heart transplantation. Renal Fail. 2009;31:633–40. doi: 10.3109/08860220903100689. [DOI] [PubMed] [Google Scholar]
- 42.Shroyer AL, Grover FL, Hattler B, et al. On-pump versus off-pump coronary artery bypass surgery. N Engl J Med. 2009;361:1827–37. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- 43.Filardo G, Grayburn PA, Hamilton C, Hebeler RF, Cooksey WB, Hamman B. Comparing long-term survival between patients undergoing off-pump and on-pump coronary artery bypass graft operations. Ann Thorac Surg. 2011;92:571–8. doi: 10.1016/j.athoracsur.2011.03.100. [DOI] [PubMed] [Google Scholar]
- 44.Lamy A, Devereaux PJ, Prabhakaran D, et al. for the CORONARY Investigators. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med. 2012;366:1489–97. doi: 10.1056/NEJMoa1200388. [DOI] [PubMed] [Google Scholar]
- 45.Kerendi F, Morris CD, Puskas JD. Off-pump coronary bypass surgery for high-risk patients: only in expert centers? Curr Opin Cardiol. 2008;23:573–8. doi: 10.1097/HCO.0b013e328312c311. [DOI] [PubMed] [Google Scholar]