Abstract
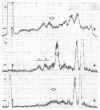
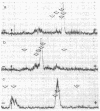
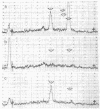
Bovine testicular β-galactosidase (β-D-galactoside galactohydrolase, EC 3.2.1.23) is rapidly and selectively assimilated by human skin fibroblasts. The assimilation of the enzyme is strongly inhibited by mannose 6-phosphate and by a glycoprotein fraction isolated from bovine testes (glycoprotein inhibitors). These results suggest that β-galactosidase and the glycoprotein inhibitors have a common recognition marker that contains mannose 6-phosphate. The presence of mannose phosphate in the glycoprotein inhibitors was demonstrated by acid hydrolysis of the glycoproteins to liberate mannose phosphate followed by reduction with NaB3H4 to give [3H]mannitol phosphate. The 3H-labeled compound was identified by paper electrophoresis and by the release of [3H]mannitol on treatment with phosphatase. The [3H]mannitol phosphate was oxidized with periodate and the resulting phosphorylated fragment, on reduction with NaB3H4, yielded [3H]ethylene glycol phosphate, indicating substitution of phosphate on carbon 6 of mannitol. Mannose 6-phosphate was also found in a major carbohydrate-containing fraction of peptides produced from the glycoprotein inhibitors by tryspin digestion. It was estimated that about 2% of the mannose residues were present as mannose 6-phosphate. Phosphorylated oligosaccharides were also identified in hydrolysates of the glycoprotein inhibitors. One, a disaccharide, was identified as α-(mannosyl-6-phosphate)-(1 → 2)-mannose. These observations suggest that the recognition marker of β-galactosidase contains α1,2-linked mannose 6-phosphate; terminal α1,2-linked mannose residues are known to occur in the high-mannose type oligosaccharides present on β-galactosidase.
Keywords: adsorptive endocytosis, recognition marker, lysosomal enzymes
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Ballou C. E., Raschke W. C. Polymorphism of the somatic antigen of yeast. Science. 1974 Apr 12;184(4133):127–134. doi: 10.1126/science.184.4133.127. [DOI] [PubMed] [Google Scholar]
- DISTLER J. J., MERRICK J. M., ROSEMAN S. Glucosamine metabolism. III. Preparation and N-acetylation of crystalline D-glucosamine- and D-galactosamine-6-phosphoric acids. J Biol Chem. 1958 Jan;230(1):497–509. [PubMed] [Google Scholar]
- Distler J. J., Jourdian G. W. beta-Galactosidase from bovine testes. Methods Enzymol. 1978;50:514–520. doi: 10.1016/0076-6879(78)50055-4. [DOI] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- Hieber V., Distler J., Myerowitz R., Schmickel R. D., Jourdian G. W. The role of glycosidically bound mannose in the assimilation of beta-galactosidase by generalized gangliosidosis fibroblasts. Biochem Biophys Res Commun. 1976 Dec 6;73(3):710–717. doi: 10.1016/0006-291x(76)90868-8. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Hirs C. H. The primary structure of porcine pancreatic ribonuclease. I. The distribution and sites of carbohydrate attachment. J Biol Chem. 1970 Feb 10;245(3):624–636. [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Fischer D., Achord D., Sly W. Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J Clin Invest. 1977 Nov;60(5):1088–1093. doi: 10.1172/JCI108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Fischer D., Sly W. S. Correlation of structural features of phosphomannans with their ability to inhibit pinocytosis of human beta-glucuronidase by human fibroblasts. J Biol Chem. 1978 Feb 10;253(3):647–650. [PubMed] [Google Scholar]
- LLOYD A. G. Fractionation of the products of the direct sulphation of monosaccharides on anion-exchange resin. Biochem J. 1962 Jun;83:455–460. doi: 10.1042/bj0830455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neufeld E. F., Sando G. N., Garvin A. J., Rome L. H. The transport of lysosomal enzymes. J Supramol Struct. 1977;6(1):95–101. doi: 10.1002/jss.400060108. [DOI] [PubMed] [Google Scholar]
- Porter W. H. Application of nitrous acid deamination of hexosamines to the simultaneous GLC determination of neutral and amino sugars in glycoproteins. Anal Biochem. 1975 Jan;63(1):27–43. doi: 10.1016/0003-2697(75)90186-4. [DOI] [PubMed] [Google Scholar]
- Robison R. Hexosemonophosphoric esters: mannosemonophosphate. Biochem J. 1932;26(6):2191–2202. doi: 10.1042/bj0262191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Slodki M. E., Ward R. M., Boundy J. A. Concanavalin A as a probe of phosphomannan molecular structure. Biochim Biophys Acta. 1973 Apr 28;304(2):449–456. doi: 10.1016/0304-4165(73)90264-x. [DOI] [PubMed] [Google Scholar]
- Ullrich K., Mersmann G., Weber E., Von Figura K. Evidence for lysosomal enzyme recognition by human fibroblasts via a phosphorylated carbohydrate moiety. Biochem J. 1978 Mar 15;170(3):643–650. doi: 10.1042/bj1700643. [DOI] [PMC free article] [PubMed] [Google Scholar]