Abstract
A growing literature suggests the association of low tissue levels and/or dietary intake of n-polyunsaturated fatty acids (PUFA) with depressive illnesses. Animal studies show that low tissue and/or dietary n-3 PUFAs can lead to behaviors and neurobiological effects associated with depression, and can potentiate the consequences of stress. Higher n- 3 PUFA tissue levels or intake have the opposite effect. These data support the involvement of n-3 PUFAs levels in the disease processes underlying depression. In addition, these pre-clinical findings indicate neurobiological mechanisms whereby n-3 PUFAs may contribute to the disease including control of serotonergic and dopaminergic function, modulation of brain-derived neurotrophic factor (BDNF) in the hippocampus, regulation of the hypothalamic-pituitary-adrenal axis, and effects on neuroinflammation. This pre-clinical evidence for a role for n-3 PUFA in the pathophysiology and treatment of depressive illness are reviewed. The implications of these finding for future pre-clinical research and clinical application are discussed.
Keywords: rain-derived neurotrophic factor, corticosterone, docosahexaenoic acid, dopamine, elevated plus maze, forced swim test, neuroimmune, serotonin
INTRODUCTION
Depression, a disease with symptoms such as depressed mood, lack of interest, anhedonia, feelings of guilt or worthlessness, suicidal thoughts, and sometimes attempted or actual suicide [1], has a lifetime prevalence of about 20%, and occurs roughly twice as often in women than in men [2]. Although not fully understood, the underlying pathology of the disease involves as variety of neurobiological changes such as altered monoamine neurotransmission, decreased expression of brain-derived neurotrophic factor (BDNF) in the hippocampus, and dysregulation of the hypothalamic-pituitary- adrenal axis [3–5]. The involvement of neuroinflammation in depression is also becoming increasingly recognized [5, 6]. Genetic and environmental factors are involved [7]; however, other factors almost certainly contribute. Clinical and epidemiologic studies suggest that inadequate tissue or dietary n-3 polyunsaturated fatty acids (PUFA) may increase susceptibility to several psychiatric disorders, particularly depression [8–10]. The diets consumed by most North Americans are quite low in these fatty acids; as such, there is a strong possibility that an individual could have sub-optimal levels of these fatty acids [11]. Pre-clinical evidence for the involvement of n-3 PUFAs in the disease processes underlying depression are reviewed here.
N-3 PUFAS AND THE BRAIN
Long-chain PUFAs, double bond-containing fatty acids of at least 20 carbons in length, are part of the phospholipids that form the cell membrane. Brain phospholipids contain high concentrations of these fatty acids. Docosahexaenoic acid (DHA, 22:6n-3) and arachidonic acid (20:4n-6) are the most abundant PUFA in the brain, representing approximately 15% and 10%, respectively, of the total fatty acids in that tissue [12]. These long-chain PUFAs are synthesized endogenously through elongation and Desaturation of α-linolenic acid (18:3n-3) and linoleic acid (18:2n-6), respectively [13] (Fig. 1). Other long chain PUFAs are also present in the brain, but at much lower levels. In addition, the fatty acid composition of brain phospholipids differs between brain regions. The highest concentrations of DHA are found in the frontal cortex and other cortical regions, whereas the lowest concentrations are found in regions such as the midbrain [14–16].
Figure 1. Biosynthesis of long-chain PUFAs.
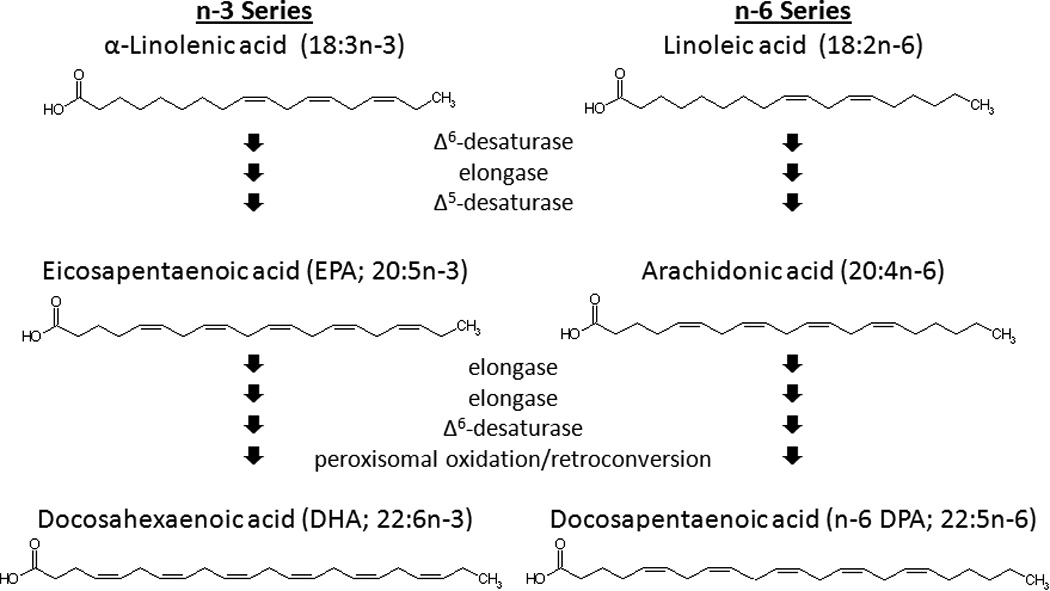
A variety of long-chain n-3 and n-6 PUFAs are derived from α-linolenic acid and linoleic acid, respectively.
The accretion of DHA into brain phospholipids occurs primarily during late gestation and early neonatal life, though this varies somewhat between species [17–19]. During this time, DHA is delivered to the developing offspring by the mother in utero prior to birth, and in breast milk, which is enriched in DHA, after birth [20, 21]. Low availability of DHA during early development, due to inadequate n-3 PUFAs in the mother’s diet or feeding an infant formula that does not contain DHA, results in less DHA being incorporated into brain membranes and the compensatory incorporation of the 22-carbon n-6 PUFA docosapentaenoic acid (n-6 DPA; 22:5n-6) into the phospholipids [22]. Studies in adult male rats indicated that brain DHA levels were not reduced by dietary n- 3 PUFA deficiency [23]. However, recent studies in adult female rats or adult male mice found that prolonged feeding of a diet containing inadequate n-3 PUFA can decrease the DHA in the adult brain, at least in those particular experimental subjects [24, 25]. Interestingly, reduction in brain DHA occurs even more rapidly in reproducing females. Of note, females fed an n-3 PUFA-deficient diet had a decrease in brain DHA content of about 25% after gestating and nursing a single litter, most likely as a result of supplying DHA to their offspring [24]. These findings show that brain n-3 PUFA status can be altered by either a failure of initial DHA accretion during development, or the loss of DHA later in life. Consistent with a potential role in depression, regardless of whether DHA failed to accumulate during development or was lost later in life, the greatest deficits in DHA occur in the frontal cortex. However, in other brain regions, the magnitude of effect varies depending on the point in the life span when a deficiency occurred, suggesting that the resulting neurobiological changes in these models likely also varies [14–16, 26].
The relative abundance of specific fatty acids in membrane phospholipids affects cell physiology in a variety of ways. First, the fatty acid composition affects the biophysical properties of the membrane. Thus changes in membrane composition impact the function of lipid rafts and proteins embedded in the membrane such as receptors, ion channels, and transporters [27, 28]. Second, when cleaved from the membrane by phospholipases, DHA and other long chain PUFAs modulate gene expression by activating nuclear receptors, such as the retinoid X receptor (RXR) and the peroxisome proliferator-activated receptors (PPAR) [32, 33]. These fatty acids can also be metabolized into a variety of signaling mediators including as prostanoids, resolvins, and neuroprotectin D1 [27, 29–31]. Accordingly, variation in membrane composition can alter the relative abundance of the various signaling molecules produced. Thus, there are multiple ways in which changes in the availability of these fatty acids can functional consequences in the brain.
On an organismal level, insufficient pre- and postnatal accretion of DHA during development does not produce any gross deficits in general health [34]. However, some deficits in visual, attentional, and intellectual development are reported [8, 35–37], as well as other neurobiological alterations that could predispose an individual towards depression (see below) or other psychiatric disorders [8]. Likewise, treatments that reduce the DHA content of the adult brain also produce neurobiological changes at least some of which could increase susceptibility to depression.
EFFECTS OF N-3 PUFAS ON DEPRESSION-RELATED NEUROBIOLOGY
Experimental manipulation of dietary, and consequently tissue, n-3 PUFAs affects many of the neurobiological systems implicated in the pathogenesis of depression. These manipulations vary widely in terms of the fatty acid compositions of the diets or other treatments, the treatment duration, the age of the animals at the time the treatment was initiated, and other aspects of the experiment (e.g., particular physiological states such as pregnancy and lactation). Despite the great diversity in experimental design, lower dietary and tissue levels of n-3 PUFAs generally result in outcomes that are similar to those found in depression, while higher dietary or tissue levels of these fatty acids tend to have the opposite effect. These findings, as well as those that differ from findings in depression are discussed below.
Monoaminergic Neurotransmission
Decreased activity of the monoamine neurotransmitters serotonin and norepinephrine has been hypothesized to contribute to the pathophysiology of depression, with dopamine also playing a minor role [3, 4]. Variation in n-3 PUFA status affects these systems in a number of ways, at least some of which are consistent with findings in depression.
Serotonin
Important changes in the serotonergic system observed in depression include lower brainstem concentrations of serotonin of postmortem depressives and suicide completers [38–40]. The densities of 5-HT1A and 5-HT2A serotonin receptors were higher in the prefrontal cortex also suggesting decreased serotonergic neurotransmission [41–44]. The increased availability of serotonin produced by antidepressant drugs causes these receptors to down regulate, further suggesting a role serotonin in both the disease process and the mechanism of action of antidepressants [45].
In animals studies, low tissue and/or dietary n-3 PUFA levels result in a number of alterations in the serotonergic system that are similar to the neurobiological alterations reported in depression. Decreased serotonin concentrations in the frontal cortex were detected in adult female rats with a diet-induced reduction in brain DHA content of about 25%, similar to that observed in depression [46, 47]. Male rats with a 61% decrease in brain DHA, induced by feeding an diet deficient in n-3 PUFA from birth, exhibited lower expression of the serotonin synthesizing enzyme tryptophan hydroxylase in the midbrain, and higher serotonin turnover in the prefrontal cortex, compared to controls [48, 49]. Moreover, feeding a diet containing α-linoleic acid reversed the effect on serotonin turnover [49]. In another study, decreased cortical concentrations of serotonin were observed in piglets fed formula that did not contain α-linolenic or linoleic acids [50], further implicating long-chain PUFA in the modulation of the serotonin system, though the effects of individual fatty acids were not tested in that study. Consistent with the consequences of low n-3 PUFA intake on the serotonin system, adult rats a fed fish oil-supplemented diet for 90 days, which supplied DHA and EPA, exhibited increased concentrations of serotonin in the frontal cortex and hippocampus [51]. Similarly, in mice, the decreases in brain serotonin levels induced by unpredictable chronic mild stress, a rodent model of depression, were reversed by feeding a diet supplemented with n-3 PUFAs [52]. However, in postpartum female rats that had a 25% reduction in brain DHA produced by feeding a diet with inadequate n-3 PUFAs during pregnancy and lactation, no alterations in serotonin concentration were detected in any brain region, including the frontal cortex, perhaps due to the interaction of the reproductive and brain DHA stasuses [46]
Expression of serotonin receptors is also affected by experimentally altering tissue or dietary n-3 PUFA status. Similar to the receptor alterations found in depression, rats with brain DHA levels 70% below normal, resulting from inadequate consumption of n-3 PUFAs for two generations, had higher densities of 5-HT2A receptors in the frontal cortex [53, 54]. However, perhaps due to differences in the extent of the change in brain fatty acid composition and/or the developmental stage of the rats at the time of treatment, neither adult virgin females nor postpartum female rats with brain DHA levels about 25% below controls exhibited any alterations in 5-HT1A or 5-HT2A receptors in the frontal cortex [46]. However, increased expression of hipopocampal 5-HT1A receptors was detected in postpartum female rats, with a 25% reduction in brain DHA when compared to postpartum females fed a control diet to maintain brain DHA levels [46]. This finding, however, differs from observations in the hippocampus of humans with depression where the densities of 5-HT1A receptors were either decreased or were unaltered [55–58].
Norephinephrine
Alterations in the noradrenergic system are noted in postmortem brains of suicide completers. Several studies report increased density of β-adrenergic receptors in the frontal cortex, although alterations in α1 and α2 receptors have also been observed in some studies [41, 59]. Similar to serotonin receptors, cortical β-receptors down-regulate in depressed patients after treatment with tricyclic antidepressants [60].
The role of n-3 PUFAs in modulation of noradrenergic neurotransmission has received relatively little attention. Studies in cultured SH-SY5Y neuroblastoma cells suggest that either brief exposure to, or incorporation of, DHA increased basal, but not KCl-evoked release of [3H]- norepinephrine by a mechanism involving enhanced exocytosis [61]. DHA treatment also increased the density of β-receptors on rat astrocytes in primary culture [62]. In animal studies, decreased levels of norepinephrine were observed in the cortex, hippocampus, and striatum of rats raised from conception on a diet containing inadequate n-3 PUFA [63]. However, no alterations in regional norepinephrine concentration were found in adult virgin female or postpartum female rats with a 25% reduction in brain DHA, or in adult male rats with a 70% reduction in DHA [46, 54]. Effects of in vivo manipulations of n-3 PUFAs on noradrenergic receptors remain to be determined.
Dopamine
A role for decreased dopaminergic function in depression is indicated by a variety of findings including decreased concentrations of the dopamine metabolite homovanillic acid in the cerebrospinal fluid patients depression and suicide completers [64–67]. In addition, comorbid depression is common in patients with Parkinson’s disease, which involves the loss of nigrostriatal dopamine neurons [68, 69]. Furthermore, in several animal models, hypoactivity of the mesolimbic dopamine projection appears to underlie decreased participation in reward-oriented behaviors s, and is thus thought to contribute to the anhedonia and decreased motivation observed in depressed patients [70–74].
In animal models, the CNS dopamine systems are also affected by variation in tissue and/or dietary n-3 PUFA content. Postpartum female rats with a 25% reduction in brain DHA level resulting from the combined effects of gestating and nursing offspring while consuming a diet deficient in n-3 PUFA, had a decrease in the number of D2 dopamine receptors in the ventral striatum [75]. A near-significant decrease in these receptors was also observed in virgin females that also had a 25% decrease DHA [75]. This decrease in expression of ventral striatal D2 receptors concurs with the hypoactivity of the mesolimbic dopamine system that is hypothesized to occur in depression, and is also consistent with the decreases in the density of the D2 receptor found rat models of depression such as learned helplessness, chronic mild stress-induced anhedonia, and the socially-isolated Flinders sensitive line rat [76–78]. Although no alterations in the number of D2 receptors the ventral striatu, of postmortem depressed patients [79], a PET imaging study found lower D2/3 dopamine receptor density in the striatum of depressed women [80]. Thus, the effects of a reduction of DHA in the brain of the adult rat are consistent with rodent models of depression, and perhaps also with depressed humans.
In contrast to the effects on the dopaminergic systems that result when an adult animal experiences a reduction of brain DHA content, the effects of inadequate pre- and postnatal accretion of brain DHA on the dopamine system vary considerably. Rats with 70% reduction in brain DHA content, induced by feeding diet containing inadequate n-3 PUFAs for multiple generations, had higher levels of D2 receptors in the nucleus accumbens. These rats also exhibited increased basal dopamine release, decreased vesicular monoamine amine transporter VMAT2 density, and lower levels of tyramine-stimulated dopamine release [81]. Decreased D2 receptor mRNA, levels of VMAT2 mRNA, and cortical tyramine-stimulated dopamine release were also observed in these rats, but no changes in dopaminergic neurochemistry in the striatum [54, 82, 83]. In another study, rats a fed diet containing inadequate n-3 PUFAs from the second day after conception, which decreased the DHA:arachidonic acid ratio by about 80% at birth, had increased densities of both D1 and D2 receptors in brain regions including the nucleus accumbens, striatum and frontal cortex [84, 85]. On the other hand, first or second litter rats from the same dam that were raised from conception on a diet containing inadequate n-3 PUFAs, resulting in decreased brain DHA levels of 48% and 65%, respectively, had no alterations in D1 or D2 receptor density or dopamine content in the nucleus accumbens, frontal cortex, or striatum compared to controls [86]. Taken together, these findings suggest that decreased brain n-3 PUFA levels may promote depressive illness in various ways based on the timing and extent of the manipulation. These data also tend to indicate that larger decreases in brain DHA content during prenatal and early postnatal development are associated with altered dopamine receptor expression in the nucleus accumbens, frontal cortex, and perhaps other brain regions. This could indicate that smaller deficits in n-3 PUFAs during early development may be predisposing towards depression whereas larger deficits during this period may lead to tendencies towards conditions such as schizophrenia or attention deficit hyperactivity disorder, in which aberrations in dopaminergic function are believed to play notable roles [8, 87].
Hippocampal BDNF Expression
The hippocampus is a part of the limbic system that is involved in memory, affect, and regulation of the hypothalamic-pituitary-adrenal axis [88]. It is also one of two primary regions in the mature brain that exhibits neurogenesis [89]. Depression is strongly associated with decreased levels of BDNF in this brain region [5]. Of note, lower levels of hippocampal BDNF were observed in postmortem samples from suicide completers than in normal controls [90, 91]. BDNF supports hippocampal neurogenesis [92]; thus decreased expression of BDNF appears to contribute to atrophy of the hippocampus found in postmortem depressives [93]. Furthermore, higher levels of BDNF in the hippocampus were found in postmortem samples from depressed patients that had been treated with antidepressant drugs than those who were not [94]. Preclinical studies using depression models and/or antidepressant drugs have yielded similar findings [95–101]. Together these data support a role for hippocampal BDNF not only in the pathophysiology of depression, but also in the mechanism of action of antidepressant drugs.
Low tissue and/or dietary n-3 PUFA levels lead to decreased expression of hippocampal BDNF. Female rats fed a diet containing inadequate n-3 PUFAs to reduce brain DHA by about 25%, exhibited decreased hippocampal BDNF mRNA levels that were accompanied by a near-significant decrease in BDNF peptide concentration [46]. Interestingly, postpartum female rats, with a 25% reduction in brain DHA resulting from gestating and nursing offspring while consuming inadequate n-3 PUFAs, had lower levels of both hippocampal mRNA encoding BDNF and BDNF peptide [46]. Furthermore, in these animals, these DHA-reducing treatments decreased levels of BDNF mRNA by roughly 32%, which is similar to that reported in suicide completers [90, 91]. This suggests the decrease in BDNF produced by the reduction of brain DHA in these rat models is relevant to the disease.
Consistent with the findings for low n-3 PUFAs, higher tissue and dietary n-3 PUFA levels result in increased expression of hippocampal BDNF. Several studies indicate that rats or mice fed diets supplemented with n-3 PUFAs, or injected with α-linoleic acid, had higher BDNF expression in the hippocampus [51, 102–105]. In another rat study, a DHA-enriched diet resulted in higher levels of calmodulin kinase II and activated Akt, which are involved in BDNF signaling [106]. Higher levels of mediators involved antidepressant-induced increases in BDNF, such as cAMP response element binding protein (CREB) are also reported after dietary supplementation [107]. In addition, the volume of the hippocampus was increased in mice fed an α-linoleic acid-enriched diet [104]. Likewise, increased hippocampal neurogenesis has been observed in rats fed diets supplemented with DHA and EPA, as well as in mutant mice expressing fat-1 that are able to metabolize n-6 PUFA into n-3 PUFA, and thus do not require n-3 PUFAs in the diet [108, 109]. These observations of increased neurogenesis suggest that manipulations that increase availability of n-3 PUFAs increase in BDNF expression; however, neither study [108, 109] determined the effects of the treatments on BDNF expression itself. Taken together, these findings suggest that higher levels of tissue and dietary n-3 PUFAs support BDNF expression in the hippocamus, which in turn fosters hippocampal neurogenesis and other functions.
Hypothalamic-Pituitary-Adrenal Axis Function
Another important observation in depression is dysregulation of the hypothalamic-pituitary-adrenal axis [5]. Noteworthy alterations in this system include increased basal levels of corticotrophin-releasing factor in cerebral spinal fluid and cortisol in the serum [110–112]. In addition, negative feedback mechanisms are disrupted [113], resulting at least in part from altered glucocorticoid receptor expression [114].
Several preclinical findings implicate n-3 PUFAs in the regulation of the hypothalamic-pituitary-adrenal axis. In particular, postpartum female rats with a 25% decrease in brain DHA, resulting from gestating and nursing a litter while consuming inadequate n-3 PUFAs, had higher stress-induced serum corticosterone concentrations and exhibited a greater stress-induced increase of corticosterone secretion over baseline induced by forced swimming than postpartum females fed a diet that maintained normal brain DHA levels [46]. Virgin female rats fed a diet containing inadequate n-3 PUFAs to reduce brain DHA by 25% also exhibited a larger increase in corticosterone secretion over baseline when subjected to forced swimming, although their basal and stressed serum corticosterone concentrations were not different from virgin females with normal brain DHA levels [46]. These findings indicate that lowered DHA levels in the adult brain can contribute to dysregulation of the hypothalamic-pituitary-adrenal axis and that this effect may be augmented by the endocrine and other physiological changes associated with pregnancy and lactation.
Long-term behavioral responses to stress also appear to be augmented in rats with low dietary and/or tissue DHA. Several studies have examined the effects of maternal separation during the pre-weaning period in rats with a 70% decrease in brain DHA resulting from being fed a diet containing inadequate n-3 PUFAs for multiple generations. At adulthood, rats subjected to both treatments exhibited augmented stress-induced responses including greater reaction to novelty in the open field and increased sucrose consumption than rats subjected maternal separation without n-3 PUFA deficiency [115]. N-3 deficient rats subjected to maternal separation also exhibited increased anxiety as adults in the elevated plus maze and classical fear conditioning tests, which was not observed after either treatment alone [116]. In another study, mice with a 50% reduction in brain DHA resulting from being fed a diet containing inadequate n-3 PUFAs for two generations, exhibited greater novelty suppressed feeding test after being subjected to the stress of individual housing, indicating higher levels of anxiety [117]. That these effects were not seen, or were less pronounced, in stressed animals not subjected to n-3 PUFA deficiency, suggests that n-3 PUFA deficiency confers increased susceptibility to stress.
Consistent with these findings, diets supplemented with n-3 PUFAs appear to mitigate the physiological consequences of various stressors that activate the hypothalamic-pituitary-adrenal axis. For example, in the open field test and elevated plus maze, rats fed a diet enriched in n-3 PUFA exhibited lower levels of stress- and anxiety-like behavioral effects after treatment with interleukin-1, which corticosterone levels [118]. Similarly, the effects of restraint stress were attenuated in rats fed a fish oil-supplemented diet for 3 months beginning at weaning with respect to stressed plasma corticosterone levels and behavior in the elevated plus maze and forced swim test, as well as in the Morris water maze, a test of cognitive function [119].
Neuroinflammation
Neuroinflammation also appears to be a mediator in the underlying pathology of depressive illnesses [6]. Notably, depressed patients exhibited greater serum concentrations, or gene expression levels in brain, of several inflammatory mediators in the NFκB-mediated cascade including tumor necrosis factor-α, interferon-γ, interleukin-6, and interleukin- 1β [120–126]. In addition, a meta-analysis of 22 studies associated reduced serum levels of interleukin 1β in the improvement in depressive symptoms after treatment with antidepressant drugs, providing relatively strong evidence for this cytokine as a contributor to the pathobiology of the disease [127].
N-3 PUFAs mitigate inflammation in a variety of ways [128]. DHA and EPA are precursors of the D- and E-series resolvins, respectively, which modulate the level and length of the inflammatory response [129]. In addition, these fatty acids also decrease inflammation initiated through the NFκB cascade via actions at PPARs and the toll-like 4 receptor [130, 131]. Another DHA metabolite, neuroprotectin D1, decreases the production of tumor necrosis factor-α and interferon-γ by activated T cells [30, 132]. Moreover, in cultured BV-2 microglia, both DHA and EPA increased expression of heme oxygenase-1, and decreased expression of tumor necrosis factor-α, interleukin-6, nitric oxide synthase, and cyclo-oxygenase 2 [133]. Finally, rats that consumed inadequate n-3 PUFAs from birth had higher plasma levels of interleukin-6, C-reactive protein, and tumor necrosis factor-α, a, which was reversed by subsequently feeding an α-linoleic-acid-containing diet [49]. Thus, n-3 PUFAs could contribute to antidepressant effects and/or resistance to depression through anti-inflammatory mechanisms
EFFECTS OF N-3 PUFAS ON DEPRESSION-RELATED BEHAVIORS
Debate exists as to the extent to which rodents can manifest depression [134]. Nevertheless, behaviors relevant to depression can be assessed in rodents using a variety of test paradigms. In addition, behavioral tests in rodents have proven to be highly reliable screens used to identify compounds with antidepressant efficacy, and are thus used as putative depression models. Experimental findings in rodents using these models suggest that n-3 PUFAs may have antidepressant efficacy, and that at least some treatments involving inadequate consumption of n-3 PUFAs and/or decreased tissue n-3 PUFA status produce the opposite effect. These findings, as well as those that are inconsistent with a role in depression, are discussed. Effects on anxiety-related behaviors are also presented.
Rodent Models of Depression
The forced swim test is a well validated drug screen for antidepressant activity [135]. Treatments that decrease circulating and/or tissue n-3 PUFA levels produce effects in the forced swim test that are opposite those associated with antidepressant efficacy. For example, adult rats with a 36% reduction brain DHA resulting from diet treatments initiated at weaning, spent more time immobile than those with normal DHA levels [137]. Likewise, when tested 21 days after parturition, postpartum female rats with a 25% decrease in brain DHA exhibited immobility earlier in the test than postpartum rats fed a control diet that maintained normal brain DHA levels [46].
Consistent with the findings on the effects of decreased DHA in the forced swim test, higher tissue and dietary n-3 PUFA levels resulted in behavioral effects that are consistent with the prediction of antidepressant efficacy. Administration of various n-3 PUFA-supplemented diets whether for multiple generations [138], during pre- and perinatal development [51, 139], at weaning for 30 days or more [119, 139] or for at least 16 days in adults [104, 107, 140–142], resulted in less immobility than exhibited by controls. Similarly, when injections of α-linolenic acid were given to adult male mice, they spent less time immobile [103]. In addition, treatment with fish oil potentiated the immobility-reducing effects of the antidepressant drugs fluoxetine and mirtazepine in the test [142]. Other studies, however, found diets containing inadequate n-3 PUFAs on behavior in the forced swim [46, 115].
Anhedonia, another symptom of depression, also appears to be increased by some treatments involving decreased tissue and/or dietary n-3 PUFAs. In one study, mice that consumed a diet containing inadequate n-3 PUFAs from conception displayed reduced preference for sucrose, suggesting decreased pleasure [143, 144]. In another study, however, rats with a 70% decrease in brain DHA level resulting from consuming an n-3 PUFA-deficient diet for multiple generations, did not exhibit altered sucrose consumption at adulthood [115].
Rodent Models of Anxiety
Several studies suggest that inadequate n-3 PUFAs may contribute to anxiety. In the elevated plus maze [145], mice and rats fed inadequate n-3 PUFAs from conception spent less time in the open arms of the maze than control mice, which is interpreted as greater anxiety [146–147]. In the mouse study this outcome was partially reversed by an n-3 PUFA-supplemented diet initiated at 7 weeks of age, which restored brain DHA levels in all regions except the frontal cortex [146]. Similarly in the rat study, the effect was fully reversed by supplementation with DHA for 1 week [147]. Consistent with these findings, when tested 15 days after giving birth, lactating female rats fed a diet containing inadequate n-3 PUFAs also spent less time in the open arms of the maze and made fewer entries into the open arms compared to lactating females fed a fish-oil supplemented diet [148]. Similar to findings in the elevated plus maze, adult rats with dietary n-3 PUFA-deficiency from conception exhibited more freezing behavior, indicative of a higher level of anxiety, in the conditioned fear-induced freezing test than rats fed a DHA-supplemented diet [147]. Similarly, mice with a 50% reduction in brain DHA resulting from feeding an n-3 PUFA-deficient diet for two generations, exhibited decreased feeding in the early phase (first 5 min) of the novelty suppressed feeding test, also consistent with anxiety [117].
Other studies, however, do not support an anti-anxiety effect for n-3 PUFAs. Two studies of mice fed n-3 PUFA-deficient diets from conception, found that the deficient mice spent a longer duration on the open arms of the maze than control mice [149, 150]. A number of other studies in mice and rats found no effect of either n-3 PUFA deficiency or supplementation using the elevated plus maze or other tests such as classical fear conditioning [116, 119, 139, 151–154].
SUMMARY AND CONCLUSION
Depression appears to result from the interaction of genetic and environmental factors. The pre-clinical literature demonstrates that low tissue and/or dietary n-3 PUFA levels lead to behavioral changes in animals that are consistent with current models of depression. These findings also point to mechanisms by which n-3 PUFAs affect neurobiological substrates of depression including regulation of serotonergic and dopaminergic neurotransmission, hippocampal BDNF expression, the hypothalamic-pituitary-adrenal axis, and neuroinflammation. In keeping with diathesis-stress theories of mental illness [155], low dietary or tissue n-3 PUFAs alone do not need to cause depression in humans; however, they likely create a vulnerability that increases susceptibility to depression when the other contributing factors (e.g., specific genotypes, stessors, etc.) are also present. Thus, when viewed in this context, the body of pre-clinical neurobiological and behavioral data supports a role for low tissue and/or dietary n-3 PUFAs as one of many potential contributing factors in the pathogenesis of depression.
Critical issues regarding the role of n-3 PUFAs in depression remain to be resolved. The magnitude of change in brain fatty acid composition and the point in the life span when it occurs appear to affect the type and extent of the resulting neurobiological changes. This in turn likely determines the tendency of those changes to predispose an individual towards depression or the other psychiatric disorders in which low n-3 PUFAs are implicated. Thus, we must systematically assess the effects of variation in PUFA status on the nervous system at various points in development, and with an emphasis on using clinically-relevant animal models. We must also elucidate the cellular mechanisms by which low tissue and dietary n-3 PUFA levels produce depression-associated behavioral and neurobiological effects, and determine which n-3 PUFAs are of critical importance (i.e., DHA, EPA, etc.). Finally, we must determine which effects can be reversed by subsequent n-3 PUFA administration and if so, if full restoration of brain PUFA composition is required. Should it be found that the effects of low n-3 PUFAs that are important for depression are reversible, the use of n-3 PUFA supplementation for the treatment depression would be supported. Alternatively, should the effects prove be irreversible, this will support the importance of ensuring adequate nutrition with respect to n-3 PUFAs as a means of prevention.
ACKNOWLEDGEMENTS
The author thanks Heather Spalding for assistance in the preparation of this manuscript. Supported by NIH MH071599, P30 HD02528, and P20 RR016475 from the INBRE Program of the National Center for Research Resources.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- DHA
docosahexaenoic acid
- n-6 DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- PPAR
peroxisome proliferator-activated receptor
- PUFA
polyunsaturated fatty acid
- RXR
retinoid X receptor
- VMAT
vesicular monoamine transporter
Footnotes
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Nemeroff CB, Kelsey JE. Affective disorders. In: Enna SJ, Coyle JT, editors. Pharmacological Management of Neurological and Psychiatric Disorders. New York: McGraw Hill; 1998. pp. 95–136. [Google Scholar]
- 2.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch. Gen. Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 3.Duman RS. The neurochemistry of mood disorders: preclinical studies. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2004. pp. 421–339. [Google Scholar]
- 4.Garlow SJ, Musselman DL, Nemeroff CB. The neurochemistry of mood disorders: clinical studies. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2004. pp. 440–460. [Google Scholar]
- 5.Schmidt H, Shelton R, Duman R. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36:2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci. STKE. 2004;2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- 8.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Freeman MP, Rapaport MH. Omega-3 fatty acids and depression:from cellular mechanisms to clinical care. J. Clin. Psychiatry. 2011;72:258–259. doi: 10.4088/JCP.11ac06830. [DOI] [PubMed] [Google Scholar]
- 10.McNamara RK. Evaluation of docosahexaenoic acid deficiency as a preventable risk factor for recurrent affective disorders: Current status, future directions, and dietary recommendations. Prostaglandins Leukot. Essent. Fatty Acids. 2009 doi: 10.1016/j.plefa.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair AJ. Long chain polyunsaturated fatty acids in the mammalian brain. Proc. Nutr. Soc. 1975;34:287–291. doi: 10.1079/pns19750051. [DOI] [PubMed] [Google Scholar]
- 13.Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta. 2000;1486:219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 14.Carrie I, Clement M, de Javel D, Frances H, Bourre JM. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000;41:465–472. [PubMed] [Google Scholar]
- 15.Xiao Y, Huang Y, Chen ZY. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br. J. Nutr. 2005;94:544–550. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]
- 16.Levant B, Ozias MK, Jones KA, Carlson SE. Differential effects of modulation of docosahexaenoic acid content during development in specific regions of rat brain. Lipids. 2006;41:407–414. doi: 10.1007/s11745-006-5114-6. [DOI] [PubMed] [Google Scholar]
- 17.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum. Dev. 1980;4:131–138. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- 18.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum. Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 19.Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids. 1996;31:859–865. doi: 10.1007/BF02522981. [DOI] [PubMed] [Google Scholar]
- 20.Innis SM. Human milk and formula fatty acids. J. Pediatrics. 1992;120:S56–S61. doi: 10.1016/s0022-3476(05)81237-5. [DOI] [PubMed] [Google Scholar]
- 21.Innis SM. Polyunsaturated fatty acids in human milk: an essential role in infant development. Adv. Exp. Med. Biol. 2004;554:27–43. doi: 10.1007/978-1-4757-4242-8_5. [DOI] [PubMed] [Google Scholar]
- 22.Galli C, Trzeciak HI, Paoletti R. Effects of dietary fatty acids on the fatty acid composition of brain ethanolamine phosphoglyceride: reciprocal replacement of n-6 and n-3 polyunsaturated fatty acids. Biochim. Biophys. Acta. 1971;248:449–454. [Google Scholar]
- 23.Bourre JM, Dumont OS, Piciotti MJ, Pascal GA, Durand GA. Dietary alpha-linolenic acid deficiency in adult rats for 7 months does not alter brain docosahexaenoic acid content, in contrast to liver, heart and testes. Biochim. Biophys. Acta. 1992;1124:119–122. doi: 10.1016/0005-2760(92)90087-c. [DOI] [PubMed] [Google Scholar]
- 24.Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. J. Nutr. 2006;136:2236–2242. doi: 10.1093/jn/136.8.2236. [DOI] [PubMed] [Google Scholar]
- 25.McNamara RK, Sullivan J, Richtand NM, Jandacek R, Rider T, Tso P, Campbell N, Lipton J. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult DBA/2J mice: Relationship with ventral striatum dopamine concentrations. Synapse. 2008;62:725–735. doi: 10.1002/syn.20542. [DOI] [PubMed] [Google Scholar]
- 26.Levant B, Ozias MK, Carlson SE. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J. Nutr. 2007;137:130–134. doi: 10.1093/jn/137.1.130. [DOI] [PubMed] [Google Scholar]
- 27.Salem N, Jr., Litman B, Kim H-Y, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–960. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 28.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod. Nutr. Dev. 2005;45:559–579. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- 29.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bannenberg G, Arita M, Serhan CN. Endogenous receptor agonists: resolving inflammation. ScientificWorldJournal. 2007;7:1440–1462. doi: 10.1100/tsw.2007.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 33.Clarke SD, Thuillier P, Baillie RA, Sha X. Peroxisome proliferator-activated receptors: a family of lipid-activated transcription factors. Am. J. Clin. Nutr. 1999;70:566–571. doi: 10.1093/ajcn/70.4.566. [DOI] [PubMed] [Google Scholar]
- 34.Gordon N. Nutrition and cognitive function. Brain & Dev. 1997;19:165–167. doi: 10.1016/s0387-7604(96)00560-8. [DOI] [PubMed] [Google Scholar]
- 35.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000;42:174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- 36.Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Influence of long-chain polyunsaturated fatty acids on infant cognitive function. Lipids. 1998;33:973–980. doi: 10.1007/s11745-998-0294-7. [DOI] [PubMed] [Google Scholar]
- 37.Makrides M, Smithers LG, Gibson RA. Role of long-chain polyunsaturated fatty acids in neurodevelopment and growth. Nestle Nutr. Workshop Ser. Pediatr. Program. 2010;65:123–133. doi: 10.1159/000281154. discussion 133-126. [DOI] [PubMed] [Google Scholar]
- 38.Beskow J, Gottfries CG, Roos BE, Winblad B. Determination of monoamine and monoamine metabolites in the human brain: post mortem studies in a group of suicides and in a control group. Acta. Psychiatry Scand. 1976;53:7–20. doi: 10.1111/j.1600-0447.1976.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd KG, Farley IJ, Deck JHN, Hornykiewicz O. Serotonin and 5-hydroxyindoleactetic acid in discrete areas of the brain stem in suicide victims and control patients. Adv. Biochem. Psychopharmacol. 1974;11:387–397. [PubMed] [Google Scholar]
- 40.Shaw DM, Camps FE, Eccleston EG. 5-hydroxytryptamine in the hind-brain of depressive suicides. Br. J. Psychiatry. 1967;113:1407–1411. doi: 10.1192/bjp.113.505.1407. [DOI] [PubMed] [Google Scholar]
- 41.Arango V, Ernsberger P, Marzuk J, Chen S, Tierney H, Stanley M, Reis DJ, Mann JJ. Autoradiographic demonstration of increased serotonin 5-HT2 and b-adrenergic receptor binding sites in the brain of suicide victims. Arch. Gen. Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 42.Mann JJ, Arango V. Abnormalities of brain structure and function in mood disorders. In: Charney DS, Neslter EJ, Bunney BS, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 1999. pp. 385–393. [Google Scholar]
- 43.Yates M, Ferrier IN. 5-HT1A receptor in major depression. J. Psychopharmacol. 1990;4:59–67. doi: 10.1177/026988119000400204. [DOI] [PubMed] [Google Scholar]
- 44.Yates M, Leake A, Candy JM, Fairbairn AF, McKeith IG, Ferrier IN. 5-HT2 receptor changes in major depression. Biol. Psychiatry. 1990;27:489–496. doi: 10.1016/0006-3223(90)90440-d. [DOI] [PubMed] [Google Scholar]
- 45.Baldessarini RJ. Drugs and the treatment of psychiatric disorders: depression and anxiety disorders. In: Hardman JG, Limbird LE, Gilman AG, editors. The Pharmacological Basis of Therapeutics. 10th ed. New York: McGraw-Hill; 2001. pp. 447–484. [Google Scholar]
- 46.Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, Reed GA, McCarson KE. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33:1279–1292. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol. Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 48.McNamara RK, Able J, Liu Y, Jandacek R, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: dissociation from estrogenic effects. J. Psychiatr. Res. 2009;43:656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive proinflammatory cytokine production in rats: relationship with central serotonin turnover. Prostaglandins Leukot. Essent. Fatty Acids. 2010;83:185–191. doi: 10.1016/j.plefa.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de la Presa Owens S, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alphalinolenic acid deficient diet in formula-fed piglets. J. Nutr. 1999;129:2088–2093. doi: 10.1093/jn/129.11.2088. [DOI] [PubMed] [Google Scholar]
- 51.Vines A, Delattre AM, Lima MM, Rodrigues LS, Suchecki D, Machado RB, Tufik S, Pereira SI, Zanata SM, Ferraz AC. The role of 5-HT(1A) receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism. Neuropharmacology. 2011;62:184–191. doi: 10.1016/j.neuropharm.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 52.Vancassel S, Leman S, Hanonick L, Denis S, Roger J, Nollet M, Bodard S, Kousignian I, Belzung C, Chalon S. n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J. Lipid Res. 2008;49:340–348. doi: 10.1194/jlr.M700328-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Delion S, Chalon S, Herault J, Guilloteau D, Bresnard JC, Durand G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotonergic neurotransmission in rats. J. Nutr. 1994;124:2466–2475. doi: 10.1093/jn/124.12.466. [DOI] [PubMed] [Google Scholar]
- 54.Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. alpha-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. J. Neurochem. 1996;66:1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- 55.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J. Psychiatr. Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 56.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch. Gen. Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 57.Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nucl. Med. Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 58.Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biegon A, Israeli M. Regionally selective increased in b-adrenergic receptor density in the brains of suicide victims. Brain Res. 1988;442:199–203. doi: 10.1016/0006-8993(88)91453-9. [DOI] [PubMed] [Google Scholar]
- 60.Vetulani J, Sulser F. Action of various antidepressant treatments reduces reactivity of noradrenergic cAMP generating system in limbic forebrain. Nature. 1975;22:181. doi: 10.1038/257495a0. [DOI] [PubMed] [Google Scholar]
- 61.Mathieu G, Denis S, Langelier B, Denis I, Lavialle M, Vancassel S. DHA enhances the noradrenaline release by SH-SY5Y cells. Neurochem. Int. 2010;56:94–100. doi: 10.1016/j.neuint.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Joardar A, Sen AK, Das S. Docosahexaenoic acid facilitates cell maturation and beta-adrenergic transmission in astrocytes. J. Lipid Res. 2006;47:571–581. doi: 10.1194/jlr.M500415-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Takeuchi T, Fukumoto Y, Harada E. Influence of a dietary n-3 fatty acid deficiency on the cerebral catecholamine contents, EEG and learning ability in rat. Behav. Brain Res. 2002;131:193–203. doi: 10.1016/s0166-4328(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 64.Post RM, Kotin J, Goodwin FK, Gordon E. Psychomotor activity and cerebrospinal fluid metabolites in affective illness. Am. J. Psychiatry. 1973;130:67–72. doi: 10.1176/ajp.130.1.67. [DOI] [PubMed] [Google Scholar]
- 65.Roy A, Karoum F, Pollack S. Marked reduction in indexes of dopamine transmission among patients with depression who attempted suicide. Arch. Gen. Psychiatry. 1992;49:447–450. doi: 10.1001/archpsyc.1992.01820060027004. [DOI] [PubMed] [Google Scholar]
- 66.Reddy PL, Khanna S, Subash MN, Channabasavanna SM, Sridhara Rama Rao BS. CSF amine metabolites in depression. Biol. Psychiatry. 1992;31:112–118. doi: 10.1016/0006-3223(92)90198-9. [DOI] [PubMed] [Google Scholar]
- 67.Brown AS, Gershon S. Dopamine and depression. J. Neural Trans. 1993;91:75–109. doi: 10.1007/BF01245227. [DOI] [PubMed] [Google Scholar]
- 68.Van Praag HM, Korf J, Lakke J, Schut T. Dopamine metabolism in depression, psychoses, and Parkinson’s disease: the problem of specificity of biological variables in behavior disorders. Psychol. Med. 1975;4:21–29. doi: 10.1017/s0033291700056385. [DOI] [PubMed] [Google Scholar]
- 69.Guze BH, Barrio JC. The etiology of depression in Parkinson’s disease patients. Psychosomatics. 1991;32:390–394. doi: 10.1016/S0033-3182(91)72039-2. [DOI] [PubMed] [Google Scholar]
- 70.Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 72.Gershon AA, Vishne T, Grunhaus L. Dopamine D2-like receptors and the antidepressant response. Biol. Psychiatry. 2007;61:145–153. doi: 10.1016/j.biopsych.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 73.aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180:305–313. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol. Psychiatry. 2007;12:703, 767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- 75.Davis PF, Ozias MK, Carlson SE, Reed GA, Winter MK, McCarson KE, Levant B. Dopamine receptor alterations in female rats with diet-induced decreased brain docosahexaenoic acid (DHA): interactions with reproductive status. Nutr. Neurosci. 2010;13:161–169. doi: 10.1179/147683010X12611460764282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papp M, Klimek V, Willner P. Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stressinduced anhedonia and its reversal by imipramine. Psychopharmacology (Berl) 1994;115:441–446. doi: 10.1007/BF02245566. [DOI] [PubMed] [Google Scholar]
- 77.Bjornebekk A, Mathe AA, Brene S. Isolated Flinders Sensitive Line rats have decreased dopamine D2 receptor mRNA. Neuroreport. 2007;18:1039–1043. doi: 10.1097/WNR.0b013e3281668bf7. [DOI] [PubMed] [Google Scholar]
- 78.Kram ML, Kramer GL, Ronan PJ, Steciuk M, Petty F. Dopamine receptors and learned helplessness in the rat: an autoradiographic study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:639–645. doi: 10.1016/s0278-5846(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 79.Hirvonen J, Karlsson H, Kajander J, Markkula J, Rasi-Hakala H, Nagren K, Salminen JK, Hietala J. Striatal dopamine D2 receptors in medication-naive patients with major depressive disorder as assessed with [11C]raclopride PET. Psychopharmacology (Berl) 2008;197:581–590. doi: 10.1007/s00213-008-1088-9. [DOI] [PubMed] [Google Scholar]
- 80.Moses-Kolko EL, Price JC, Wisner KL, Hanusa BH, Meltzer CC, Berga SL, Grace AA, di Scalea TL, Kaye WH, Becker C, Drevets WC. Postpartum and depression status are associated with lower [(1)(1)C]raclopride BP(ND) in reproductive-age women. Neuropsychopharmacology. 2012;37:1422–1432. doi: 10.1038/npp.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmer L, Delion S, Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, Chalon S. Modification of dopamineneurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J. Lipid Res. 2000;41:32–40. [PubMed] [Google Scholar]
- 82.Zimmer L, Hembert S, Durand G, Breton P, Guilloteau D, Besnard JC, Chalon S. Chronic n-3 polyunsaturated fatty acid dietdeficiency acts on dopamine metabolism in the rat frontal cortex: a microdialysis study. Neurosci. Lett. 1998;240:177–181. doi: 10.1016/s0304-3940(97)00938-5. [DOI] [PubMed] [Google Scholar]
- 83.Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am. J. Clin. Nutr. 2002;75:662–667. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]
- 84.Kuperstein F, Yakubov E, Dinerman P, Gil S, Eylam R, Salem N, Jr., Yavin E. Overexpression of dopamine receptor genes and their products in the postnatal rat brain following maternal n-3 fatty acid dietary deficiency. J. Neurochem. 2005;95:1550–1562. doi: 10.1111/j.1471-4159.2005.03513.x. [DOI] [PubMed] [Google Scholar]
- 85.Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J. Neurochem. 2008;106:662–671. doi: 10.1111/j.1471-4159.2008.05418.x. [DOI] [PubMed] [Google Scholar]
- 86.Levant B, Zarcone TJ, Fowler SC. Developmental effects of dietary n-3 fatty acids on activity and response to novelty. Physiol. Behav. 2010;101:176–183. doi: 10.1016/j.physbeh.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chalon S. The role of fatty acids in the treatment of ADHD. Neuropharmacology. 2009;57:636–639. doi: 10.1016/j.neuropharm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 88.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol. Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 89.Curtis MA, Kam M, Faull RL. Neurogenesis in humans. Eur. J. Neurosci. 2011;33:1170–1174. doi: 10.1111/j.1460-9568.2011.07616.x. [DOI] [PubMed] [Google Scholar]
- 90.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res. Mol. Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 91.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 92.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 93.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 95.Smith MA, Makino S, Kim SY, Kvetnansky R. Stress increases brain-derived neurotropic factor messenger ribonucleic acid in the hypothalamus and pituitary. Endocrinology. 1995;136:3743–3750. doi: 10.1210/endo.136.9.7649080. [DOI] [PubMed] [Google Scholar]
- 96.Gronli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, Ursin R, Portas CM. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 2006;85:842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 97.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121:1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- 99.Siuciak JA, Lewis D, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor. Pharmacol. Biochem. Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 100.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol. Psychiatry. 2007;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 103.Blondeau N, Nguemeni C, Debruyne DN, Piens M, Wu X, Pan H, Hu X, Gandin C, Lipsky RH, Plumier JC, Marini AM, Heurteaux C. Subchronic alpha-linolenic acid treatment enhances brainplasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology. 2009;34:2548–2559. doi: 10.1038/npp.2009.84. [DOI] [PubMed] [Google Scholar]
- 104.Venna VR, Deplanque D, Allet C, Belarbi K, Hamdane M, Bordet R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology. 2009;34:199–211. doi: 10.1016/j.psyneuen.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 105.Cysneiros RM, Ferrari D, Arida RM, Terra VC, de Almeida AC, Cavalheiro EA, Scorza FA. Qualitative analysis of hippocampal plastic changes in rats with epilepsy supplemented with oral omega-3 fatty acids. Epilepsy Behav. 2010;17:33–38. doi: 10.1016/j.yebeh.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 106.Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma. 2007;24:1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- 107.Park Y, Moon HJ, Kim SH. N-3 polyunsaturated fatty acid consumption produces neurobiological effects associated with prevention of depression in rats after the forced swimming test. J Nutr. Biochem. 2012;23:924–928. doi: 10.1016/j.jnutbio.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 108.He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11370–11375. doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dyall SC, Michael GJ, Michael-Titus AT. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J. Neurosci. Res. 2010;88:2091–2102. doi: 10.1002/jnr.22390. [DOI] [PubMed] [Google Scholar]
- 110.Carpenter WT, Jr., Bunney WE., Jr. Adrenal cortical activity in depressive illness. Am. J. Psychiatr. 1971;128:31–40. doi: 10.1176/ajp.128.1.31. [DOI] [PubMed] [Google Scholar]
- 111.Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. Urinary free cortisol excretion in depression. J. Psychol. Med. 1976;6:43–50. doi: 10.1017/s0033291700007480. [DOI] [PubMed] [Google Scholar]
- 112.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 113.Carroll BJ, Martin FI, Davies B. Pituitary-adrenal function in depression. Lancet. 1968;1:1373–1374. doi: 10.1016/s0140-6736(68)92072-2. [DOI] [PubMed] [Google Scholar]
- 114.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 115.Mathieu G, Denis S, Lavialle M, Vancassel S. Synergistic effects of stress and omega-3 fatty acid deprivation on emotional response and brain lipid composition in adult rats. Prostaglandins Leukot. Essent. Fatty Acids. 2008;78:391–401. doi: 10.1016/j.plefa.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 116.Mathieu G, Oualian C, Denis I, Lavialle M, Gisquet-Verrier P, Vancassel S. Dietary n-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot. Essent. Fatty. Acids. 2011;85:129–136. doi: 10.1016/j.plefa.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 117.Harauma A, Moriguchi T. Dietary n-3 fatty acid deficiency in mice enhances anxiety induced by chronic mild stress. Lipids. 2011;46:409–416. doi: 10.1007/s11745-010-3523-z. [DOI] [PubMed] [Google Scholar]
- 118.Song C, Leonard BE, Horrobin DF. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress. 2004;7:43–54. doi: 10.1080/10253890410001667188. [DOI] [PubMed] [Google Scholar]
- 119.Ferraz AC, Delattre AM, Almendra RG, Sonagli M, Borges C, Araujo P, Andersen ML, Tufik S, Lima MM. Chronic omega-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav. Brain Res. 2011;219:116–122. doi: 10.1016/j.bbr.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 120.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J. Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 121.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 122.Dinan T, Siggins L, Scully P, O’Brien S, Ross P, Stanton C. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. J. Psychiatr. Res. 2009;43:471–476. doi: 10.1016/j.jpsychires.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 123.Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Moller HJ, Chen HH, Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr. Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dean B, Tawadros N, Scarr E, Gibbons AS. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J. Affect. Disord. 2010;120:245–248. doi: 10.1016/j.jad.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 125.Gaiteri C, Guilloux JP, Lewis DA, Sibille E. Altered gene synchrony suggests a combined hormone-mediated dysregulated state in major depression. PLoS One. 2010;5:e9970. doi: 10.1371/journal.pone.0009970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry. 2010;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hannestad J, Dellagioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J. Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 129.Bannenberg GL. Resolvins: Current understanding and future potential in the control of inflammation. Curr. Opin. Drug Discov. Devel. 2009;12:644–658. [PubMed] [Google Scholar]
- 130.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 131.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J. Biol. Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 133.Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase- 1 expression in BV-2 microglia: iImplications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology. 2010;35:2238–2248. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Frazer A, Morilak DA. What should animal models of depression model? Neurosci. Biobehav. Rev. 2005;29:515–523. doi: 10.1016/j.neubiorev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 135.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 136.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J. Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.DeMar JC, Jr., Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 138.Naliwaiko K, Araujo RL, da Fonseca RV, Castilho JC, Andreatini R, Bellissimo MI, Oliveira BH, Martins EF, Curi R, Fernandes LC, Ferraz AC. Effects of fish oil on the central nervous system: a new potential antidepressant? Nutr. Neurosci. 2004;7:91–99. doi: 10.1080/10284150410001704525. [DOI] [PubMed] [Google Scholar]
- 139.Ferraz AC, Kiss A, Araujo RL, Salles HM, Naliwaiko K, Pamplona J, Matheussi F. The antidepressant role of dietary longchain polyunsaturated n-3 fatty acids in two phases in the developing brain. Prostaglandins Leukot. Essent. Fatty Acids. 2008;78:183–188. doi: 10.1016/j.plefa.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 140.Lakhwani L, Tongia SK, Pal VS, Agrawal RP, Nyati P, Phadnis P. Omega-3 fatty acids have antidepressant activity in forced swimming test in Wistar rats. Acta Pol. Pharm. 2007;64:271–276. [PubMed] [Google Scholar]
- 141.Huang SY, Yang HT, Chiu CC, Pariante CM, Su KP. Omega- 3 fatty acids on the forced-swimming test. J. Psychiatr. Res. 2008;42:58–63. doi: 10.1016/j.jpsychires.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Laino CH, Fonseca C, Sterin-Speziale N, Slobodianik N, Reines A. Potentiation of omega-3 fatty acid antidepressant-like effects with low non-antidepressant doses of fluoxetine and mirtazapine. Eur. J. Pharmacol. 2010;648:117–126. doi: 10.1016/j.ejphar.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 143.Frances H, Drai P, Smirnova M, Carrie I, Debray M, Bourre JM. Nutritional (n-3) polyunsaturated fatty acids influence the behavioral responses to positive events in mice. Neurosci. Lett. 2000;285:223–227. doi: 10.1016/s0304-3940(00)01065-x. [DOI] [PubMed] [Google Scholar]
- 144.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 145.File SE. Animal models of anxiety. In: Crawley JN, Gerfen CR, McKay R, Rogawski MA, Sibley DR, Skolnik P, editors. Current Protocols in Neuroscience. New York: John Wiley & Sons; 1997. pp. 8.3.1–8.3.15. [Google Scholar]
- 146.Carrie I, Clement M, de Javel D, Frances H, Bourre JM. Phospholipid supplementation reverses behavioral and biochemical alterations induced by n-3 polyunsaturated fatty acid deficiency in mice. J. Lipid Res. 2000;41:473–480. [PubMed] [Google Scholar]
- 147.Takeuchi T, Iwanaga M, Harada E. Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res. 2003;964:136–143. doi: 10.1016/s0006-8993(02)04113-6. [DOI] [PubMed] [Google Scholar]
- 148.Chen HF, Su HM. Fish oil supplementation of maternal rats on an n- 3 fatty acid-deficient diet prevents depletion of maternal brain regional docosahexaenoic acid levels and has a postpartum anxiolytic effect. J. Nutr. Biochem. 2011;23:299–305. doi: 10.1016/j.jnutbio.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 149.Nakashima Y, Yuasa S, Hukamizu Y, Okuyama H, Ohhara T, Kameyama T, Nabeshima T. Effect of a high linoleate and a high alpha-linolenate diet on general behavior and drug sensitivity in mice. J. Lipid Res. 1993;34:239–247. [PubMed] [Google Scholar]
- 150.Frances H, Monier C, Bourre JM. Effects of dietary alpha-linolenic acid deficiency on neuromuscular and cognitive functions in mice. Life. Sci. 1995;57:1935–1947. doi: 10.1016/0024-3205(95)02180-q. [DOI] [PubMed] [Google Scholar]
- 151.Belzung C, Leguisquet AM, Barreau S, Delion Vancassel S, Chalon S, Durand G. Alpha-linolenic acid deficiency modifies distractibility but not anxiety and locomotion in rats during aging. J. Nutr. 1998;128:1537–1542. doi: 10.1093/jn/128.9.1537. [DOI] [PubMed] [Google Scholar]
- 152.Moriguchi T, Sheaff-Greiner R, Salem N., Jr. Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 153.Fedorova I, Hussein N, Di Martino C, Moriguchi T, Hoshiba J, Majchrzak S, Salem N., Jr. An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins. Leukot. Essent. Fatty Acids. 2007;77:269–277. doi: 10.1016/j.plefa.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trofimiuk E, Braszko JJ. Long-term administration of cod liver oil ameliorates cognitive impairment induced by chronic stress in rats. Lipids. 2011;46:417–423. doi: 10.1007/s11745-011-3551-3. [DOI] [PubMed] [Google Scholar]
- 155.Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol. Bull. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]


