Abstract
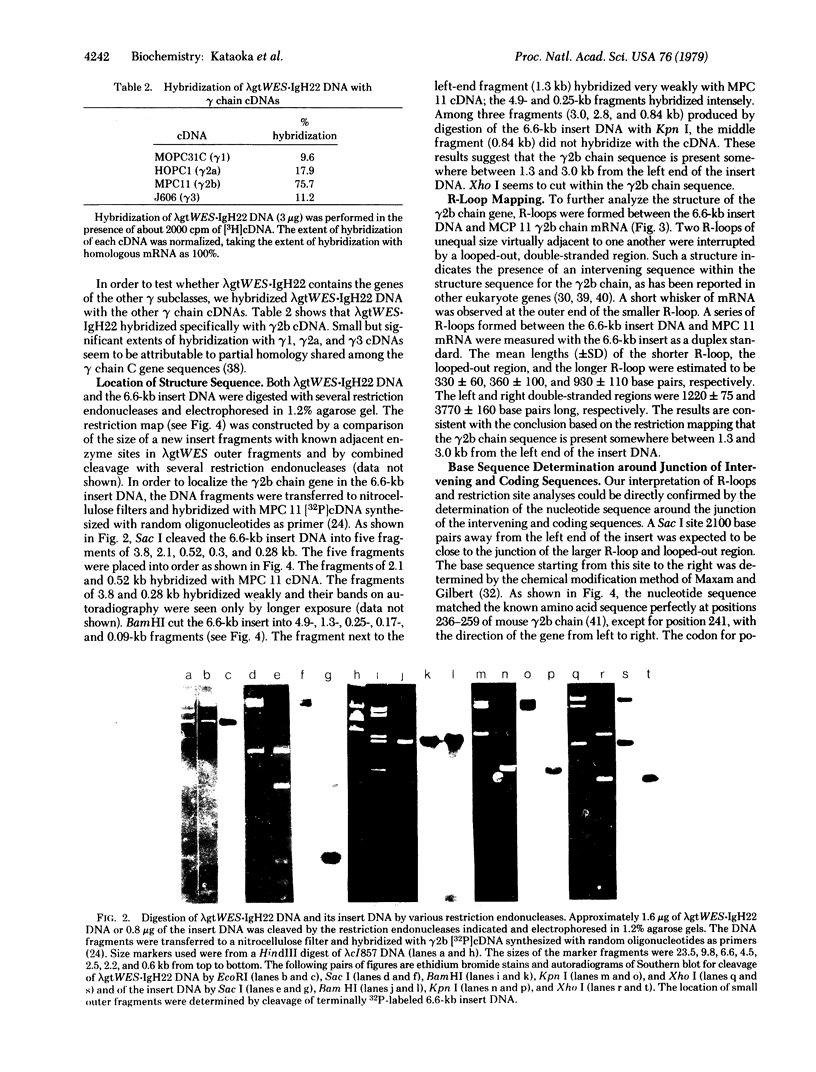
DNA from newborn mice was digested with restriction endonuclease EcoRI, and a 6.6-kilobase fragment encoding immunoglobulin gamma 2b chain mRNA derived from MPC 11 myeloma was enriched about 100-fold by RPC-5 column chromatography and agarose gell electrophoresis. The 6.6-kilobase fragment was cloned with lambda gt WES.lambda B as EK2 vector. The cloned phage (lambda WES.IgH22) contained the constant region gene of the gamma 2b chain but not the variable region gene of MPC 11 mRNA. The constant region genes of the other gamma chains (i.e., gamma 1, gamma 2a, and gamma 3) were not present in lambda gt WES.IgH22 DNA. R-loop mapping indicates that the gamma 2b chain structural gene is divided into two parts (330 +/- 60 SD base pairs and 930 +/- 110 SD base pairs) by an intervening sequence (360 +/- 100 SD base pairs). The nucleotide sequence around the junction of the hinge region and CH2 domain was determined and shown to match the amino acid sequence of the initial part of the CH2 domain of the gamma 2b chain. The base sequence upstream from the junction, however, is unrelated to the amino acid sequence of the CH1 domain and the hinge region of all the gamma chains whose sequences have been determined. These results indicate that the gamma 2b chain gene is interrupted at the junction of the hinge region and CH2 domain by an intervening sequence. The existence of two more intervening sequences, one between the CH1 domain and the hinge region and the other between the CH2 and CH3 domains, is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birshtein B. K., Hussain Q. Z., Cebra J. J. Structure of heavy chain from strain 13 guinea pig immunoglobulin-G(2). 3. Amino acid sequence of the region around the half-cystine joining heavy and light chains. Biochemistry. 1971 Jan 5;10(1):18–25. doi: 10.1021/bi00777a003. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brack C., Tonegawa S. Variable and constant parts of the immunoglobulin light chain gene of a mouse myeloma cell are 1250 nontranslated bases apart. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5652–5656. doi: 10.1073/pnas.74.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Colberg J. E., Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966 Mar 1;123(3):547–558. doi: 10.1084/jem.123.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Steiner L. A., Porter R. R. The partial sequence of two large peptides from the N-terminal half of heavy chains from normal rabbit immunoglobulin G. Biochem J. 1968 Mar;107(1):79–88. doi: 10.1042/bj1070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. M., Franklin E. C., Frangione B. Molecular defect in a gamma-2 heavy chain. Science. 1972 Apr 14;176(4031):187–189. doi: 10.1126/science.176.4031.187. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science. 1978 Dec 22;202(4374):1257–1260. doi: 10.1126/science.364651. [DOI] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fougereau M., Bourgois A., de Preval C., Rocca-Serra J., Schiff C. The complete sequence of the murine monoclonal immunoglobulin MOPC 173 (IgG2a): genetic implications. Ann Immunol (Paris) 1976 Sep-Oct;127(5):607–631. [PubMed] [Google Scholar]
- Francus T., Birshtein B. K. An IgG2a-producing variant of an IgG2b-producing mouse myeloma cell line. Structural studies on the Fc region of parent and variant heavy chains. Biochemistry. 1978 Oct 3;17(20):4324–4331. doi: 10.1021/bi00613a033. [DOI] [PubMed] [Google Scholar]
- Frangione B., Lee L., Haber E., Bloch K. J. Protein Hal: partial deletion of a " " immunoglobulin gene(s) and apparent reinitiation at an internal AUG codon. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1073–1077. doi: 10.1073/pnas.70.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Partial deletion in the heavy chain disease protein ZUC. Nature. 1969 Nov 8;224(5219):597–599. doi: 10.1038/224597a0. [DOI] [PubMed] [Google Scholar]
- Franklin E. C., Frangione B. The molecular defect in a protein (CRA) found in gamma-1 heavy chain disease, and its genetic implications. Proc Natl Acad Sci U S A. 1971 Jan;68(1):187–191. doi: 10.1073/pnas.68.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruchter R. G., Jackson S. A., Mole L. E., Porter R. R. Sequence studies of the Fd section of the heavy chain of rabbit immunoglobulin G. Biochem J. 1970 Jan;116(2):249–259. doi: 10.1042/bj1160249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Kataoka T. Organization of immunoglobulin heavy chain genes and allelic deletion model. Proc Natl Acad Sci U S A. 1978 May;75(5):2140–2144. doi: 10.1073/pnas.75.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Packman S. Quantitation of constant and variable region genes for mouse immunoglobulin lambda chains. Biochemistry. 1976 Jun 29;15(13):2780–2785. doi: 10.1021/bi00658a012. [DOI] [PubMed] [Google Scholar]
- Honjo T., Packman S., Swan D., Nau M., Leder P. Organization of immunoglobulin genes: reiteration frequency of the mouse kappa chain constant region gene. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3659–3663. doi: 10.1073/pnas.71.9.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kindt T. J., Mandy W. J., Todd C. W. Association of allotypic specificities of group a with allotypic specificities A11 and A12 in rabbit immunoglobulin. Biochemistry. 1970 Apr 28;9(9):2028–2032. doi: 10.1021/bi00811a026. [DOI] [PubMed] [Google Scholar]
- Kunkel H. G., Natvig J. B., Joslin F. G. A "Lepore" type of hybrid gamma-globulin. Proc Natl Acad Sci U S A. 1969 Jan;62(1):144–149. doi: 10.1073/pnas.62.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mäkelä O., Cross A. M. The diversity and specialization of immunocytes. Prog Allergy. 1970;14:145–207. doi: 10.1159/000289379. [DOI] [PubMed] [Google Scholar]
- Natvig J. B., Kunkel H. G. A HYBRID IgG4-IgG2 immunoglobulin. J Immunol. 1974 Apr;112(4):1277–1284. [PubMed] [Google Scholar]
- Ono M., Kondo T., Kawakami M., Honjo T. Purification of immunoglobulin heavy chain messenger RNA by immunoprecipitation from the mouse myeloma tumor, MOPC-31C. J Biochem. 1977 Apr;81(4):949–954. doi: 10.1093/oxfordjournals.jbchem.a131560. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsky F., Edgell M. H., Seidman J. G., Leder P. High capacity gel preparative electrophoresis for purification of fragments of genomic DNA. Anal Biochem. 1978 Jul 1;87(2):397–410. doi: 10.1016/0003-2697(78)90689-9. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. A., Smith D. F., Appella E. Chemical characterization of a mouse immunoglobulin A heavy chain with a 100-residue deletion. Amino acid and carbohydrate compositions and NH2-and COOH-terminal sequences. J Biol Chem. 1974 Oct 25;249(20):6605–6610. [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Takanami M. Specific cleavage of coliphage fd DNA by five different restriction endonucleases from Haemophilus genus. FEBS Lett. 1973 Aug 15;34(2):318–322. doi: 10.1016/0014-5793(73)80821-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Polsky F., Edgell M. H., Seidman J. G., Leder A., Enquist L. W., Norman B., Leder P. Cloning specific segments of the mammalian genome: bacteriophage lambda containing mouse globin and surrounding gene sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4406–4410. doi: 10.1073/pnas.74.10.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S. L., Dubiski S., Mage R. G. Distribution of allotypic specificities A1, A2, A14, and A15 among immunoglobulin G molecules. J Immunol. 1970 Mar;104(3):641–647. [PubMed] [Google Scholar]
- Turner K. J., Cebra J. J. Structure of heavy chain from strain 13 guinea pig immunoglobulin-G(2). II. Amino acid sequence of the carboxyl-terminal and hinge region cyanogen bromide fragments. Biochemistry. 1971 Jan 5;10(1):9–17. doi: 10.1021/bi00777a002. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Inokuchi H., Ozeki H. Excision and duplication of su3+-transducing fragments carried by bacteriophage phi 80. I. Novel structure of phi 80sus2psu3+ DNA molecule. J Virol. 1976 Jun;18(3):1016–1023. doi: 10.1128/jvi.18.3.1016-1023.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Sato K., Shimizu A., Kataoka T., Mano Y., Ono M., Kawakami M., Honjo T. Mutual homology of mouse immunoglobulin gamma-chain gene sequences. Biochemistry. 1979 Feb 6;18(3):490–494. doi: 10.1021/bi00570a018. [DOI] [PubMed] [Google Scholar]