Abstract
Background
Atrial fibrillation (AF) prediction models have unclear clinical utility given the absence of AF prevention therapies and the immutability of many risk factors. Premature atrial contractions (PACs) play a critical role in AF pathogenesis and may be modifiable.
Objective
To investigate whether PAC count improves model performance for AF risk.
Design
Prospective cohort study.
Setting
4 U.S. communities.
Patients
A random subset of 1260 adults without prevalent AF enrolled in the Cardiovascular Health Study between 1989 and 1990.
Measurements
The PAC count was quantified by 24-hour electrocardiography. Participants were followed for the diagnosis of incident AF or death. The Framingham AF risk algorithm was used as the comparator prediction model.
Results
In adjusted analyses, doubling the hourly PAC count was associated with a significant increase in AF risk (hazard ratio, 1.17 [95% CI, 1.13 to 1.22]; P < 0.001) and overall mortality (hazard ratio, 1.06 [CI, 1.03 to 1.09]; P < 0.001). Compared with the Framingham model, PAC count alone resulted in similar AF risk discrimination at 5 and 10 years of follow-up and superior risk discrimination at 15 years. The addition of PAC count to the Framingham model resulted in significant 10-year AF risk discrimination improvement (c-statistic, 0.65 vs. 0.72; P < 0.001), net reclassification improvement (23.2% [CI, 12.8% to 33.6%]; P < 0.001), and integrated discrimination improvement (5.6% [CI, 4.2% to 7.0%]; P < 0.001). The specificity for predicting AF at 15 years exceeded 90% for PAC counts more than 32 beats/h.
Limitation
This study does not establish a causal link between PACs and AF.
Conclusion
The addition of PAC count to a validated AF risk algorithm provides superior AF risk discrimination and significantly improves risk reclassification. Further study is needed to determine whether PAC modification can prospectively reduce AF risk.
Primary Funding Source
American Heart Association, Joseph Drown Foundation, and National Institutes of Health.
More than 3 million adults in the United States are living with atrial fibrillation (AF) (1). This common arrhythmia is associated with increased morbidity (2), excess mortality (3), and substantial health care costs (4). The considerable medical and economic effects of AF have generated interest in prediction algorithms to estimate AF risk in an individual patient (5–7). However, such models are of unclear clinical utility given the absence of primary AF prevention therapies and the immutability of many identified risk factors.
Premature atrial contractions (PACs) have been shown to initiate episodes of AF (8, 9). In addition, targeted ablation of atrial ectopy can eliminate or substantially reduce AF recurrence (8). Evidence also shows that PACs are associated with incident AF in certain patient populations without known baseline arrhythmias (10–12). Together, these findings suggest that PACs play a critical role in AF pathogenesis. Further study of the association between PACs and AF is especially compelling because PAC burden can theoretically be modified by catheter ablation.
The contribution of PACs to AF risk prediction has not been reported. We therefore examined the association between PACs and incident AF among participants enrolled in the Cardiovascular Health Study (CHS).
Methods
Study Design
The CHS is a prospective, community-based cohort study sponsored by the National Heart, Lung, and Blood Institute. Details about eligibility, enrollment, and follow-up have been published (13–15). In brief, 5201 persons aged 65 years or older were recruited between 1989 and 1990 from a random sample of Medicare beneficiaries at 4 academic centers (Johns Hopkins University, Baltimore, Maryland; Wake Forest University, Winston-Salem, North Carolina; University of Pittsburgh, Pittsburgh, Pennsylvania; and University of California, Davis, Davis, California). All participants had a medical history, physical examination, laboratory testing, and 12-lead electrocardiography (ECG). Participants were followed with annual clinic visits and semiannual telephone contact for 10 years. Telephone contact was continued every 6 months thereafter.
Study Cohort
Our analysis was restricted to the subset of 1429 persons randomly assigned to 24-hour ambulatory ECG (Holter) monitoring during their initial assessment. Baseline cardiovascular comorbid conditions were ascertained by participant history and validated by physical examination, physician report, and medical record review. Participants with prevalent AF (defined as a reported history of AF at their first study encounter, on baseline 12-lead ECG, or on baseline Holter monitoring) were excluded.
PAC Assessment
Baseline Holter data were analyzed at the Washington University School of Medicine Heart Rate Variability Laboratory using a MARS 8000 Holter scanner (GE Healthcare, Milwaukee, Wisconsin) and manually reviewed to ensure accuracy. Participants with atrial pacing, wandering atrial pacemaker, or poor-quality Holter data were excluded. Participants selected for baseline Holter monitoring were invited to undergo repeated testing after 5 years of follow-up.
Covariate Ascertainment
Self-identified race was dichotomized as white persons or nonwhite persons. Hypertension was defined as a reported history of physician-diagnosed hypertension and use of antihypertensive medications, systolic blood pressure of 140 mm Hg or more, or diastolic pressure of 90 mm Hg or more. Diabetes was present if the participant used an antihyperglycemic medication or had a fasting glucose concentration of 126 mmol/L (2270.27 mg/dL) or more. Heart failure and myocardial infarction were diagnosed by participant self-report and were confirmed by medical record verification (16). Coronary artery disease was defined as angina, previous myocardial infarction, previous coronary artery bypass grafting, or previous angioplasty. The ECGs were recorded using MAC PC ECG machines (GE Healthcare) at baseline and annually over the first 10 years of follow-up. They were initially processed using the Dalhousie ECG program in a central laboratory at the EPICORE Center (University of Alberta, Edmonton, Alberta, Canada) and later using the GE Marquette 12-SL program (2001 version) at the EPICARE Center (Wake Forest University School of Medicine). After visual inspection for technical errors and adequate quality, ECG waveform amplitudes and durations were automatically measured.
Event Ascertainment
Medical records were obtained for all hospitalizations after study enrollment. Incident AF was diagnosed by annual study ECG or hospital discharge diagnosis codes. Death was ascertained by reviewing medical records, death certificates, autopsies, coroner reports, and obituaries and by searching the Social Security Death Index. Cardiovascular mortality was defined as death secondary to coronary artery disease, heart failure, peripheral arterial disease, or cerebrovascular disease and was adjudicated by the CHS event subcommittee (15, 16).
Statistical Analysis
Continuous variables with normal distribution are presented as means with SDs and were compared by using the t test. Nonnormally distributed continuous variables are presented as medians with interquartile ranges (IQRs) and were compared by using the Kruskal–Wallis test. The association between categorical variables was measured by the chi-square test. A Fine–Gray model treating death as a competing risk (17) was used to determine the association between PAC count and incident AF before and after controlling for confounders identified a priori. We used log base 2 and spline transformations of PAC count to meet model linearity assumptions. Models incorporating log-transformed PAC count showed a higher log-likelihood than those using an untransformed PAC term, indicating that this transformation was appropriate. Spline analyses used restricted cubic splines with 4 knots. A Wald test was used to confirm that the nonlinear spline terms were statistically significant.
Prediction model performance was evaluated according to consensus recommendations for novel cardiovascular risk marker assessment (18). The Framingham AF risk algorithm (6) was reestimated by using a Fine–Gray competing risk model to predict 10-year AF risk. For these analyses, CHS follow-up was censored at 10 years. The Framingham model has been validated in the CHS cohort and contained the following terms: age, age2, male sex, body mass index, current treatment for hypertension, PR interval, history of heart failure, male sex × age2, and age × history of heart failure (5). Unless specifically noted, all prediction analyses used competing risk models with spline-transformed PAC count.
Discrimination of the prediction models was measured by using the Wolbers-adapted c-statistic (19). The CIs for this statistic were obtained by using bootstrap resampling with 1000 repetitions. Model calibration was determined by visual inspection of observed versus predicted risk plots in which participants were grouped according to decile of predicted risk.
Risk reclassification was assessed by using net reclassification and integrated discrimination improvement (20). Bias-corrected clinical net reclassification improvement was calculated to determine the proportion of persons in the intermediate AF risk group (10-year risk of 15% to 20%) who were reclassified as low (<15%) or high (>20%) risk (21).
Several sensitivity analyses were done to further explore the utility of PAC count for AF risk prediction, including comparison of prediction model discrimination after censoring follow-up at 5 and 15 years. In addition, successive analyses were done after stratifying the cohort by using either the 50th or 75th percentile of age (71 and 75 years, respectively). Because we reasoned that study ECGs would be more specific than hospital discharge coding for the diagnosis of AF, we assessed risk model performance after stratifying by AF diagnosis method (study ECG vs. hospital discharge coding). Participants with AF diagnosed both by ECG and hospital coding were included only in the ECG diagnosis category. Finally, we did prediction model comparisons by using Cox proportional hazards models that did not account for the competing risk for death.
To determine the test characteristics of PAC count as a predictor of AF, a competing risk prediction model incorporating log-transformed PAC count was used to generate a predicted 15-year AF risk for each participant. This 15-year time point was chosen to take advantage of the extended CHS duration of follow-up.
Data were analyzed by using Stata 12 (StataCorp, College Station, Texas). A 2-tailed P value less than 0.05 was considered statistically significant.
Role of the Funding Source
This study was funded by the American Heart Association; Joseph Drown Foundation; National Heart, Lung, and Blood Institute; National Institute of Neurological Disorders and Stroke; and the National Institute on Aging. The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Results
Analyzable PAC data were available for 1260 persons without prevalent AF. The CHS participants with Holter data were significantly younger, were more likely to be male, and had longer PR intervals compared with the remainder of the cohort (Appendix Table 1, available at www.annals.org). Over a median Holter monitoring duration of 22.2 hours (IQR, 21.7 to 22.8 hours), the median PAC count was 2.5 beats/h (IQR, 0.8 to 9.5 beats/h). Among participants without AF who were willing and able to have repeated Holter monitoring after 5 years (n = 726), median PAC count increased by 0.5 beats/h (IQR, −0.6 to 4.4 beats/h) between baseline and the 5-year Holter assessment.
PACs and Incident AF
During a median follow-up of 13.0 years (IQR, 7.3 to 18.1 years), 343 participants (27%) developed incident AF and 573 (45%) died without known AF. Participants with incident AF were significantly older; were more likely to be male; and had a higher prevalence of hypertension, heart failure, and coronary artery disease (Table 1). Median hourly PAC count at baseline was significantly higher in participants with incident AF (5.3 beats/h [IQR, 2.1 to 18.0 beats/h] vs. 1.8 beats/h [IQR, 0.6 to 6.1 beats/h]; P < 0.001). In unadjusted and adjusted competing risk models using a categorical or log-transformed variable, PAC count was significantly associated with incident AF (Table 2). When the association was modeled by using restricted cubic splines, all spline terms were statistically significant (P < 0.001) in unadjusted and adjusted competing risk models.
Table 1.
Baseline Characteristics of Participants With and Without Incident AF
| Characteristic | Entire Cohort (n = 1260) | Without AF (n = 917) | Incident AF (n = 343) | P Value* |
|---|---|---|---|---|
| Median age (IQR), y | 71 (68–75) | 70 (68–74) | 71 (68–75) | 0.002 |
| Female, n (%) | 691 (55) | 519 (57) | 172 (50) | 0.041 |
| White, n (%) | 1200 (95) | 873 (95) | 327 (95) | 0.92 |
| Mean BMI (SD), kg/m2 | 26.7 (4.1) | 26.6 (4.1) | 26.8 (4.2) | 0.50 |
| Hypertension, n (%) | 686 (55) | 476 (52) | 210 (61) | 0.003 |
| Diabetes, n (%) | 186 (15) | 125 (14) | 61 (18) | 0.066 |
| Heart failure, n (%) | 31 (2) | 16 (2) | 15 (4) | 0.007 |
| Coronary disease, n (%) | 245 (19) | 161 (18) | 84 (25) | 0.006 |
| Myocardial infarction, n (%) | 132 (10) | 84 (9) | 48 (14) | 0.013 |
| Mean PR interval (SD), ms | 171 (31) | 170 (29) | 174 (35) | 0.036 |
| Median PAC count (IQR), beats/h | 2.5 (0.8–9.5) | 1.8 (0.6–6.1) | 5.3 (2.1–18.0) | <0.001 |
AF = atrial fibrillation; BMI = body mass index; IQR = interquartile range; PAC = premature atrial contraction.
For the comparison of the indicated characteristic in participants with vs. those without incident AF.
Table 2.
Association Between PAC Count and Outcome Events, by Quartile*
| PAC Count | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI)† | P Value |
|---|---|---|---|---|
| Incident AF | ||||
| Quartile 1 | 1.00 (reference) | – | 1.00 (reference) | – |
| Quartile 2 | 2.13 (1.44–3.15) | <0.001 | 2.17 (1.46–3.22) | <0.001 |
| Quartile 3 | 2.80 (1.93–4.08) | <0.001 | 2.79 (1.90–4.09) | <0.001 |
| Quartile 4‡ | 5.01 (3.50–7.17) | <0.001 | 4.92 (3.39–7.16) | <0.001 |
| Beats per hour§ | 1.18 (1.14–1.22) | <0.001 | 1.17 (1.13–1.22) | <0.001 |
| Overall mortality | ||||
| Quartile 1 | 1.00 (reference) | – | 1.00 (reference) | – |
| Quartile 2 | 1.13 (0.92–1.39) | 0.25 | 0.96 (0.77–1.18) | 0.68 |
| Quartile 3 | 1.46 (1.19–1.78) | <0.001 | 1.14 (0.93–1.40) | 0.21 |
| Quartile 4‡ | 1.93 (1.59–2.34) | <0.001 | 1.35 (1.10–1.66) | 0.005 |
| Beats per hour§ | 1.10 (1.07–1.13) | <0.001 | 1.06 (1.03–1.09) | <0.001 |
| Cardiovascular mortality║ | ||||
| Quartile 1 | 1.00 (reference) | – | 1.00 (reference) | – |
| Quartile 2 | 1.01 (0.72–1.42) | 0.94 | 0.88 (0.62–1.24) | 0.47 |
| Quartile 3 | 1.37 (0.99–1.90) | 0.054 | 1.10 (0.79–1.54) | 0.57 |
| Quartile 4‡ | 2.15 (1.58–2.91) | <0.001 | 1.50 (1.08–2.08) | 0.014 |
| Beats per hour§ | 1.12 (1.08–1.17) | <0.001 | 1.08 (1.03–1.13) | 0.001 |
AF = atrial fibrillation; HR = hazard ratio; PAC = premature atrial contraction.
Quartile 1, 0–0.8 beats/h; quartile 2, 0.8–2.5 beats/h; quartile 3, 2.5–9.4 beats/h; and quartile 4, 9.5–965.4 beats/h.
Adjusted for age; sex; race; body mass index; PR interval; and history of hypertension, diabetes, myocardial infarction, coronary artery disease, and heart failure.
P value for trend <0.01 for unadjusted and adjusted comparisons.
Log-transformed with the resultant HR interpreted as the increased hazard for each doubling in ectopic beats per hour.
Death secondary to coronary artery disease, heart failure, peripheral arterial disease, or cerebrovascular disease.
PACs and Mortality
A total of 837 of the 1260 participants died during follow-up: 323 (39%) died of a cardiovascular cause, 512 (61%) died of a noncardiovascular cause, and 2 (0.2%) died of an unknown cause. Participants with PACs in the highest quartile had a significantly increased risk for death, and each doubling of the hourly PAC count was associated with significantly increased risk for cardiovascular and overall death (Table 2).
AF Risk Discrimination
The c-statistic for AF risk discrimination at 10 years using the Framingham model was 0.65 (95% CI, 0.61 to 0.67). The PAC count alone resulted in a higher c-statistic for AF risk discrimination (0.69 [CI, 0.66 to 0.72]), although this difference was of borderline statistical significance (P = 0.055). Atrial fibrillation discrimination after the addition of PAC count to the Framingham algorithm (c-statistic, 0.72 [CI, 0.68 to 0.74]) significantly improved compared with the Framingham model alone (P < 0.001). The PAC count remained a significant predictor of incident AF when added to the Framingham AF risk algorithm (P < 0.001 for all spline terms).
The c-statistic for each risk model was reduced as the observation window was extended from 5 to 15 years (Appendix Figure, available at www.annals.org). Discrimination was better for the PAC model compared with the Framingham model at each time point, although this difference only became statistically significant at 15 years. The addition of PAC count to the Framingham model resulted in a significant improvement in AF risk discrimination at each time point. When the cohort was stratified by age, the c-statistic was higher among younger participants regardless of the prediction model or age cutoff (Appendix Table 2, available at www.annals.org). The c-statistic for AF risk discrimination at 5 years using a Cox proportional hazards model that did not account for the competing risk for death was 0.68 (CI, 0.62 to 0.72) for the Framingham model, 0.73 (CI, 0.68 to 0.77) for the PAC model, and 0.76 (CI, 0.72 to 0.79) for the combined model.
AF Risk Calibration
Visual comparison of observed versus model-predicted 10-year AF risk suggested reasonable agreement for both the Framingham model and the PAC model (Figure 1). Accuracy improved after adding the PAC term to the Framingham model.
Figure 1. Observed versus predicted 10-year AF risk.
Participants are grouped into deciles of predicted risk. In the setting of perfect model calibration, observed and predicted risk would be equal (dashed line). AF = atrial fibrillation; PAC = premature atrial contraction. Top. The Framingham model. Middle. PAC. Bottom. Framingham and PAC risk models.
AF Risk Reclassification
With the addition of PAC count to the Framingham risk model, the difference between the proportion of participants diagnosed with AF who moved up a risk category and the proportion who moved down, plus the difference between the proportion of participants without AF who moved down a risk category and the proportion who moved up (net reclassification improvement), was 23.2% (CI, 12.8% to 33.6%; P < 0.001). This improvement in risk reclassification was entirely driven by enhanced prediction among persons diagnosed with AF. For participants not diagnosed with AF, overall risk prediction slightly worsened after the addition of PAC count to the Framingham model (Table 3). The difference in average predicted probability of AF between case and control participants (integrated discrimination improvement) significantly increased after adding PAC count to the Framingham model (5.6% [CI, 4.2% to 7.0%]; P < 0.001). The adjusted clinical net reclassification improvement, which quantifies the proportion of intermediate-risk participants (10-year AF risk between 15% and 20%) reclassified after the addition of PAC count, was 31.8% (CI, 4.1% to 59.6%; P = 0.025).
Table 3.
Predicted 10-Year AF Risk Using the Framingham Model With and Without Inclusion of PAC Count*
| Framingham Model | Framingham Model and PAC Count |
Total | ||
|---|---|---|---|---|
| <15% | 15%–20% | >20% | ||
| Participants with AF, n | ||||
| <15% | 25 | 25 | 19 | 69 |
| 15%–20% | 11 | 7 | 35 | 53 |
| >20% | 11 | 5 | 65 | 81 |
| Total | 47 | 37 | 119 | – |
| Participants without AF, n | ||||
| <15% | 435 | 67 | 60 | 562 |
| 15%–20% | 92 | 42 | 88 | 222 |
| >20% | 84 | 20 | 148 | 252 |
| Total | 611 | 129 | 296 | – |
AF = atrial fibrillation; PAC = premature atrial contraction.
Net reclassification improvement was 23.2% (95% CI, 12.8%–33.6%; P < 0.001).
Risk Prediction by Method of AF Diagnosis
Among the 343 participants with incident AF, 70 were diagnosed by study ECG and 273 by hospital coding. Although PACs predicted AF regardless of the AF ascertainment method, the adjusted association between log-transformed PAC count and AF differed when restricted to participants diagnosed by study ECG (hazard ratio, 1.26 [CI, 1.18 to 1.35]; P < 0.001) or hospital discharge coding (hazard ratio, 1.16 [CI, 1.11 to 1.21]; P < 0.001). The relative performance of risk model discrimination was consistent between diagnosis methods, although the c-statistics were consistently higher among participants diagnosed by study ECG (Appendix Table 3, available at www.annals.org). Integrated discrimination and net reclassification improvement also remained significantly improved after the addition of PAC count to the Framingham model regardless of the AF ascertainment method.
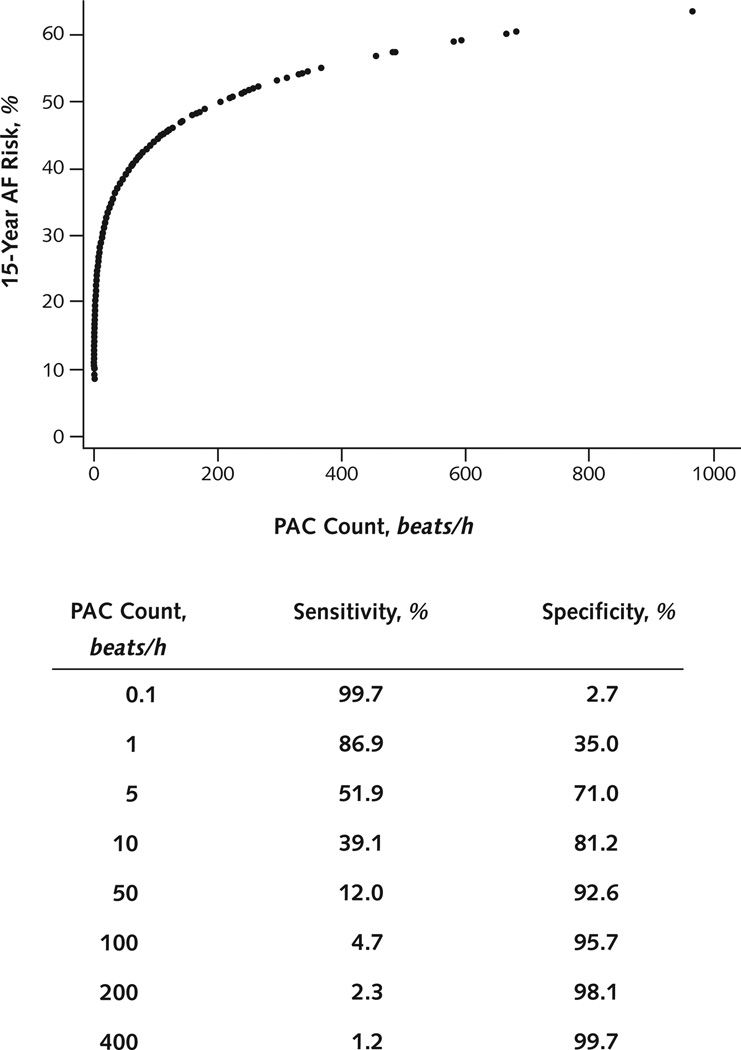
PAC Count Test Characteristics
The relationship between PAC count and 15-year AF risk showed an initial sharp increase in risk with rising PAC count that became less steep at extreme PAC frequencies (Figure 2). The specificity for predicting AF at 15 years exceeded 90% for PAC counts greater than 32 beats/h.
Figure 2. Predicted AF risk and PAC count.
The predicted 15-y risk for AF (using the log-transformed PAC model) is plotted against the hourly PAC count. The sensitivity and specificity for the diagnosis of AF at 15 y for an individual patient are listed for various PAC cutoff values. AF = atrial fibrillation; PAC = premature atrial contraction.
Discussion
In a multicenter cohort of more than 1200 participants aged 65 years or older, PAC count did as well as or better than the validated Framingham model in discriminating AF risk. The addition of PAC count to the Framingham AF prediction model resulted in significant improvements in 10-year risk discrimination, net reclassification, and integrated discrimination. Furthermore, PAC count is highly specific in predicting diagnosis of incident AF.
Assessment of atrial ectopy is readily available and easily quantifiable via ambulatory telemetry monitoring. Excess PACs have been associated with AF in patients without known cardiovascular disease (11, 12) and after the diagnosis of acute stroke (10, 11). Among patients with AF, PACs from the pulmonary veins frequently trigger AF (8). Ablation of these PACs has been shown to reduce AF recurrence (8), and empirical electrical isolation of the pulmonary veins has become the cornerstone of catheter-based therapy for AF (22, 23). These PACs could therefore be an important and modifiable risk factor for arrhythmia.
Whereas CHS participants were randomly assigned to Holter monitoring, there were identifiable differences between the monitored group and the remainder of the cohort. It should be recognized, however, that Holter monitoring was not systematically biased in favor of participants with greater AF risk factors. Although Holter participants were more likely to be male and have higher body mass indices and longer PR intervals, these persons were also younger and were less likely to have heart failure than the nonmonitored group. As such, to what degree these small differences affect the generalizability of our results remains unclear.
Although previous reports describing the association between PACs and AF have emphasized a dichotomized PAC count (10–12), our findings show that this association is continuous and independent of an arbitrary PAC cut point. However, it is clear that the specificity for identifying 15-year AF risk exceeded 90% for those with more than 32 PACs per hour. The small median change in PAC count between those participants with baseline and 5-year Holter monitoring suggests that serial assessment may not provide further information on AF risk. Our study was not designed to specifically address this question, however, and the clinical utility of repeated PAC assessment for AF risk prediction is not known.
In addition to incident AF, baseline PAC count was also associated with overall and cardiovascular mortality. Whereas a dichotomized PAC count has previously been shown to predict a composite end point of ischemic stroke, heart failure, or death (11), to our knowledge this is the first study to identify an association between PACs and mortality. Although the mechanism underlying this association remains unclear, we believe these findings substantiate the need to account for the competing risk of death during prediction of AF risk in older populations with a high mortality rate.
We used variables from the Framingham AF risk algorithm to derive the referent AF risk model. The Framingham model was originally developed to predict 10-year AF risk in the Framingham Heart Study (6) and was subsequently validated for 5-year AF prediction in CHS and the Age, Gene/Environment Susceptibility–Reykjavik Study (5). In our sensitivity analysis, discrimination of the 5-year Framingham algorithm without competing risks was identical to that reported in the previous validation study, suggesting the subset of participants in CHS with Holter data was not substantially different from the overall cohort. Interestingly, both the Framingham model and the PAC model showed improved discrimination among younger participants. Our findings imply that differences in AF risk model performance between studies can potentially be explained by duration of follow-up, age distribution of the examined cohort, and whether the analysis accounted for the competing risk of death. Notably, the significant improvement in net reclassification improvement after addition of PAC count to the Framingham model was entirely due to better risk reclassification among patients ultimately diagnosed with AF. The PAC count may therefore be most clinically useful for identifying certain high-risk patients that are not classified as such by traditional prediction algorithms.
The increased morbidity and mortality associated with AF underscores the appeal of primary prevention strategies to treat persons at high risk for the disease (3, 7). Development and assessment of preventive interventions will require accurate prediction models to identify and follow at-risk patients (7). In this capacity, PAC count substantially enhances the performance of the established Framingham AF model. Beyond improvement in risk classification for a hypothetical primary prevention intervention, the high specificity of frequent PACs for incident AF invites speculation whether selected patients with a high PAC burden may be candidates for PAC ablation (or other treatments, such as antiarrhythmic drugs) to reduce long-term risk for AF. It should be stressed, however, no current data support such a therapeutic strategy. Because age is a significant, independent risk factor for AF, our cohort of participants 65 years of age or older represents a population in which PAC screening may have substantial utility for identifying high-risk patients. Further studies are warranted to determine the relative risk and benefit profile of invasive or pharmacologic therapies to treat PACs.
Limitations of this analysis should be acknowledged. We did not attempt to characterize the location of PAC origin, nor did we determine whether more than 1 atrial focus was present. Although this limitation does not weaken our findings, it may have implications if catheter-based ablation strategies are considered in future intervention studies. It is also possible that some participants with a high baseline PAC count may have been misdiagnosed during follow-up because of misinterpretation of frequent atrial ectopy as AF. We believe this is less likely given the results of our sensitivity analyses showing a consistent PAC–AF association and risk model performance independent of diagnosis method (ECG vs. hospital discharge coding). It is important to recognize that this study does not establish a causal link between PACs and AF, and the ability to prevent AF by means of PAC suppression is currently speculative. Furthermore, CHS participants who had Holter analysis were enrolled between 1989 and 1990, permitting a long duration of follow-up. Although methods for PAC assessment and AF diagnosis have not substantially changed over this interval, it remains possible that secular trends could modify the association between these variables in a more contemporary population. Finally, this study investigated the association between atrial ectopy and AF in a single, community-based longitudinal cohort of older, predominantly white participants. Our results should not be extrapolated to younger or multiracial populations.
In conclusion, PAC count is a significant predictor of incident AF. This biomarker provides significantly superior AF risk discrimination and improved risk reclassification when added to a previously validated AF risk model. The specificity of atrial ectopy for the long-term diagnosis of AF is high in the setting of a modestly elevated PAC count. In light of prior investigations indicating that atrial ectopy may be modifiable by catheter ablation, these results provide enthusiasm for future research investigating the role of PAC modification for primary AF prevention.
Context
Models to predict incident atrial fibrillation (AF) would have enhanced clinical utility if they identified potentially modifiable risks factors.
Contribution
This study found that increases in the hourly number of premature atrial contractions (PACs) were associated with an increased risk for incident AF and overall mortality. Addition of PAC count improved the performance of the Framingham AF prediction model.
Caution
These data do not establish causation or whether suppressing PACs is appropriate for primary prevention of AF.
Implication
The PAC count improves the prediction of incident AF. Additional studies should assess whether PAC modification can reduce the occurrence of AF.
—The Editors
Acknowledgments
Grant Support: By grants 12POST11810036 (Dr. Dewland) and 12GRNT11780061 (Dr. Marcus) from the American Heart Association, the Joseph Drown Foundation (Dr. Marcus), and grant R01HL116747 (Dr. Sotoodehnia) from the National Heart, Lung, and Blood Institute; contracts HHSN268201200036C, N01HC85239, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke; and grant AG023629 from the National Institute on Aging.
Appendix
Appendix Table 1. Baseline Characteristics of CHS Participants With and Without Holter Monitoring*
| Characteristic | Holter (n = 1429) |
No Holter (n = 3772) |
P Value |
|---|---|---|---|
| Median age (IQR), y | 71 (68–75) | 72 (68–77) | <0.001 |
| Female, n (%) | 763 (53) | 2199 (58) | 0.001 |
| White, n (%) | 1358 (95) | 3567 (95) | 0.50 |
| Mean BMI (SD), kg/m2 | 26.6 (4.2) | 26.3 (4.6) | 0.033 |
| Hypertension, n (%) | 783 (55) | 2159 (57) | 0.113 |
| Diabetes, n (%) | 217 (15) | 571 (15) | 0.99 |
| Heart failure, n (%) | 49 (3) | 181 (5) | 0.032 |
| Coronary disease, n (%) | 287 (20) | 735 (19) | 0.63 |
| Myocardial infarction, n (%) | 159 (11) | 356 (9) | 0.069 |
| Mean PR interval (SD), ms | 167 (41) | 163 (45) | 0.004 |
BMI = body mass index; CHS = Cardiovascular Health Study; IQR = interquartile range.
Participants were randomly selected to have Holter monitoring at study enrollment. From the 1429 participants with Holter monitoring, 1260 were included in incident atrial fibrillation analyses.
Appendix Figure. AF risk discrimination, by follow-up time.
The c-statistics for AF risk discrimination using the Framingham (square), PAC (circle), and Framingham and PAC (triangle) models are shown after censoring follow-up at 5, 10, and 15 y. All models used competing risk method with spline-transformed PAC counts. Error bars denote 95% CIs. AF = atrial fibrillation; PAC = premature atrial contraction.
* For comparison of the Framingham model c-statistic with the PAC model c-statistic.
† For comparison of the Framingham model c-statistic with the combined Framingham and PAC model c-statistic.
Appendix Table 2. 10-Year AF Risk Discrimination, by Age*
| Model | C-Statistic at 10-y Follow-Up | |||||
|---|---|---|---|---|---|---|
| Age <71 y (n = 629) | Age ≥71 y (n = 631) | P Value* | Age <75 y (n = 942) | Age ≥75 y (n = 318) | P Value† | |
| Framingham | 0.71 | 0.57 | <0.001 | 0.67 | 0.58 | 0.012 |
| PAC | 0.72 | 0.66 | 0.070 | 0.69 | 0.66 | 0.46 |
| Framingham and PAC | 0.81 | 0.67 | <0.001 | 0.75 | 0.66 | 0.013 |
AF = atrial fibrillation; PAC = premature atrial contraction.
Cohort stratified by 50th and 75th age percentiles (71 and 75 y, respectively).
For comparison of c-statistic between age strata in each model.
Appendix Table 3. Risk Discrimination and Reclassification at 10 Years, by Method of AF Diagnosis*
| Variable | Study ECG (n = 70) |
Discharge Coding (n = 138) |
||
|---|---|---|---|---|
| Score (95% CI) | P Value | Score (95% CI) | P Value | |
| C-statistic | ||||
| Framingham | 0.72 (0.65–0.76) | 1.00 (reference) | 0.62 (0.55–0.65) | 1.00 (reference) |
| PAC | 0.77 (0.72–0.81) | 0.142† | 0.66 (0.60–0.69) | 0.28† |
| Framingham and PAC | 0.82 (0.76–0.85) | <0.001† | 0.69 (0.63–0.71) | 0.001† |
| NRI | 26.9 (12.2–41.6) | <0.001 | 13.9 (2.8–25.0) | 0.014 |
| IDI | 7.9 (5.2–10.6) | <0.001 | 2.3 (1.2–3.4) | <0.001 |
AF = atrial fibrillation; ECG = electrocardiogram; IDI = integrated discrimination improvement; NRI = net reclassification improvement; PAC = premature atrial contraction.
At 10-y follow-up, 70 of the 208 AF diagnoses had been made by using study ECGs and 138 by hospital coding.
For comparison of the designated model c-statistic with the Framingham model c-statistic in the same AF diagnosis stratum.
Footnotes
Publisher's Disclaimer: Disclaimer: Drs. Dewland and Marcus had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A full list of principal Cardiovascular Health Study investigators and institutions can be found at www.chs-nhlbi.org. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M13-1229.
Reproducible Research Statement: Study protocol and statistical code: Available from Dr. Marcus (marcusg@medicine.ucsf.edu). Data set: Available through established CHS procedures for obtaining and analyzing data (see https://chs-nhlbi.org/).
Author Contributions: Conception and design: T.A. Dewland, M.C. Mandyam, G.M. Marcus.
Analysis and interpretation of the data: T.A. Dewland, E. Vittinghoff, M.C. Mandyam, S.R. Heckbert, D.S. Siscovick, P.K. Stein, B.M. Psaty, N. Sotoodehnia, J.S. Gottdeiner, G.M. Marcus.
Drafting of the article: T.A. Dewland, E. Vittinghoff, M.C. Mandyam, G.M. Marcus.
Critical revision of the article for important intellectual content: T.A. Dewland, E. Vittinghoff, M.C. Mandyam, S.R. Heckbert, D.S. Siscovick, P.K. Stein, B.M. Psaty, N. Sotoodehnia, J.S. Gottdeiner, G.M. Marcus.
Final approval of the article: T.A. Dewland, E. Vittinghoff, M.C. Mandyam, S.R. Heckbert, D.S. Siscovick, P.K. Stein, B.M. Psaty, N. Sotoodehnia, J.S. Gottdiener, G.M. Marcus.
Provision of study materials or patients: S.R. Heckbert, D.S. Siscovick, P.K. Stein, B.M. Psaty, N. Sotoodehnia, J.S. Gottdeiner.
Statistical expertise: T.A. Dewland, E. Vittinghoff, G.M. Marcus.
Obtaining of funding: T.A. Dewland, G.M. Marcus.
Administrative, technical, or logistic support: T.A. Dewland, S.R. Heckbert, D.S. Siscovick, P.K. Stein, B.M. Psaty, N. Sotoodehnia, J.S. Gottdeiner, G.M. Marcus.
Collection and assembly of data: T.A. Dewland, M.C. Mandyam, S.R. Heckbert, D.S. Siscovick, P.K. Stein, B.M. Psaty, N. Sotoodehnia, J.S. Gottdeiner, G.M. Marcus.
References
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [PMID: 19932788] [DOI] [PubMed] [Google Scholar]
- 2.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [PMID: 8841325] [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [PMID: 9737513] [DOI] [PubMed] [Google Scholar]
- 4.Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda JG, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace. 2011;13:1375–1385. doi: 10.1093/europace/eur194. [PMID: 21757483] [DOI] [PubMed] [Google Scholar]
- 5.Schnabel RB, Aspelund T, Li G, Sullivan LM, Suchy-Dicey A, Harris TB, et al. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170:1909–1917. doi: 10.1001/archinternmed.2010.434. [PMID: 21098350] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D’Agostino RB, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [PMID: 19249635] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus GM. Predicting incident atrial fibrillation: an important step toward primary prevention [Editorial] Arch Intern Med. 2010;170:1874–1875. doi: 10.1001/archinternmed.2010.426. [PMID: 21098344] [DOI] [PubMed] [Google Scholar]
- 8.Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [PMID: 9725923] [DOI] [PubMed] [Google Scholar]
- 9.Kolb C, Nürnberger S, Ndrepepa G, Zrenner B, Schömig A, Schmitt C. Modes of initiation of paroxysmal atrial fibrillation from analysis of spontaneously occurring episodes using a 12-lead Holter monitoring system. Am J Cardiol. 2001;88:853–857. doi: 10.1016/s0002-9149(01)01891-4. [PMID: 11676946] [DOI] [PubMed] [Google Scholar]
- 10.Wallmann D, Tüller D, Wustmann K, Meier P, Isenegger J, Arnold M, et al. Frequent atrial premature beats predict paroxysmal atrial fibrillation in stroke patients: an opportunity for a new diagnostic strategy. Stroke. 2007;38:2292–2294. doi: 10.1161/STROKEAHA.107.485110. [PMID: 17585079] [DOI] [PubMed] [Google Scholar]
- 11.Chong BH, Pong V, Lam KF, Liu S, Zuo ML, Lau YF, et al. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. doi: 10.1093/europace/eur389. [PMID: 22183750] [DOI] [PubMed] [Google Scholar]
- 12.Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. [PMID: 20404258] [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [PMID: 1669507] [DOI] [PubMed] [Google Scholar]
- 14.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [PMID: 8275211] [DOI] [PubMed] [Google Scholar]
- 15.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [PMID: 8520709] [DOI] [PubMed] [Google Scholar]
- 16.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [PMID: 8520708] [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Amer Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [PMID: 19364974] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [PMID: 19367167] [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-12. [PMID: 17569110] [DOI] [PubMed] [Google Scholar]
- 21.Cook NR, Paynter NP. Performance of reclassification statistics in comparing risk prediction models. Biom J. 2011;53:237–258. doi: 10.1002/bimj.201000078. [PMID: 21294152] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, end-points, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632.e21–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [PMID: 22386883] [DOI] [PubMed] [Google Scholar]
- 23.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, et al. ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [PMID: 20103757] [DOI] [PubMed] [Google Scholar]